Phospholipid Opportunities in Non-invasive Health Monitoring
JUL 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phospholipid Sensing Background and Objectives
Phospholipids have emerged as a promising frontier in non-invasive health monitoring, offering a unique window into cellular processes and overall physiological status. The evolution of phospholipid sensing technologies has been driven by the increasing demand for accessible, real-time health data and the growing understanding of lipids' role in various biological functions.
The primary objective of phospholipid sensing in non-invasive health monitoring is to develop reliable, user-friendly methods for detecting and analyzing phospholipid profiles in easily accessible biological samples, such as saliva, sweat, or exhaled breath condensate. These methods aim to provide valuable insights into an individual's health status without the need for invasive procedures or complex laboratory analysis.
Historically, phospholipid analysis has been confined to laboratory settings, relying on sophisticated techniques like mass spectrometry and chromatography. However, recent advancements in biosensor technologies, microfluidics, and nanotechnology have paved the way for more compact, portable, and sensitive phospholipid detection systems. These developments align with the broader trend towards personalized medicine and point-of-care diagnostics.
The potential applications of phospholipid sensing in health monitoring are vast and diverse. Researchers are exploring its use in early disease detection, particularly for conditions associated with altered lipid metabolism, such as cardiovascular diseases, neurodegenerative disorders, and certain types of cancer. Additionally, phospholipid profiles could serve as biomarkers for stress, inflammation, and overall metabolic health, offering a comprehensive snapshot of an individual's physiological state.
One of the key technological goals in this field is to achieve high sensitivity and specificity in phospholipid detection while maintaining the non-invasive nature of the sampling process. This involves overcoming challenges related to sample collection, processing, and analysis in real-world conditions. Researchers are also focusing on developing algorithms and data interpretation methods to translate complex phospholipid profiles into actionable health insights.
As the field progresses, there is a growing emphasis on integrating phospholipid sensing capabilities into wearable devices and smart health monitoring systems. This integration aims to provide continuous, real-time health data to users and healthcare providers, potentially revolutionizing preventive healthcare and chronic disease management.
The development of phospholipid sensing technologies for non-invasive health monitoring represents a convergence of multiple scientific disciplines, including biochemistry, nanotechnology, materials science, and data analytics. As research in this area continues to advance, it holds the promise of transforming our approach to health monitoring, making it more accessible, comprehensive, and personalized than ever before.
The primary objective of phospholipid sensing in non-invasive health monitoring is to develop reliable, user-friendly methods for detecting and analyzing phospholipid profiles in easily accessible biological samples, such as saliva, sweat, or exhaled breath condensate. These methods aim to provide valuable insights into an individual's health status without the need for invasive procedures or complex laboratory analysis.
Historically, phospholipid analysis has been confined to laboratory settings, relying on sophisticated techniques like mass spectrometry and chromatography. However, recent advancements in biosensor technologies, microfluidics, and nanotechnology have paved the way for more compact, portable, and sensitive phospholipid detection systems. These developments align with the broader trend towards personalized medicine and point-of-care diagnostics.
The potential applications of phospholipid sensing in health monitoring are vast and diverse. Researchers are exploring its use in early disease detection, particularly for conditions associated with altered lipid metabolism, such as cardiovascular diseases, neurodegenerative disorders, and certain types of cancer. Additionally, phospholipid profiles could serve as biomarkers for stress, inflammation, and overall metabolic health, offering a comprehensive snapshot of an individual's physiological state.
One of the key technological goals in this field is to achieve high sensitivity and specificity in phospholipid detection while maintaining the non-invasive nature of the sampling process. This involves overcoming challenges related to sample collection, processing, and analysis in real-world conditions. Researchers are also focusing on developing algorithms and data interpretation methods to translate complex phospholipid profiles into actionable health insights.
As the field progresses, there is a growing emphasis on integrating phospholipid sensing capabilities into wearable devices and smart health monitoring systems. This integration aims to provide continuous, real-time health data to users and healthcare providers, potentially revolutionizing preventive healthcare and chronic disease management.
