How to Combine Phospholipid Insights with Genomics?
JUL 16, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phospholipid-Genomics Integration Background
The integration of phospholipid insights with genomics represents a cutting-edge approach in the field of molecular biology and personalized medicine. This convergence of two distinct yet interconnected areas of study has the potential to revolutionize our understanding of cellular processes and disease mechanisms.
Phospholipids, the primary components of cell membranes, play crucial roles in cellular signaling, membrane trafficking, and overall cell function. Traditionally, phospholipid research has focused on their structural and functional properties within cellular systems. On the other hand, genomics has been at the forefront of understanding the genetic basis of biological processes and diseases.
The synergy between phospholipid research and genomics has emerged as a promising avenue for comprehensive biological insights. This integration allows researchers to explore the complex relationships between genetic variations and phospholipid metabolism, composition, and function. By combining these two fields, scientists can gain a more holistic view of how genetic factors influence lipid profiles and how lipid alterations may, in turn, affect gene expression and cellular processes.
Recent technological advancements have facilitated this integration. High-throughput lipidomics techniques, coupled with next-generation sequencing and bioinformatics tools, have enabled researchers to analyze vast amounts of data on both phospholipids and genomic information simultaneously. This has led to the identification of novel genetic variants associated with lipid metabolism disorders and the discovery of lipid-related biomarkers for various diseases.
The potential applications of this integrated approach are far-reaching. In the realm of personalized medicine, understanding the interplay between an individual's genetic makeup and their phospholipid profile could lead to more targeted and effective treatments for metabolic disorders, cardiovascular diseases, and even certain types of cancer. Moreover, this integration could provide new insights into drug development, as many pharmaceuticals interact with or target lipid-based cellular components.
As research in this field progresses, we are witnessing a paradigm shift in how we approach complex biological questions. The combination of phospholipid insights with genomics is not just a merger of two disciplines but a new frontier in biomedical research that promises to unravel the intricate relationships between genes, lipids, and human health.
Phospholipids, the primary components of cell membranes, play crucial roles in cellular signaling, membrane trafficking, and overall cell function. Traditionally, phospholipid research has focused on their structural and functional properties within cellular systems. On the other hand, genomics has been at the forefront of understanding the genetic basis of biological processes and diseases.
The synergy between phospholipid research and genomics has emerged as a promising avenue for comprehensive biological insights. This integration allows researchers to explore the complex relationships between genetic variations and phospholipid metabolism, composition, and function. By combining these two fields, scientists can gain a more holistic view of how genetic factors influence lipid profiles and how lipid alterations may, in turn, affect gene expression and cellular processes.
Recent technological advancements have facilitated this integration. High-throughput lipidomics techniques, coupled with next-generation sequencing and bioinformatics tools, have enabled researchers to analyze vast amounts of data on both phospholipids and genomic information simultaneously. This has led to the identification of novel genetic variants associated with lipid metabolism disorders and the discovery of lipid-related biomarkers for various diseases.
The potential applications of this integrated approach are far-reaching. In the realm of personalized medicine, understanding the interplay between an individual's genetic makeup and their phospholipid profile could lead to more targeted and effective treatments for metabolic disorders, cardiovascular diseases, and even certain types of cancer. Moreover, this integration could provide new insights into drug development, as many pharmaceuticals interact with or target lipid-based cellular components.
As research in this field progresses, we are witnessing a paradigm shift in how we approach complex biological questions. The combination of phospholipid insights with genomics is not just a merger of two disciplines but a new frontier in biomedical research that promises to unravel the intricate relationships between genes, lipids, and human health.
Market Demand Analysis
The convergence of phospholipid research and genomics represents a burgeoning field with significant market potential. This intersection addresses critical needs in personalized medicine, drug development, and nutritional science. The global market for precision medicine, which encompasses genomics and lipidomics, is projected to reach $141.3 billion by 2026, with a compound annual growth rate of 11.5%.
Pharmaceutical companies are increasingly investing in phospholipid-genomic research to develop targeted therapies. This approach allows for more effective drug design by understanding how genetic variations influence lipid metabolism and drug response. The demand for such tailored treatments is driven by the growing prevalence of chronic diseases and the push for more efficient, personalized healthcare solutions.
