Phospholipid Contributions to Early Disease Detection
JUL 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phospholipid Biomarkers
Phospholipid biomarkers have emerged as powerful tools in the field of early disease detection, offering unique insights into cellular processes and pathological changes. These biomolecules, essential components of cell membranes, undergo alterations in their composition and concentration during disease progression, making them valuable indicators of various health conditions.
Recent advancements in lipidomics and mass spectrometry techniques have significantly enhanced our ability to identify and quantify specific phospholipid species associated with different diseases. For instance, changes in phosphatidylcholine and phosphatidylethanolamine levels have been linked to neurodegenerative disorders such as Alzheimer's disease, potentially serving as early diagnostic markers.
In cancer research, phospholipid biomarkers have shown promise in detecting malignancies at early stages. Alterations in phosphatidylserine externalization and increased levels of lysophosphatidylcholine have been observed in various cancer types, offering potential for non-invasive screening methods through blood-based tests.
Cardiovascular diseases have also benefited from phospholipid biomarker research. Elevated levels of oxidized phospholipids and specific phosphatidylcholine species have been associated with atherosclerosis and acute coronary syndromes, providing opportunities for improved risk assessment and early intervention strategies.
The field of metabolic disorders has seen significant progress in utilizing phospholipid biomarkers. Changes in sphingomyelin and ceramide levels have been linked to insulin resistance and type 2 diabetes, offering new avenues for early detection and monitoring of these conditions.
One of the key advantages of phospholipid biomarkers is their stability and abundance in biological fluids, making them suitable for routine clinical testing. Additionally, their involvement in multiple cellular processes allows for the development of multi-marker panels, enhancing the specificity and sensitivity of diagnostic approaches.
However, challenges remain in standardizing phospholipid biomarker analysis across different laboratories and platforms. Efforts are underway to establish robust protocols and reference standards to ensure reproducibility and clinical applicability. Furthermore, ongoing research aims to elucidate the precise mechanisms linking phospholipid alterations to disease pathogenesis, which will further refine their use as diagnostic tools.
As the field continues to evolve, integrating phospholipid biomarker data with other omics approaches, such as genomics and proteomics, holds promise for developing comprehensive disease signatures. This holistic approach may lead to more accurate and personalized early disease detection strategies, ultimately improving patient outcomes through timely interventions.
Recent advancements in lipidomics and mass spectrometry techniques have significantly enhanced our ability to identify and quantify specific phospholipid species associated with different diseases. For instance, changes in phosphatidylcholine and phosphatidylethanolamine levels have been linked to neurodegenerative disorders such as Alzheimer's disease, potentially serving as early diagnostic markers.
In cancer research, phospholipid biomarkers have shown promise in detecting malignancies at early stages. Alterations in phosphatidylserine externalization and increased levels of lysophosphatidylcholine have been observed in various cancer types, offering potential for non-invasive screening methods through blood-based tests.
Cardiovascular diseases have also benefited from phospholipid biomarker research. Elevated levels of oxidized phospholipids and specific phosphatidylcholine species have been associated with atherosclerosis and acute coronary syndromes, providing opportunities for improved risk assessment and early intervention strategies.
The field of metabolic disorders has seen significant progress in utilizing phospholipid biomarkers. Changes in sphingomyelin and ceramide levels have been linked to insulin resistance and type 2 diabetes, offering new avenues for early detection and monitoring of these conditions.
One of the key advantages of phospholipid biomarkers is their stability and abundance in biological fluids, making them suitable for routine clinical testing. Additionally, their involvement in multiple cellular processes allows for the development of multi-marker panels, enhancing the specificity and sensitivity of diagnostic approaches.
However, challenges remain in standardizing phospholipid biomarker analysis across different laboratories and platforms. Efforts are underway to establish robust protocols and reference standards to ensure reproducibility and clinical applicability. Furthermore, ongoing research aims to elucidate the precise mechanisms linking phospholipid alterations to disease pathogenesis, which will further refine their use as diagnostic tools.
