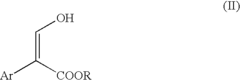How to Innovate Propionic Acid-Based Synthetic Routes?
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Propionic Acid Synthesis Background and Objectives
Propionic acid synthesis has been a cornerstone in the chemical industry for decades, with applications spanning from food preservatives to pharmaceuticals. The evolution of synthetic routes for this valuable compound has been driven by the need for more efficient, cost-effective, and environmentally friendly processes. Historically, propionic acid production relied heavily on petrochemical feedstocks, primarily through the oxidation of propanol or the hydrocarboxylation of ethylene.
As global demand for propionic acid continues to rise, projected to reach over 500,000 tons annually by 2025, the imperative for innovation in synthetic routes has never been more pressing. This growth is fueled by expanding applications in various sectors, including agriculture, food and beverage, and pharmaceuticals. The increasing focus on sustainability and green chemistry has also spurred research into bio-based production methods, presenting both challenges and opportunities for technological advancement.
The primary objectives of innovating propionic acid-based synthetic routes are multifaceted. First and foremost is the enhancement of process efficiency, aiming to reduce energy consumption and improve yield. This is closely tied to the goal of cost reduction, crucial for maintaining competitiveness in a global market. Equally important is the development of more sustainable production methods, aligning with global efforts to reduce carbon footprints and minimize environmental impact.
Another key objective is the exploration of alternative feedstocks, particularly renewable resources, to decrease dependence on fossil fuels. This includes investigating biotechnological approaches, such as fermentation processes using various microorganisms. The potential for utilizing waste streams or byproducts from other industrial processes as feedstock for propionic acid synthesis is also a promising area of research.
Improving product purity and reducing byproduct formation are additional technical goals, as these factors significantly impact downstream applications and overall process economics. Furthermore, there is a growing interest in developing catalytic systems that offer higher selectivity and longer lifetimes, potentially revolutionizing current synthetic routes.
The trajectory of propionic acid synthesis innovation is expected to follow broader trends in chemical engineering and green chemistry. This includes the integration of continuous flow processes, the application of novel reactor designs, and the utilization of advanced separation technologies. The convergence of biotechnology and chemical engineering may also lead to hybrid processes that combine the best aspects of biological and chemical synthesis routes.
As global demand for propionic acid continues to rise, projected to reach over 500,000 tons annually by 2025, the imperative for innovation in synthetic routes has never been more pressing. This growth is fueled by expanding applications in various sectors, including agriculture, food and beverage, and pharmaceuticals. The increasing focus on sustainability and green chemistry has also spurred research into bio-based production methods, presenting both challenges and opportunities for technological advancement.
The primary objectives of innovating propionic acid-based synthetic routes are multifaceted. First and foremost is the enhancement of process efficiency, aiming to reduce energy consumption and improve yield. This is closely tied to the goal of cost reduction, crucial for maintaining competitiveness in a global market. Equally important is the development of more sustainable production methods, aligning with global efforts to reduce carbon footprints and minimize environmental impact.
Another key objective is the exploration of alternative feedstocks, particularly renewable resources, to decrease dependence on fossil fuels. This includes investigating biotechnological approaches, such as fermentation processes using various microorganisms. The potential for utilizing waste streams or byproducts from other industrial processes as feedstock for propionic acid synthesis is also a promising area of research.
Improving product purity and reducing byproduct formation are additional technical goals, as these factors significantly impact downstream applications and overall process economics. Furthermore, there is a growing interest in developing catalytic systems that offer higher selectivity and longer lifetimes, potentially revolutionizing current synthetic routes.
The trajectory of propionic acid synthesis innovation is expected to follow broader trends in chemical engineering and green chemistry. This includes the integration of continuous flow processes, the application of novel reactor designs, and the utilization of advanced separation technologies. The convergence of biotechnology and chemical engineering may also lead to hybrid processes that combine the best aspects of biological and chemical synthesis routes.