The development of phospholipid sensing technologies for non-invasive health monitoring represents a convergence of multiple scientific disciplines, including biochemistry, nanotechnology, materials science, and data analytics. As research in this area continues to advance, it holds the promise of transforming our approach to health monitoring, making it more accessible, comprehensive, and personalized than ever before.
Market Analysis for Non-invasive Health Monitoring
The non-invasive health monitoring market has experienced significant growth in recent years, driven by increasing health consciousness, aging populations, and advancements in wearable technology. This market segment encompasses a wide range of devices and technologies designed to track various health parameters without invasive procedures, offering convenience and continuous monitoring capabilities to users.
The global non-invasive health monitoring market was valued at approximately $12 billion in 2020 and is projected to reach $23 billion by 2025, growing at a compound annual growth rate (CAGR) of 13.8%. This robust growth is attributed to several factors, including the rising prevalence of chronic diseases, increasing healthcare costs, and a growing emphasis on preventive healthcare.
Key market segments within non-invasive health monitoring include wearable devices, remote patient monitoring systems, and smartphone-based health apps. Wearable devices, such as smartwatches and fitness trackers, dominate the market, accounting for over 60% of the total market share. These devices offer continuous monitoring of vital signs, activity levels, and sleep patterns.
The integration of phospholipid-based sensors in non-invasive health monitoring devices presents a significant opportunity for market expansion. Phospholipids, being biocompatible and capable of mimicking cell membranes, can potentially enhance the accuracy and reliability of non-invasive sensing technologies. This innovation could lead to the development of more sophisticated and sensitive monitoring devices, particularly in areas such as glucose monitoring, stress detection, and early disease diagnosis.
Consumer demand for non-invasive health monitoring solutions is driven by several factors. These include the desire for proactive health management, the need for continuous monitoring of chronic conditions, and the growing trend of personalized healthcare. Additionally, the COVID-19 pandemic has accelerated the adoption of remote monitoring technologies, further boosting market growth.
Geographically, North America leads the non-invasive health monitoring market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its advanced healthcare infrastructure, high healthcare expenditure, and early adoption of new technologies. However, the Asia-Pacific region is expected to witness the fastest growth, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness of preventive healthcare.
Key players in the non-invasive health monitoring market include Apple, Fitbit (now part of Google), Garmin, Samsung, and Philips Healthcare. These companies are continuously innovating to enhance their product offerings and expand their market presence. The integration of phospholipid-based sensing technologies could provide a competitive edge for companies looking to differentiate their products in this rapidly evolving market.
The global non-invasive health monitoring market was valued at approximately $12 billion in 2020 and is projected to reach $23 billion by 2025, growing at a compound annual growth rate (CAGR) of 13.8%. This robust growth is attributed to several factors, including the rising prevalence of chronic diseases, increasing healthcare costs, and a growing emphasis on preventive healthcare.
Key market segments within non-invasive health monitoring include wearable devices, remote patient monitoring systems, and smartphone-based health apps. Wearable devices, such as smartwatches and fitness trackers, dominate the market, accounting for over 60% of the total market share. These devices offer continuous monitoring of vital signs, activity levels, and sleep patterns.
The integration of phospholipid-based sensors in non-invasive health monitoring devices presents a significant opportunity for market expansion. Phospholipids, being biocompatible and capable of mimicking cell membranes, can potentially enhance the accuracy and reliability of non-invasive sensing technologies. This innovation could lead to the development of more sophisticated and sensitive monitoring devices, particularly in areas such as glucose monitoring, stress detection, and early disease diagnosis.
Consumer demand for non-invasive health monitoring solutions is driven by several factors. These include the desire for proactive health management, the need for continuous monitoring of chronic conditions, and the growing trend of personalized healthcare. Additionally, the COVID-19 pandemic has accelerated the adoption of remote monitoring technologies, further boosting market growth.
Geographically, North America leads the non-invasive health monitoring market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its advanced healthcare infrastructure, high healthcare expenditure, and early adoption of new technologies. However, the Asia-Pacific region is expected to witness the fastest growth, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness of preventive healthcare.
Key players in the non-invasive health monitoring market include Apple, Fitbit (now part of Google), Garmin, Samsung, and Philips Healthcare. These companies are continuously innovating to enhance their product offerings and expand their market presence. The integration of phospholipid-based sensing technologies could provide a competitive edge for companies looking to differentiate their products in this rapidly evolving market.