In the field of diagnostics, the combination of phospholipid analysis and genomics offers promising applications for early disease detection and risk assessment. This is particularly relevant for cardiovascular diseases, neurological disorders, and certain cancers, where lipid profiles and genetic markers play crucial roles. The global biomarkers market, which benefits from these advancements, is expected to reach $78.9 billion by 2027.
Nutritional science and the wellness industry are also capitalizing on this integrated approach. Consumers are increasingly seeking personalized nutrition plans based on their genetic makeup and lipid profiles. This trend is fueling the growth of the nutrigenomics market, which is anticipated to expand at a CAGR of 16.48% from 2021 to 2028.
Academic and research institutions are showing heightened interest in combining phospholipid insights with genomics. This is evidenced by the increasing number of publications and research grants in this area. The demand for advanced analytical tools and bioinformatics platforms that can integrate genomic and lipidomic data is also on the rise, creating opportunities for technology providers.
The agricultural sector is another area where this combined approach is gaining traction. Understanding the interplay between plant genetics and lipid composition is crucial for developing crops with enhanced nutritional profiles and stress resistance. This application aligns with the growing demand for sustainable and nutritionally optimized food sources.
Despite the promising outlook, challenges remain in terms of data integration, standardization of methodologies, and the need for interdisciplinary expertise. Addressing these challenges presents market opportunities for companies specializing in data analytics, machine learning, and systems biology approaches tailored to phospholipid-genomic research.
Pharmaceutical companies are increasingly investing in phospholipid-genomic research to develop targeted therapies. This approach allows for more effective drug design by understanding how genetic variations influence lipid metabolism and drug response. The demand for such tailored treatments is driven by the growing prevalence of chronic diseases and the push for more efficient, personalized healthcare solutions.
In the field of diagnostics, the combination of phospholipid analysis and genomics offers promising applications for early disease detection and risk assessment. This is particularly relevant for cardiovascular diseases, neurological disorders, and certain cancers, where lipid profiles and genetic markers play crucial roles. The global biomarkers market, which benefits from these advancements, is expected to reach $78.9 billion by 2027.
Nutritional science and the wellness industry are also capitalizing on this integrated approach. Consumers are increasingly seeking personalized nutrition plans based on their genetic makeup and lipid profiles. This trend is fueling the growth of the nutrigenomics market, which is anticipated to expand at a CAGR of 16.48% from 2021 to 2028.
Academic and research institutions are showing heightened interest in combining phospholipid insights with genomics. This is evidenced by the increasing number of publications and research grants in this area. The demand for advanced analytical tools and bioinformatics platforms that can integrate genomic and lipidomic data is also on the rise, creating opportunities for technology providers.
The agricultural sector is another area where this combined approach is gaining traction. Understanding the interplay between plant genetics and lipid composition is crucial for developing crops with enhanced nutritional profiles and stress resistance. This application aligns with the growing demand for sustainable and nutritionally optimized food sources.
Despite the promising outlook, challenges remain in terms of data integration, standardization of methodologies, and the need for interdisciplinary expertise. Addressing these challenges presents market opportunities for companies specializing in data analytics, machine learning, and systems biology approaches tailored to phospholipid-genomic research.
Current Challenges
The integration of phospholipid insights with genomics presents several significant challenges that researchers and scientists are currently grappling with. One of the primary obstacles is the complexity of data integration. Phospholipid profiles and genomic data are inherently different in nature, scale, and structure, making it difficult to establish meaningful correlations and draw comprehensive conclusions.
Another major challenge lies in the development of robust analytical tools and algorithms capable of processing and interpreting the vast amounts of data generated from both phospholipid and genomic analyses. Current bioinformatics platforms often lack the sophistication needed to handle the multidimensional nature of combined phospholipid-genomic datasets effectively.
The issue of data standardization also poses a significant hurdle. The lack of uniform protocols for data collection, processing, and reporting across different laboratories and research institutions hampers the ability to compare and integrate findings from various studies. This inconsistency in data formats and quality control measures makes it challenging to build comprehensive, reliable databases that can serve as a foundation for advanced analyses.