As the field continues to evolve, integrating phospholipid biomarker data with other omics approaches, such as genomics and proteomics, holds promise for developing comprehensive disease signatures. This holistic approach may lead to more accurate and personalized early disease detection strategies, ultimately improving patient outcomes through timely interventions.
Market for Early Diagnostics
The market for early diagnostics has been experiencing significant growth in recent years, driven by increasing awareness of the importance of preventive healthcare and the rising prevalence of chronic diseases. This sector encompasses a wide range of diagnostic tools and technologies aimed at detecting diseases in their earliest stages, often before symptoms become apparent. The global early diagnostics market was valued at approximately $50 billion in 2020 and is projected to reach $90 billion by 2026, growing at a CAGR of around 8-10% during this period.
One of the key factors driving this market growth is the aging population worldwide, which is more susceptible to various diseases and requires frequent health monitoring. Additionally, the increasing incidence of cancer, cardiovascular diseases, and neurological disorders has created a strong demand for early detection methods. Governments and healthcare organizations are also promoting early diagnosis programs to reduce the overall healthcare burden and improve patient outcomes.
The market for early diagnostics can be segmented based on technology, including molecular diagnostics, point-of-care testing, immunodiagnostics, and imaging diagnostics. Among these, molecular diagnostics and point-of-care testing are experiencing the fastest growth due to their accuracy, speed, and convenience. Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the highest growth rates in the coming years due to improving healthcare infrastructure and rising healthcare expenditure.
In the context of phospholipid-based early disease detection, there is a growing interest in using lipid biomarkers for diagnostic purposes. This approach offers several advantages, including non-invasiveness and the potential for detecting a wide range of diseases at early stages. The market for phospholipid-based diagnostics is still in its nascent stages but shows promising growth potential. It is estimated to be a subset of the broader biomarker-based diagnostics market, which was valued at around $30 billion in 2020 and is expected to grow at a CAGR of 12-15% over the next five years.
Key players in the early diagnostics market include established healthcare companies like Roche, Abbott, and Siemens Healthineers, as well as innovative startups focusing on specific diagnostic technologies. These companies are investing heavily in research and development to create more accurate, faster, and cost-effective diagnostic tools. The competitive landscape is characterized by frequent collaborations, mergers, and acquisitions as companies seek to expand their technological capabilities and market reach.
One of the key factors driving this market growth is the aging population worldwide, which is more susceptible to various diseases and requires frequent health monitoring. Additionally, the increasing incidence of cancer, cardiovascular diseases, and neurological disorders has created a strong demand for early detection methods. Governments and healthcare organizations are also promoting early diagnosis programs to reduce the overall healthcare burden and improve patient outcomes.
The market for early diagnostics can be segmented based on technology, including molecular diagnostics, point-of-care testing, immunodiagnostics, and imaging diagnostics. Among these, molecular diagnostics and point-of-care testing are experiencing the fastest growth due to their accuracy, speed, and convenience. Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the highest growth rates in the coming years due to improving healthcare infrastructure and rising healthcare expenditure.
In the context of phospholipid-based early disease detection, there is a growing interest in using lipid biomarkers for diagnostic purposes. This approach offers several advantages, including non-invasiveness and the potential for detecting a wide range of diseases at early stages. The market for phospholipid-based diagnostics is still in its nascent stages but shows promising growth potential. It is estimated to be a subset of the broader biomarker-based diagnostics market, which was valued at around $30 billion in 2020 and is expected to grow at a CAGR of 12-15% over the next five years.
Key players in the early diagnostics market include established healthcare companies like Roche, Abbott, and Siemens Healthineers, as well as innovative startups focusing on specific diagnostic technologies. These companies are investing heavily in research and development to create more accurate, faster, and cost-effective diagnostic tools. The competitive landscape is characterized by frequent collaborations, mergers, and acquisitions as companies seek to expand their technological capabilities and market reach.
Current Challenges
The field of phospholipid-based early disease detection faces several significant challenges that hinder its widespread adoption and clinical implementation. One of the primary obstacles is the complexity of phospholipid metabolism and its intricate relationship with various diseases. The vast diversity of phospholipid species and their dynamic changes in response to different pathological conditions make it challenging to identify specific biomarkers with high sensitivity and specificity.