Current Phospholipid Detection Technologies and Challenges
Phospholipid detection technologies have made significant strides in recent years, yet they still face considerable challenges in the context of non-invasive health monitoring. Current methods primarily rely on mass spectrometry, nuclear magnetic resonance spectroscopy, and chromatography techniques. These approaches offer high sensitivity and specificity but are often limited by their invasive nature, requiring blood or tissue samples.
Mass spectrometry-based methods, such as MALDI-TOF and LC-MS/MS, provide excellent resolution and quantification capabilities for phospholipids. However, they typically necessitate complex sample preparation and are not suitable for real-time, non-invasive monitoring. Similarly, NMR spectroscopy offers detailed structural information but requires specialized equipment and is not easily adaptable for portable or wearable devices.
Chromatography techniques, including high-performance liquid chromatography (HPLC) and thin-layer chromatography (TLC), are widely used for phospholipid separation and analysis. While these methods are reliable, they are time-consuming and require laboratory settings, making them impractical for continuous health monitoring applications.
Recent advancements in biosensor technologies have shown promise for non-invasive phospholipid detection. Electrochemical sensors and optical biosensors have been developed to detect specific phospholipids in biological fluids. However, these technologies still struggle with sensitivity, selectivity, and stability issues when applied to complex biological matrices like saliva or sweat.
A major challenge in non-invasive phospholipid detection is the low concentration of these molecules in easily accessible biological fluids. This necessitates the development of highly sensitive detection methods that can accurately measure trace amounts of phospholipids without interference from other biomolecules.
Another significant hurdle is the lack of standardization in phospholipid analysis methods. Different techniques often yield varying results, making it difficult to establish reliable reference ranges for health monitoring purposes. This inconsistency hampers the clinical interpretation of phospholipid profiles and their correlation with specific health conditions.
The complexity and diversity of phospholipid structures pose additional challenges. With numerous subclasses and molecular species, developing a comprehensive detection method that can simultaneously identify and quantify multiple phospholipids remains a formidable task.
Stability and degradation of phospholipids during sample collection and analysis are also critical concerns. Non-invasive sampling methods must ensure the preservation of phospholipid integrity to provide accurate results. This requires innovative approaches to sample handling and rapid analysis techniques.
As research progresses, there is a growing need for miniaturization and integration of phospholipid detection technologies. The development of portable, user-friendly devices that can perform real-time analysis is crucial for the widespread adoption of phospholipid-based health monitoring. However, achieving this level of technological sophistication while maintaining accuracy and reliability remains a significant challenge in the field.
Mass spectrometry-based methods, such as MALDI-TOF and LC-MS/MS, provide excellent resolution and quantification capabilities for phospholipids. However, they typically necessitate complex sample preparation and are not suitable for real-time, non-invasive monitoring. Similarly, NMR spectroscopy offers detailed structural information but requires specialized equipment and is not easily adaptable for portable or wearable devices.
Chromatography techniques, including high-performance liquid chromatography (HPLC) and thin-layer chromatography (TLC), are widely used for phospholipid separation and analysis. While these methods are reliable, they are time-consuming and require laboratory settings, making them impractical for continuous health monitoring applications.
Recent advancements in biosensor technologies have shown promise for non-invasive phospholipid detection. Electrochemical sensors and optical biosensors have been developed to detect specific phospholipids in biological fluids. However, these technologies still struggle with sensitivity, selectivity, and stability issues when applied to complex biological matrices like saliva or sweat.
A major challenge in non-invasive phospholipid detection is the low concentration of these molecules in easily accessible biological fluids. This necessitates the development of highly sensitive detection methods that can accurately measure trace amounts of phospholipids without interference from other biomolecules.
Another significant hurdle is the lack of standardization in phospholipid analysis methods. Different techniques often yield varying results, making it difficult to establish reliable reference ranges for health monitoring purposes. This inconsistency hampers the clinical interpretation of phospholipid profiles and their correlation with specific health conditions.
The complexity and diversity of phospholipid structures pose additional challenges. With numerous subclasses and molecular species, developing a comprehensive detection method that can simultaneously identify and quantify multiple phospholipids remains a formidable task.