Furthermore, the biological complexity of phospholipid-genomic interactions presents a formidable challenge. The dynamic nature of phospholipid metabolism and its intricate relationship with gene expression and regulation are not fully understood. This knowledge gap makes it difficult to develop accurate models that can predict how genetic variations might influence phospholipid profiles or vice versa.
The temporal aspect of phospholipid-genomic interactions adds another layer of complexity. While genomic data remains relatively stable over time, phospholipid profiles can fluctuate rapidly in response to environmental factors, diet, and physiological states. Capturing and accounting for these temporal variations in integrated analyses remains a significant technical challenge.
Lastly, the interdisciplinary nature of combining phospholipid insights with genomics requires expertise from diverse fields, including biochemistry, genetics, bioinformatics, and systems biology. The shortage of researchers with cross-disciplinary knowledge and skills in these areas limits the pace of progress in developing integrated approaches and interpreting complex datasets.
Addressing these challenges will require concerted efforts in developing advanced computational tools, establishing standardized protocols, enhancing our understanding of phospholipid-genomic interactions, and fostering interdisciplinary collaboration. Overcoming these hurdles will be crucial for unlocking the full potential of integrating phospholipid insights with genomics in advancing our understanding of biological systems and improving healthcare outcomes.
Another major challenge lies in the development of robust analytical tools and algorithms capable of processing and interpreting the vast amounts of data generated from both phospholipid and genomic analyses. Current bioinformatics platforms often lack the sophistication needed to handle the multidimensional nature of combined phospholipid-genomic datasets effectively.
The issue of data standardization also poses a significant hurdle. The lack of uniform protocols for data collection, processing, and reporting across different laboratories and research institutions hampers the ability to compare and integrate findings from various studies. This inconsistency in data formats and quality control measures makes it challenging to build comprehensive, reliable databases that can serve as a foundation for advanced analyses.
Furthermore, the biological complexity of phospholipid-genomic interactions presents a formidable challenge. The dynamic nature of phospholipid metabolism and its intricate relationship with gene expression and regulation are not fully understood. This knowledge gap makes it difficult to develop accurate models that can predict how genetic variations might influence phospholipid profiles or vice versa.
The temporal aspect of phospholipid-genomic interactions adds another layer of complexity. While genomic data remains relatively stable over time, phospholipid profiles can fluctuate rapidly in response to environmental factors, diet, and physiological states. Capturing and accounting for these temporal variations in integrated analyses remains a significant technical challenge.
Lastly, the interdisciplinary nature of combining phospholipid insights with genomics requires expertise from diverse fields, including biochemistry, genetics, bioinformatics, and systems biology. The shortage of researchers with cross-disciplinary knowledge and skills in these areas limits the pace of progress in developing integrated approaches and interpreting complex datasets.
Addressing these challenges will require concerted efforts in developing advanced computational tools, establishing standardized protocols, enhancing our understanding of phospholipid-genomic interactions, and fostering interdisciplinary collaboration. Overcoming these hurdles will be crucial for unlocking the full potential of integrating phospholipid insights with genomics in advancing our understanding of biological systems and improving healthcare outcomes.
Existing Integration Methods
01 Phospholipid-based genomic analysis methods
This category focuses on methods that utilize phospholipids for genomic analysis. These techniques may involve using phospholipids as carriers or markers in DNA sequencing, gene expression profiling, or other genomic applications. The integration of phospholipids in genomic analysis can enhance sensitivity, specificity, or efficiency of various genomic techniques.- Phospholipid-based genomic analysis methods: Methods utilizing phospholipids for genomic analysis, including DNA sequencing, gene expression profiling, and genetic variant detection. These techniques leverage the unique properties of phospholipids to enhance the efficiency and accuracy of genomic studies.
- Liposome-mediated gene delivery systems: Development of liposome-based delivery systems for gene therapy and genomic editing applications. These systems use phospholipid-based liposomes to encapsulate and deliver genetic material to target cells, improving transfection efficiency and reducing off-target effects.
- Phospholipid-based biomarkers in genomics: Identification and characterization of phospholipid-based biomarkers for genomic analysis and disease diagnosis. This approach combines lipidomics with genomics to discover novel biomarkers and improve understanding of gene-lipid interactions in various biological processes.