Another major hurdle is the lack of standardized analytical methods for phospholipid profiling. Current techniques, such as mass spectrometry and nuclear magnetic resonance spectroscopy, require sophisticated equipment and expertise, limiting their accessibility in clinical settings. Moreover, the interpretation of phospholipid profiles remains a complex task, often requiring advanced bioinformatics tools and machine learning algorithms to extract meaningful information from large datasets.
The biological variability among individuals poses an additional challenge in developing reliable phospholipid-based diagnostic tools. Factors such as age, gender, diet, and lifestyle can significantly influence phospholipid composition, making it difficult to establish universal reference ranges for disease detection. This variability necessitates the development of personalized approaches and the integration of multiple biomarkers to improve diagnostic accuracy.
Furthermore, the stability and reproducibility of phospholipid measurements in biological samples present technical challenges. Pre-analytical factors, including sample collection, storage, and processing, can significantly affect phospholipid profiles, potentially leading to inconsistent results across different laboratories or studies. Standardization of these procedures is crucial for the reliable implementation of phospholipid-based diagnostics in clinical practice.
The translation of research findings into clinically validated diagnostic tests remains a significant bottleneck. Large-scale clinical trials are needed to validate the diagnostic and prognostic value of phospholipid biomarkers across diverse patient populations and disease stages. However, such studies are often costly and time-consuming, hindering the rapid advancement of this field.
Regulatory challenges also play a role in slowing the progress of phospholipid-based diagnostics. The complex nature of these biomarkers and the novel analytical approaches used in their detection may require new regulatory frameworks and guidelines for their approval and implementation in clinical settings. This regulatory uncertainty can discourage investment and innovation in the field.
Lastly, the integration of phospholipid-based diagnostics into existing clinical workflows and decision-making processes presents logistical and educational challenges. Healthcare providers need to be trained in interpreting these new biomarkers and incorporating them into their diagnostic and treatment strategies. Additionally, the cost-effectiveness of these advanced diagnostic tools must be demonstrated to ensure their adoption in healthcare systems with limited resources.
Another major hurdle is the lack of standardized analytical methods for phospholipid profiling. Current techniques, such as mass spectrometry and nuclear magnetic resonance spectroscopy, require sophisticated equipment and expertise, limiting their accessibility in clinical settings. Moreover, the interpretation of phospholipid profiles remains a complex task, often requiring advanced bioinformatics tools and machine learning algorithms to extract meaningful information from large datasets.
The biological variability among individuals poses an additional challenge in developing reliable phospholipid-based diagnostic tools. Factors such as age, gender, diet, and lifestyle can significantly influence phospholipid composition, making it difficult to establish universal reference ranges for disease detection. This variability necessitates the development of personalized approaches and the integration of multiple biomarkers to improve diagnostic accuracy.
Furthermore, the stability and reproducibility of phospholipid measurements in biological samples present technical challenges. Pre-analytical factors, including sample collection, storage, and processing, can significantly affect phospholipid profiles, potentially leading to inconsistent results across different laboratories or studies. Standardization of these procedures is crucial for the reliable implementation of phospholipid-based diagnostics in clinical practice.
The translation of research findings into clinically validated diagnostic tests remains a significant bottleneck. Large-scale clinical trials are needed to validate the diagnostic and prognostic value of phospholipid biomarkers across diverse patient populations and disease stages. However, such studies are often costly and time-consuming, hindering the rapid advancement of this field.
Regulatory challenges also play a role in slowing the progress of phospholipid-based diagnostics. The complex nature of these biomarkers and the novel analytical approaches used in their detection may require new regulatory frameworks and guidelines for their approval and implementation in clinical settings. This regulatory uncertainty can discourage investment and innovation in the field.