Stability and degradation of phospholipids during sample collection and analysis are also critical concerns. Non-invasive sampling methods must ensure the preservation of phospholipid integrity to provide accurate results. This requires innovative approaches to sample handling and rapid analysis techniques.
As research progresses, there is a growing need for miniaturization and integration of phospholipid detection technologies. The development of portable, user-friendly devices that can perform real-time analysis is crucial for the widespread adoption of phospholipid-based health monitoring. However, achieving this level of technological sophistication while maintaining accuracy and reliability remains a significant challenge in the field.
Existing Non-invasive Phospholipid Detection Methods
01 Phospholipid synthesis and modification
Various methods for synthesizing and modifying phospholipids are described, including enzymatic processes and chemical reactions. These techniques aim to produce phospholipids with specific properties or structures for use in pharmaceuticals, cosmetics, and other industries.- Phospholipid synthesis and modification: Various methods for synthesizing and modifying phospholipids are described. These include chemical synthesis routes, enzymatic modifications, and techniques for altering the structure or properties of phospholipids. Such processes can be used to create novel phospholipids with specific characteristics or to improve existing phospholipid formulations.
- Phospholipid-based drug delivery systems: Phospholipids are utilized in the development of drug delivery systems. These lipid-based carriers can enhance the solubility, stability, and bioavailability of various therapeutic compounds. Applications include liposomes, nanoparticles, and other lipid-based formulations for improved drug delivery and targeting.
- Analytical methods for phospholipid characterization: Various analytical techniques are employed for the characterization and quantification of phospholipids. These methods include chromatography, mass spectrometry, and spectroscopic approaches. Such analytical tools are crucial for quality control, research, and development in phospholipid-related fields.
- Phospholipid applications in food and nutrition: Phospholipids find applications in food and nutritional products. They are used as emulsifiers, stabilizers, and functional ingredients in various food formulations. Additionally, phospholipids play roles in nutritional supplements and functional foods, contributing to health benefits and improved product characteristics.
- Phospholipid-based materials and coatings: Phospholipids are utilized in the development of advanced materials and coatings. These applications include biocompatible surfaces, membrane-mimetic materials, and functional coatings with specific properties. Such phospholipid-based materials find use in biomedical, industrial, and technological fields.
02 Phospholipid-based drug delivery systems
Phospholipids are utilized in the development of drug delivery systems, such as liposomes and nanoparticles. These systems enhance drug solubility, stability, and targeted delivery, improving therapeutic efficacy and reducing side effects.Expand Specific Solutions03 Phospholipid analysis and characterization
Advanced analytical techniques are employed to characterize phospholipids, including mass spectrometry, chromatography, and spectroscopic methods. These techniques enable the identification and quantification of phospholipids in complex biological samples.Expand Specific Solutions04 Phospholipid applications in food and nutrition
Phospholipids are used in food and nutritional products for their emulsifying properties and potential health benefits. They are incorporated into functional foods, dietary supplements, and infant formulas to improve nutritional value and product stability.Expand Specific Solutions05 Phospholipid-based biomaterials and medical devices
Phospholipids are utilized in the development of biomaterials and medical devices, such as artificial cell membranes, biosensors, and implant coatings. These applications leverage the biocompatibility and self-assembly properties of phospholipids to create functional interfaces between biological systems and synthetic materials.Expand Specific Solutions
Key Players in Phospholipid-based Health Monitoring
The field of phospholipid-based non-invasive health monitoring is in its early stages of development, with significant potential for growth. The market is expanding as healthcare trends shift towards preventive and personalized medicine. While the technology is still evolving, several key players are making strides in this area. Companies like Masimo Corp. and Abbott Diabetes Care are leveraging their expertise in non-invasive monitoring to explore phospholipid-based solutions. Academic institutions such as The Ohio State University and Emory University are contributing to foundational research. The involvement of both established medical technology firms and innovative startups like GraphWear Technologies indicates a competitive landscape with diverse approaches to commercialization.
Masimo Corp.