- Phospholipid-DNA interactions in chromatin structure: Investigation of the role of phospholipids in chromatin structure and gene regulation. This research explores how phospholipids interact with DNA and histone proteins to influence genome organization and gene expression patterns.
- Phospholipid-based nanoparticles for genomic applications: Development of phospholipid-based nanoparticles for various genomic applications, including drug delivery, gene therapy, and diagnostic imaging. These nanoparticles offer improved stability, targeting, and cellular uptake of genetic material and other therapeutic agents.
02 Phospholipid-mediated gene delivery systems
This point covers the use of phospholipids in gene delivery systems for genomic applications. Phospholipids can be formulated into liposomes or other nanoparticles to encapsulate and deliver genetic material into cells. These systems are crucial for gene therapy, transfection experiments, and other genomic manipulations requiring efficient DNA or RNA delivery.Expand Specific Solutions03 Phospholipid-based biomarkers in genomics
This category explores the use of phospholipids as biomarkers in genomic studies. Certain phospholipid profiles or modifications may be associated with specific genetic conditions or disease states. Analyzing these phospholipid biomarkers in conjunction with genomic data can provide valuable insights into gene-environment interactions and personalized medicine approaches.Expand Specific Solutions04 Phospholipid-genomic interactions in cellular processes
This point focuses on the interplay between phospholipids and genomic elements in various cellular processes. It includes studies on how phospholipids influence gene expression, DNA replication, and other genomic functions. Understanding these interactions is crucial for comprehending cellular regulation and developing targeted therapies.Expand Specific Solutions05 Phospholipid-based platforms for genomic technology
This category covers the development of phospholipid-based platforms and technologies for genomic applications. These may include novel biosensors, microarray systems, or other analytical tools that leverage the properties of phospholipids to enhance genomic research and diagnostics. Such platforms aim to improve the accuracy, speed, or cost-effectiveness of genomic analyses.Expand Specific Solutions
Key Industry Players
The field of combining phospholipid insights with genomics is in its early stages, with significant potential for growth. The market size is expanding as researchers recognize the importance of integrating lipid metabolism with genetic information. While the technology is still developing, several key players are advancing the field. Universities like Tianjin University of Science & Technology and Shiga University of Medical Science are conducting foundational research. Companies such as Novozymes A/S and Agilent Technologies, Inc. are developing tools and technologies to support this integration. The Institute of Biophysics of Chinese Academy of Sciences is contributing to the fundamental understanding of lipid-genome interactions. As the field matures, we can expect increased collaboration between academic institutions and industry partners to drive innovation and practical applications.
Yale University
Technical Solution: Yale University has developed a multifaceted approach to combining phospholipid insights with genomics, focusing on the role of lipid-gene interactions in metabolic diseases. Their method incorporates advanced lipidomic profiling techniques, including targeted and untargeted mass spectrometry, with next-generation sequencing and epigenomic analysis. The research team has created a unique bioinformatics pipeline that integrates lipidomic data with genomic and epigenomic information, allowing for the identification of novel lipid-associated genetic variants and their potential impact on disease risk[7]. Additionally, they have implemented metabolic flux analysis techniques to study the dynamic interplay between lipid metabolism and gene regulation in real-time, providing insights into the temporal aspects of lipid-gene interactions[8]. The team has also developed in vitro and in vivo models to validate their findings and explore potential therapeutic interventions based on the identified lipid-gene networks[9].
Strengths: Comprehensive integration of multiple omics data types, focus on metabolic diseases, and development of validation models. Weaknesses: Complexity in data integration and interpretation, potential limitations in translating findings to human populations, and high resource requirements for maintaining diverse experimental models.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed a suite of integrated solutions for combining phospholipid insights with genomics, focusing on high-throughput analytical platforms and data integration tools. Their approach involves the use of advanced liquid chromatography-mass spectrometry (LC-MS) systems for comprehensive lipidomic profiling, coupled with next-generation sequencing technologies for genomic analysis. The company has created specialized software that enables seamless integration of lipidomic and genomic data, facilitating the identification of correlations between lipid profiles and genetic variations[10]. Additionally, Agilent has developed targeted assays for specific lipid-gene interactions, allowing researchers to focus on particular pathways or disease-related biomarkers. Their platform also incorporates quality control measures and standardization protocols to ensure reproducibility and comparability of results across different laboratories[11].