Lastly, the integration of phospholipid-based diagnostics into existing clinical workflows and decision-making processes presents logistical and educational challenges. Healthcare providers need to be trained in interpreting these new biomarkers and incorporating them into their diagnostic and treatment strategies. Additionally, the cost-effectiveness of these advanced diagnostic tools must be demonstrated to ensure their adoption in healthcare systems with limited resources.
Detection Methodologies
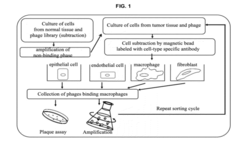
01 Phospholipid-based biosensors for early detection
Biosensors utilizing phospholipid membranes or layers can be developed for early detection of various biomarkers or analytes. These sensors often incorporate specific receptors or enzymes within the phospholipid structure, allowing for sensitive and selective detection of target molecules. The phospholipid-based biosensors can be used in medical diagnostics, environmental monitoring, and food safety applications.- Phospholipid-based biosensors for early detection: Biosensors utilizing phospholipid membranes or layers can be developed for early detection of various biomarkers or analytes. These sensors often employ electrochemical or optical detection methods, offering high sensitivity and specificity for early disease diagnosis or environmental monitoring.
- Liposome-based assays for early detection: Liposomes, composed of phospholipids, can be used as carriers or signal amplifiers in various assays for early detection of diseases or contaminants. These assays may involve fluorescence, colorimetric, or other detection methods, providing rapid and sensitive results.
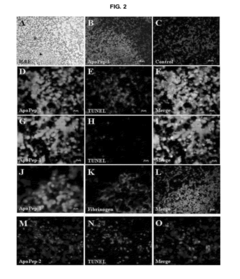
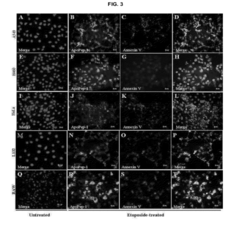
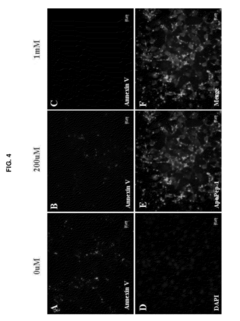
- Phospholipid analysis for disease biomarkers: Early detection of diseases can be achieved by analyzing phospholipid profiles or specific phospholipid species in biological samples. Mass spectrometry and chromatography techniques are often employed for this purpose, enabling the identification of potential biomarkers for various conditions.
- Phospholipid-based nanoparticles for diagnostics: Nanoparticles incorporating phospholipids can be designed for early detection applications. These nanoparticles may be functionalized with targeting molecules or imaging agents, allowing for improved sensitivity and specificity in diagnostic procedures.
- Phospholipid membrane-based sensors for environmental monitoring: Sensors utilizing artificial phospholipid membranes can be developed for early detection of environmental contaminants or pollutants. These sensors may employ various transduction mechanisms, such as impedance spectroscopy or surface plasmon resonance, to detect and quantify target analytes.