Technical Solution: Masimo has developed a non-invasive health monitoring system utilizing phospholipid-based sensors. Their technology employs a novel approach to detect and analyze phospholipid biomarkers in sweat and interstitial fluid. The system uses a combination of spectroscopic techniques and advanced algorithms to correlate phospholipid profiles with various health indicators[1]. Masimo's platform integrates continuous monitoring capabilities, allowing for real-time tracking of metabolic changes and potential health issues[3]. The company has also incorporated machine learning algorithms to improve the accuracy of their phospholipid-based health assessments over time[5].
Strengths: Continuous real-time monitoring, non-invasive approach, and integration of machine learning for improved accuracy. Weaknesses: May require frequent calibration and validation against traditional blood tests for certain health markers.
Abbott Diabetes Care, Inc.
Technical Solution: Abbott Diabetes Care has expanded its continuous glucose monitoring technology to include phospholipid analysis for comprehensive metabolic health assessment. Their system utilizes a minimally invasive sensor that measures interstitial fluid composition, including glucose and specific phospholipid markers[13]. Abbott's technology incorporates electrochemical sensing methods combined with advanced signal processing to detect and quantify phospholipids related to insulin sensitivity and metabolic health[15]. The company has also developed a machine learning algorithm that integrates phospholipid data with glucose measurements to provide more accurate predictions of metabolic trends and potential health risks[17].
Strengths: Integration with established glucose monitoring technology, comprehensive metabolic health assessment, and use of machine learning for improved predictions. Weaknesses: Still requires minimally invasive sensor insertion, which may be a barrier for some users.
Innovative Phospholipid Sensing Techniques
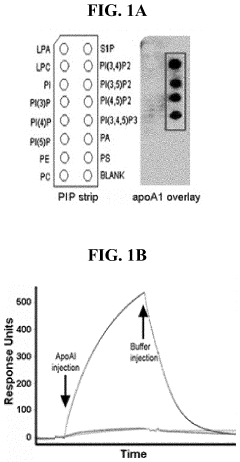
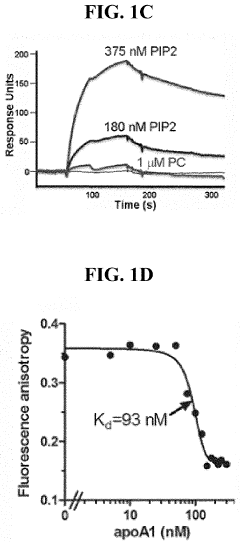
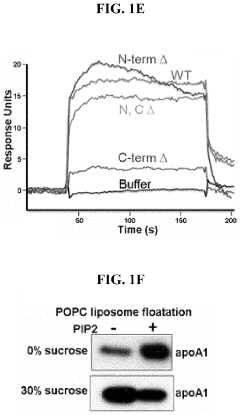
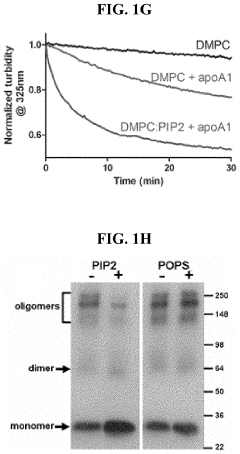
PIP2 as a marker for HDL function and cardiovascular disease risk
PatentInactiveUS20210270855A1
Innovation
- Measuring the levels of phosphatidylinositol (4,5) bis-phosphate (PIP2) in biological samples, as PIP2 serves as a marker for HDL function and is used to predict cardiovascular risk and monitor the efficacy of therapeutic agents, with PIP2 levels indicating cholesterol acceptor activity and reverse cholesterol transport potential.
Nanoparticulate probe for in vivo monitoring of tissue oxygenation
PatentInactiveUS20140170073A1
Innovation
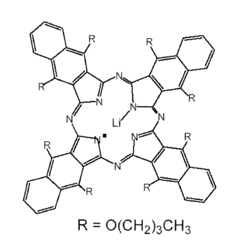
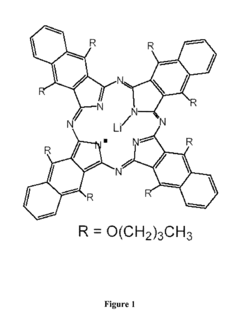
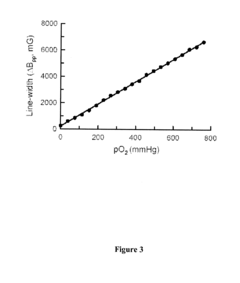
- Development of nanoparticulate and microparticulate probes comprising lithium phthalocyanine derivatives, which are paramagnetic and can be used for electron paramagnetic resonance (EPR) and magnetic resonance imaging (MRI) techniques, allowing for precise measurement of oxygen concentration and imaging of free radicals in tissues and cells.