Strengths: Comprehensive analytical solutions, integrated data analysis tools, and focus on standardization and reproducibility. Weaknesses: Potential limitations in flexibility for customized research approaches, dependence on proprietary software and hardware, and ongoing costs for equipment upgrades and maintenance.
Core Innovations
Isolation of phosphoproteins, glycoproteins and fragments thereof
PatentInactiveGB2498159A
Innovation
- The use of selective metal cations, such as lanthanum (III), to precipitate phosphoproteins and glycoproteins from complex mixtures, allowing for their controlled release and analysis, enabling the simultaneous or separate capture of these proteins through selective precipitation and subsequent enzymatic digestion.
Genes and pathways differentially expressed in bipolar disorder and/or major depressive disorder
PatentInactiveUS20170211144A1
Innovation
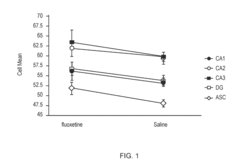
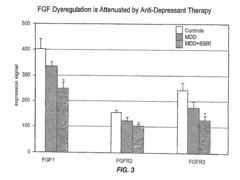
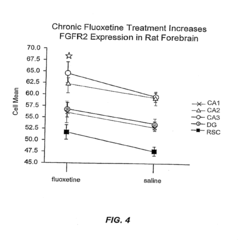
- The use of DNA microarrays to study gene expression in specific brain regions identifies differential gene expression associated with bipolar disorder and major depressive disorder, allowing for the development of methods for diagnosis and treatment by targeting specific genes and pathways, including G protein-coupled receptors, FGF pathways, and mitochondrial genes.
Data Management Strategies
Effective data management strategies are crucial for successfully combining phospholipid insights with genomics. The integration of these two complex fields generates vast amounts of heterogeneous data, requiring robust systems for storage, processing, and analysis.
One key strategy is the implementation of scalable database solutions capable of handling diverse data types. Relational databases can efficiently store structured genomic data, while NoSQL databases are better suited for the more flexible nature of phospholipid data. A hybrid approach, utilizing both types of databases, can provide the necessary versatility for this interdisciplinary research.
Data standardization and harmonization are essential for ensuring interoperability between phospholipid and genomic datasets. Adopting widely accepted ontologies and data formats, such as those proposed by the LIPID MAPS consortium for lipids and the Genomic Standards Consortium for genomics, facilitates seamless data integration and exchange among researchers and institutions.
Implementing robust data quality control measures is vital to maintain the integrity and reliability of combined phospholipid and genomic analyses. This includes automated data validation processes, outlier detection algorithms, and comprehensive metadata management to ensure proper context and traceability of all data points.
Cloud-based storage and computing solutions offer scalability and accessibility for managing the large volumes of data generated in this field. Platforms like Amazon Web Services (AWS) or Google Cloud provide powerful tools for data storage, processing, and collaboration, enabling researchers to efficiently handle complex analyses without the need for extensive local infrastructure.
Machine learning and artificial intelligence techniques can be leveraged to extract meaningful patterns and correlations from the integrated phospholipid and genomic datasets. Implementing these advanced analytics tools requires careful consideration of data preprocessing, feature selection, and model validation to ensure accurate and reproducible results.
Lastly, establishing comprehensive data governance policies is crucial for maintaining data security, privacy, and compliance with regulatory requirements. This includes implementing access controls, data encryption, and audit trails, as well as developing clear protocols for data sharing and collaboration among research teams and institutions.
One key strategy is the implementation of scalable database solutions capable of handling diverse data types. Relational databases can efficiently store structured genomic data, while NoSQL databases are better suited for the more flexible nature of phospholipid data. A hybrid approach, utilizing both types of databases, can provide the necessary versatility for this interdisciplinary research.