02 Liposome-based assays for early disease detection
Liposomes, which are composed of phospholipids, can be engineered to carry specific markers or reagents for early detection of diseases. These liposome-based assays can be designed to detect various biomarkers, pathogens, or molecular indicators associated with different health conditions. The use of liposomes enhances sensitivity and specificity in diagnostic tests, enabling earlier and more accurate disease detection.Expand Specific Solutions03 Phospholipid analysis for early cancer detection
Changes in phospholipid profiles or compositions in biological samples can be indicative of early-stage cancer. Analytical techniques such as mass spectrometry or chromatography can be employed to detect and quantify specific phospholipids or their metabolites. This approach allows for the development of non-invasive screening methods for early cancer detection, potentially improving patient outcomes through earlier intervention.Expand Specific Solutions04 Phospholipid-based nanoparticles for targeted early detection
Nanoparticles composed of or coated with phospholipids can be engineered for targeted early detection of diseases or conditions. These nanoparticles can be functionalized with specific ligands or antibodies to bind to target molecules or cells. The use of phospholipid-based nanoparticles allows for improved biocompatibility, enhanced cellular uptake, and increased sensitivity in early detection applications.Expand Specific Solutions05 Phospholipid membrane-based sensors for environmental monitoring
Sensors incorporating phospholipid membranes can be developed for early detection of environmental contaminants or pollutants. These sensors can be designed to detect specific chemicals, toxins, or microorganisms in water, air, or soil samples. The use of phospholipid membranes allows for the creation of biomimetic sensing platforms with high sensitivity and selectivity for environmental monitoring applications.Expand Specific Solutions
Key Industry Players
The field of phospholipid-based early disease detection is in its nascent stages, with significant potential for growth. The market size is expanding as researchers explore novel applications in various diseases. While the technology is still developing, several key players are advancing its maturity. Shandong Normal University and Beijing Normal University are contributing academic research, while companies like Bio-Rad Laboratories and Boehringer Ingelheim are leveraging their expertise in diagnostics and pharmaceuticals. The Hospital for Sick Children and The Cleveland Clinic Foundation are exploring clinical applications. Emerging biotech firms such as Vascular Biogenics and Stemirna Therapeutics are also entering this space, indicating a competitive and diverse landscape with both established and innovative players.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed a comprehensive phospholipid analysis platform for early disease detection. Their approach utilizes a combination of liquid chromatography-tandem mass spectrometry (LC-MS/MS) and novel phospholipid extraction techniques to achieve high-throughput screening of clinical samples. The company has also introduced automated sample preparation systems that significantly reduce processing time and improve reproducibility[4]. Bio-Rad's technology focuses on identifying specific phospholipid signatures associated with various diseases, including cardiovascular disorders and certain types of cancer[5]. They have recently patented a method for detecting phospholipid oxidation products as early markers of oxidative stress-related diseases[6].
Strengths: High-throughput capabilities, automated systems for improved efficiency, and a wide range of applications in disease detection. Weaknesses: May require substantial initial investment in equipment and training for clinical laboratories.
Vascular Biogenics Ltd.
Technical Solution: Vascular Biogenics has developed a novel approach to early disease detection using phospholipid-based nanoparticles. Their technology, known as VB-111, utilizes genetically modified endothelial cells that express a recombinant fusion protein. This protein specifically targets areas of angiogenesis, which is a common feature in many diseases, especially cancer[7]. The phospholipid nanoparticles are designed to carry imaging agents or therapeutic compounds, allowing for both early detection and potential treatment. In clinical trials, this approach has shown promise in detecting early-stage tumors and monitoring treatment response[8]. The company is also exploring the use of their phospholipid nanoparticle technology for detecting cardiovascular diseases by targeting specific markers of vascular inflammation[9].
Strengths: Dual functionality for both detection and potential treatment, high specificity for angiogenesis-related diseases. Weaknesses: Currently limited to specific disease types, may require further validation for broader applications.
Innovative Phospholipid Studies
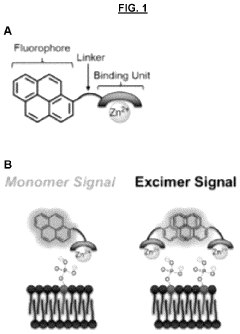
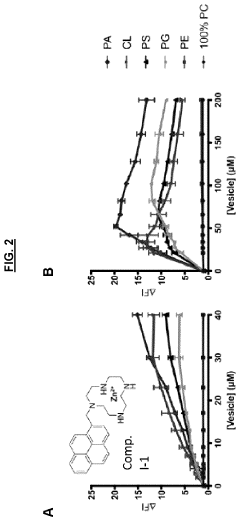
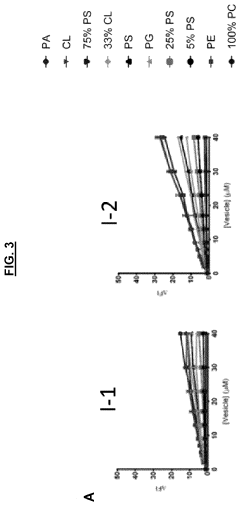
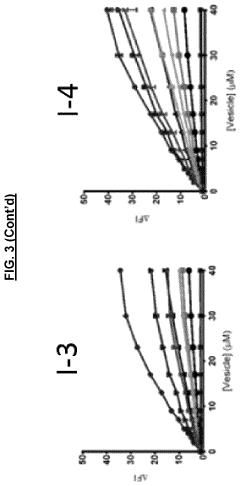
Sensors for detection of negatively charged phosphate-containing membranes and membrane components
PatentActiveUS20200270218A1
Innovation
- Development of a turn-on fluorescent sensor that selectively detects negatively charged phosphate-containing membrane components using excimer forming compounds, which do not require washing and are compatible with standard fluorometers, allowing for rapid detection of apoptosis, bacteria, and other biological processes.