Regulatory Framework for Non-invasive Diagnostic Tools
The regulatory framework for non-invasive diagnostic tools, particularly those utilizing phospholipid-based technologies for health monitoring, is a complex and evolving landscape. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing these devices. The FDA categorizes non-invasive diagnostic tools under medical devices, which are subject to different levels of regulatory control based on their intended use and risk profile.
For phospholipid-based non-invasive health monitoring devices, the regulatory pathway typically falls under the 510(k) premarket notification process. This requires manufacturers to demonstrate that their device is substantially equivalent to a legally marketed predicate device. However, if the technology is novel and without a clear predicate, it may require a De Novo classification request or even a Premarket Approval (PMA) application, depending on the level of risk associated with the device.
In the European Union, the regulatory landscape has recently undergone significant changes with the implementation of the Medical Device Regulation (MDR). This new framework places greater emphasis on clinical evidence, post-market surveillance, and traceability. Non-invasive diagnostic tools using phospholipid technology would likely be classified as Class IIa or IIb devices under the MDR, requiring conformity assessment by a Notified Body.
Globally, regulatory bodies are increasingly focusing on the validation of software and algorithms used in non-invasive diagnostic tools. This is particularly relevant for phospholipid-based technologies that may incorporate machine learning or artificial intelligence for data interpretation. Manufacturers must demonstrate the reliability and accuracy of these algorithms, as well as address potential biases in the datasets used for development.
Data privacy and security regulations also play a crucial role in the regulatory framework for these devices. In the EU, the General Data Protection Regulation (GDPR) imposes strict requirements on the handling of personal health data. Similarly, in the US, the Health Insurance Portability and Accountability Act (HIPAA) sets standards for protecting patient health information.
As the field of non-invasive health monitoring continues to advance, regulatory bodies are working to keep pace with technological innovations. There is a growing trend towards harmonization of regulatory requirements across different regions, as evidenced by initiatives like the International Medical Device Regulators Forum (IMDRF). This aims to streamline the approval process for manufacturers while maintaining high standards of safety and efficacy.
For phospholipid-based non-invasive health monitoring devices, the regulatory pathway typically falls under the 510(k) premarket notification process. This requires manufacturers to demonstrate that their device is substantially equivalent to a legally marketed predicate device. However, if the technology is novel and without a clear predicate, it may require a De Novo classification request or even a Premarket Approval (PMA) application, depending on the level of risk associated with the device.
In the European Union, the regulatory landscape has recently undergone significant changes with the implementation of the Medical Device Regulation (MDR). This new framework places greater emphasis on clinical evidence, post-market surveillance, and traceability. Non-invasive diagnostic tools using phospholipid technology would likely be classified as Class IIa or IIb devices under the MDR, requiring conformity assessment by a Notified Body.
Globally, regulatory bodies are increasingly focusing on the validation of software and algorithms used in non-invasive diagnostic tools. This is particularly relevant for phospholipid-based technologies that may incorporate machine learning or artificial intelligence for data interpretation. Manufacturers must demonstrate the reliability and accuracy of these algorithms, as well as address potential biases in the datasets used for development.
Data privacy and security regulations also play a crucial role in the regulatory framework for these devices. In the EU, the General Data Protection Regulation (GDPR) imposes strict requirements on the handling of personal health data. Similarly, in the US, the Health Insurance Portability and Accountability Act (HIPAA) sets standards for protecting patient health information.
As the field of non-invasive health monitoring continues to advance, regulatory bodies are working to keep pace with technological innovations. There is a growing trend towards harmonization of regulatory requirements across different regions, as evidenced by initiatives like the International Medical Device Regulators Forum (IMDRF). This aims to streamline the approval process for manufacturers while maintaining high standards of safety and efficacy.