Data standardization and harmonization are essential for ensuring interoperability between phospholipid and genomic datasets. Adopting widely accepted ontologies and data formats, such as those proposed by the LIPID MAPS consortium for lipids and the Genomic Standards Consortium for genomics, facilitates seamless data integration and exchange among researchers and institutions.
Implementing robust data quality control measures is vital to maintain the integrity and reliability of combined phospholipid and genomic analyses. This includes automated data validation processes, outlier detection algorithms, and comprehensive metadata management to ensure proper context and traceability of all data points.
Cloud-based storage and computing solutions offer scalability and accessibility for managing the large volumes of data generated in this field. Platforms like Amazon Web Services (AWS) or Google Cloud provide powerful tools for data storage, processing, and collaboration, enabling researchers to efficiently handle complex analyses without the need for extensive local infrastructure.
Machine learning and artificial intelligence techniques can be leveraged to extract meaningful patterns and correlations from the integrated phospholipid and genomic datasets. Implementing these advanced analytics tools requires careful consideration of data preprocessing, feature selection, and model validation to ensure accurate and reproducible results.
Lastly, establishing comprehensive data governance policies is crucial for maintaining data security, privacy, and compliance with regulatory requirements. This includes implementing access controls, data encryption, and audit trails, as well as developing clear protocols for data sharing and collaboration among research teams and institutions.
Ethical Considerations
The integration of phospholipid insights with genomics raises several ethical considerations that must be carefully addressed. Privacy concerns are paramount, as the combination of these data types could potentially reveal sensitive information about an individual's health, predispositions, and even behavioral traits. Safeguarding this data from unauthorized access and ensuring informed consent for its use in research or clinical applications is crucial.
Equity in access to the benefits of this combined approach is another significant ethical issue. The advanced nature of this technology may lead to disparities in healthcare, with only certain populations having access to the potential advantages of personalized treatments or preventive measures based on phospholipid-genomic profiles. This could exacerbate existing health inequalities and raise questions of justice in healthcare distribution.
The potential for misuse of this information also presents ethical challenges. Employers or insurance companies might seek access to such comprehensive health data, potentially leading to discrimination based on genetic and metabolic profiles. Establishing robust legal and ethical frameworks to prevent such misuse is essential.
Furthermore, the predictive power of combined phospholipid and genomic data raises questions about the right to know versus the right not to know. Individuals may face difficult decisions regarding whether to learn about potential future health risks, especially if effective interventions are not available. This could lead to psychological distress and impact life choices in unforeseen ways.
The use of this combined approach in prenatal testing and embryo selection also presents complex ethical dilemmas. It could potentially expand the criteria for genetic selection, raising concerns about eugenics and the societal implications of increased control over human traits.
Lastly, the long-term consequences of interventions based on phospholipid-genomic insights are not fully understood. Ethical considerations must include the potential for unintended effects on human evolution and biodiversity, as well as the responsibility of scientists and healthcare providers in managing these far-reaching implications.
Equity in access to the benefits of this combined approach is another significant ethical issue. The advanced nature of this technology may lead to disparities in healthcare, with only certain populations having access to the potential advantages of personalized treatments or preventive measures based on phospholipid-genomic profiles. This could exacerbate existing health inequalities and raise questions of justice in healthcare distribution.
The potential for misuse of this information also presents ethical challenges. Employers or insurance companies might seek access to such comprehensive health data, potentially leading to discrimination based on genetic and metabolic profiles. Establishing robust legal and ethical frameworks to prevent such misuse is essential.
Furthermore, the predictive power of combined phospholipid and genomic data raises questions about the right to know versus the right not to know. Individuals may face difficult decisions regarding whether to learn about potential future health risks, especially if effective interventions are not available. This could lead to psychological distress and impact life choices in unforeseen ways.
The use of this combined approach in prenatal testing and embryo selection also presents complex ethical dilemmas. It could potentially expand the criteria for genetic selection, raising concerns about eugenics and the societal implications of increased control over human traits.
Lastly, the long-term consequences of interventions based on phospholipid-genomic insights are not fully understood. Ethical considerations must include the potential for unintended effects on human evolution and biodiversity, as well as the responsibility of scientists and healthcare providers in managing these far-reaching implications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!