Peptides for targeting apoptotic cells and uses thereof
PatentActiveUS20120316101A1
Innovation
- Development of peptides with specific amino acid sequences, like Cys-X1-Val-Ala-Pro-X2, where X1 is a polar uncharged amino acid and X2 is a positively charged amino acid, that can specifically target apoptotic cells, enabling compositions for detection, drug delivery, and imaging.
Regulatory Considerations
The regulatory landscape surrounding phospholipid-based early disease detection technologies is complex and evolving. As these innovative diagnostic methods gain traction, regulatory bodies worldwide are adapting their frameworks to ensure patient safety, test accuracy, and ethical use of biological data.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing phospholipid-based diagnostic tests. These tests typically fall under the category of in vitro diagnostics (IVDs) and may be subject to premarket approval (PMA) or 510(k) clearance processes, depending on their classification and intended use. The FDA has shown increased interest in liquid biopsy technologies, which often involve phospholipid analysis, and has issued guidance documents to address the unique challenges these tests present.
European regulations, particularly the In Vitro Diagnostic Regulation (IVDR), have significant implications for phospholipid-based diagnostics. The IVDR, which came into full effect in May 2022, introduces more stringent requirements for clinical evidence, risk classification, and post-market surveillance. Manufacturers of phospholipid-based tests must navigate these new regulations, which may require additional clinical studies and technical documentation.
Privacy and data protection regulations, such as the General Data Protection Regulation (GDPR) in Europe and various state-level laws in the US, also impact the development and deployment of phospholipid-based diagnostic technologies. These regulations govern the collection, storage, and use of patient data, which is critical in early disease detection applications.
Standardization efforts are underway to ensure consistency and reliability in phospholipid analysis across different laboratories and platforms. Organizations like the Clinical and Laboratory Standards Institute (CLSI) and the International Organization for Standardization (ISO) are working on developing guidelines and standards specific to lipidomics and related technologies.
Regulatory considerations also extend to the analytical validity, clinical validity, and clinical utility of phospholipid-based tests. Demonstrating these aspects is crucial for obtaining regulatory approval and gaining acceptance in clinical practice. This often requires extensive clinical trials and long-term follow-up studies to establish the predictive value of phospholipid biomarkers for early disease detection.
As the field advances, regulators are likely to face new challenges in assessing the safety and efficacy of combination products that integrate phospholipid analysis with other diagnostic modalities or therapeutic interventions. This may necessitate the development of novel regulatory pathways or the adaptation of existing frameworks to accommodate these innovative approaches to early disease detection.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing phospholipid-based diagnostic tests. These tests typically fall under the category of in vitro diagnostics (IVDs) and may be subject to premarket approval (PMA) or 510(k) clearance processes, depending on their classification and intended use. The FDA has shown increased interest in liquid biopsy technologies, which often involve phospholipid analysis, and has issued guidance documents to address the unique challenges these tests present.
European regulations, particularly the In Vitro Diagnostic Regulation (IVDR), have significant implications for phospholipid-based diagnostics. The IVDR, which came into full effect in May 2022, introduces more stringent requirements for clinical evidence, risk classification, and post-market surveillance. Manufacturers of phospholipid-based tests must navigate these new regulations, which may require additional clinical studies and technical documentation.