Bioethics in Phospholipid-based Health Monitoring
The integration of phospholipid-based technologies in non-invasive health monitoring raises significant bioethical considerations. As these advanced monitoring systems become more prevalent, it is crucial to address the ethical implications surrounding their development, implementation, and use.
Privacy and data protection are paramount concerns in phospholipid-based health monitoring. These systems have the potential to collect vast amounts of sensitive personal health information, necessitating robust safeguards to prevent unauthorized access, data breaches, and misuse. Ensuring informed consent and maintaining transparency about data collection, storage, and usage practices are essential to uphold individual autonomy and trust.
The accuracy and reliability of phospholipid-based health monitoring technologies also present ethical challenges. False positives or negatives could lead to unnecessary anxiety, medical interventions, or a false sense of security. Developers and healthcare providers must be transparent about the limitations and potential errors of these systems, and work towards continuous improvement to minimize inaccuracies.
Equitable access to phospholipid-based health monitoring technologies is another critical bioethical consideration. As these advanced systems become available, there is a risk of exacerbating existing healthcare disparities. Ensuring fair distribution and accessibility across diverse socioeconomic groups is essential to prevent the creation of a "health monitoring divide."
The potential for discrimination based on health data collected through phospholipid monitoring is a significant concern. Employers, insurers, or other entities may seek access to this information, potentially leading to unfair treatment or denial of services. Robust legal and ethical frameworks must be established to protect individuals from such discrimination and ensure that health monitoring data is used solely for its intended purpose.
The long-term psychological impact of continuous health monitoring through phospholipid-based technologies must also be considered. While these systems can provide valuable health insights, they may also lead to increased anxiety, hypochondria, or obsessive behavior in some individuals. Balancing the benefits of proactive health management with the potential for negative psychological effects is a delicate ethical challenge.
As phospholipid-based health monitoring technologies advance, it is crucial to establish clear guidelines for their responsible development and use. This includes addressing issues of accountability, liability, and the potential for unintended consequences. Ongoing dialogue between ethicists, healthcare professionals, policymakers, and technology developers is essential to navigate these complex bioethical landscapes and ensure that the benefits of these innovations are realized while minimizing potential harms.
Privacy and data protection are paramount concerns in phospholipid-based health monitoring. These systems have the potential to collect vast amounts of sensitive personal health information, necessitating robust safeguards to prevent unauthorized access, data breaches, and misuse. Ensuring informed consent and maintaining transparency about data collection, storage, and usage practices are essential to uphold individual autonomy and trust.
The accuracy and reliability of phospholipid-based health monitoring technologies also present ethical challenges. False positives or negatives could lead to unnecessary anxiety, medical interventions, or a false sense of security. Developers and healthcare providers must be transparent about the limitations and potential errors of these systems, and work towards continuous improvement to minimize inaccuracies.
Equitable access to phospholipid-based health monitoring technologies is another critical bioethical consideration. As these advanced systems become available, there is a risk of exacerbating existing healthcare disparities. Ensuring fair distribution and accessibility across diverse socioeconomic groups is essential to prevent the creation of a "health monitoring divide."
The potential for discrimination based on health data collected through phospholipid monitoring is a significant concern. Employers, insurers, or other entities may seek access to this information, potentially leading to unfair treatment or denial of services. Robust legal and ethical frameworks must be established to protect individuals from such discrimination and ensure that health monitoring data is used solely for its intended purpose.
The long-term psychological impact of continuous health monitoring through phospholipid-based technologies must also be considered. While these systems can provide valuable health insights, they may also lead to increased anxiety, hypochondria, or obsessive behavior in some individuals. Balancing the benefits of proactive health management with the potential for negative psychological effects is a delicate ethical challenge.
As phospholipid-based health monitoring technologies advance, it is crucial to establish clear guidelines for their responsible development and use. This includes addressing issues of accountability, liability, and the potential for unintended consequences. Ongoing dialogue between ethicists, healthcare professionals, policymakers, and technology developers is essential to navigate these complex bioethical landscapes and ensure that the benefits of these innovations are realized while minimizing potential harms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!