Privacy and data protection regulations, such as the General Data Protection Regulation (GDPR) in Europe and various state-level laws in the US, also impact the development and deployment of phospholipid-based diagnostic technologies. These regulations govern the collection, storage, and use of patient data, which is critical in early disease detection applications.
Standardization efforts are underway to ensure consistency and reliability in phospholipid analysis across different laboratories and platforms. Organizations like the Clinical and Laboratory Standards Institute (CLSI) and the International Organization for Standardization (ISO) are working on developing guidelines and standards specific to lipidomics and related technologies.
Regulatory considerations also extend to the analytical validity, clinical validity, and clinical utility of phospholipid-based tests. Demonstrating these aspects is crucial for obtaining regulatory approval and gaining acceptance in clinical practice. This often requires extensive clinical trials and long-term follow-up studies to establish the predictive value of phospholipid biomarkers for early disease detection.
As the field advances, regulators are likely to face new challenges in assessing the safety and efficacy of combination products that integrate phospholipid analysis with other diagnostic modalities or therapeutic interventions. This may necessitate the development of novel regulatory pathways or the adaptation of existing frameworks to accommodate these innovative approaches to early disease detection.
Ethical Implications
The ethical implications of using phospholipid biomarkers for early disease detection are multifaceted and require careful consideration. One primary concern is the potential for discrimination based on genetic predisposition or risk factors identified through phospholipid analysis. Employers or insurance companies might use this information to make unfair decisions, leading to social and economic disparities.
Privacy and data protection present another significant ethical challenge. The sensitive nature of health information derived from phospholipid biomarkers necessitates robust safeguards to prevent unauthorized access or misuse. There is a risk that this data could be exploited for purposes beyond healthcare, such as targeted marketing or surveillance.
The issue of informed consent is particularly complex in the context of early disease detection. Patients may not fully understand the implications of phospholipid testing, including the potential for discovering incidental findings or predispositions to diseases for which there are no current treatments. This raises questions about the right to know versus the right not to know, and how to handle such information ethically.
There are also concerns about the psychological impact of early disease detection. Knowledge of a potential future illness could lead to anxiety, depression, or changes in life choices. The ethical responsibility of healthcare providers in managing this information and supporting patients through the process must be carefully considered.
The accuracy and reliability of phospholipid-based diagnostic tools raise additional ethical questions. False positives could lead to unnecessary treatments or anxiety, while false negatives might provide a false sense of security. Ensuring equitable access to these diagnostic technologies is another ethical imperative, as disparities in availability could exacerbate existing health inequalities.
Lastly, the use of phospholipid biomarkers in research and clinical trials presents ethical challenges related to participant selection, data sharing, and long-term follow-up. Balancing the potential benefits of scientific advancement with the protection of individual rights and well-being is crucial in this emerging field of biomedical research.
Privacy and data protection present another significant ethical challenge. The sensitive nature of health information derived from phospholipid biomarkers necessitates robust safeguards to prevent unauthorized access or misuse. There is a risk that this data could be exploited for purposes beyond healthcare, such as targeted marketing or surveillance.
The issue of informed consent is particularly complex in the context of early disease detection. Patients may not fully understand the implications of phospholipid testing, including the potential for discovering incidental findings or predispositions to diseases for which there are no current treatments. This raises questions about the right to know versus the right not to know, and how to handle such information ethically.
There are also concerns about the psychological impact of early disease detection. Knowledge of a potential future illness could lead to anxiety, depression, or changes in life choices. The ethical responsibility of healthcare providers in managing this information and supporting patients through the process must be carefully considered.
The accuracy and reliability of phospholipid-based diagnostic tools raise additional ethical questions. False positives could lead to unnecessary treatments or anxiety, while false negatives might provide a false sense of security. Ensuring equitable access to these diagnostic technologies is another ethical imperative, as disparities in availability could exacerbate existing health inequalities.
Lastly, the use of phospholipid biomarkers in research and clinical trials presents ethical challenges related to participant selection, data sharing, and long-term follow-up. Balancing the potential benefits of scientific advancement with the protection of individual rights and well-being is crucial in this emerging field of biomedical research.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!