Market Analysis for Propionic Acid Derivatives
The market for propionic acid derivatives has shown significant growth potential in recent years, driven by increasing demand across various industries. Propionic acid, a versatile chemical compound, serves as a precursor for numerous derivatives with wide-ranging applications. The global market for these derivatives is expected to expand steadily, with a compound annual growth rate (CAGR) projected to remain robust over the next five years.
One of the key factors contributing to market growth is the rising demand for food preservatives. Propionic acid and its salts, particularly calcium and sodium propionate, are widely used as antimicrobial agents in the food and beverage industry. With the growing consumer preference for packaged and convenience foods, coupled with stringent food safety regulations, the demand for these preservatives is anticipated to surge.
The pharmaceutical sector represents another significant market for propionic acid derivatives. These compounds find applications in the synthesis of various drugs and as excipients in pharmaceutical formulations. The expanding pharmaceutical industry, particularly in emerging economies, is likely to drive the demand for propionic acid derivatives in this sector.
In the agrochemical industry, propionic acid derivatives are utilized in the production of herbicides and plant growth regulators. As global agricultural practices evolve to meet the increasing food demand, the market for these derivatives is expected to witness substantial growth. Additionally, the shift towards sustainable and eco-friendly agricultural solutions may present new opportunities for bio-based propionic acid derivatives.
The personal care and cosmetics industry also contributes to the market demand for propionic acid derivatives. These compounds are used as preservatives and pH adjusters in various cosmetic formulations. The growing consumer awareness regarding personal hygiene and the expanding beauty industry are likely to fuel the demand in this sector.
Geographically, North America and Europe currently dominate the market for propionic acid derivatives, owing to their well-established industrial base and stringent regulations favoring the use of these compounds. However, the Asia-Pacific region is expected to emerge as a lucrative market, driven by rapid industrialization, increasing disposable incomes, and changing consumer preferences in countries like China and India.
Despite the positive outlook, the market faces challenges such as volatility in raw material prices and environmental concerns associated with the production of propionic acid. These factors may impact market growth and necessitate the development of more sustainable production methods. Nonetheless, ongoing research and development efforts aimed at improving production efficiency and exploring bio-based alternatives are likely to create new growth avenues for the propionic acid derivatives market.
One of the key factors contributing to market growth is the rising demand for food preservatives. Propionic acid and its salts, particularly calcium and sodium propionate, are widely used as antimicrobial agents in the food and beverage industry. With the growing consumer preference for packaged and convenience foods, coupled with stringent food safety regulations, the demand for these preservatives is anticipated to surge.
The pharmaceutical sector represents another significant market for propionic acid derivatives. These compounds find applications in the synthesis of various drugs and as excipients in pharmaceutical formulations. The expanding pharmaceutical industry, particularly in emerging economies, is likely to drive the demand for propionic acid derivatives in this sector.
In the agrochemical industry, propionic acid derivatives are utilized in the production of herbicides and plant growth regulators. As global agricultural practices evolve to meet the increasing food demand, the market for these derivatives is expected to witness substantial growth. Additionally, the shift towards sustainable and eco-friendly agricultural solutions may present new opportunities for bio-based propionic acid derivatives.
The personal care and cosmetics industry also contributes to the market demand for propionic acid derivatives. These compounds are used as preservatives and pH adjusters in various cosmetic formulations. The growing consumer awareness regarding personal hygiene and the expanding beauty industry are likely to fuel the demand in this sector.
Geographically, North America and Europe currently dominate the market for propionic acid derivatives, owing to their well-established industrial base and stringent regulations favoring the use of these compounds. However, the Asia-Pacific region is expected to emerge as a lucrative market, driven by rapid industrialization, increasing disposable incomes, and changing consumer preferences in countries like China and India.
Despite the positive outlook, the market faces challenges such as volatility in raw material prices and environmental concerns associated with the production of propionic acid. These factors may impact market growth and necessitate the development of more sustainable production methods. Nonetheless, ongoing research and development efforts aimed at improving production efficiency and exploring bio-based alternatives are likely to create new growth avenues for the propionic acid derivatives market.
Current Challenges in Propionic Acid Synthesis
Propionic acid synthesis faces several significant challenges that hinder its widespread industrial application and efficiency. One of the primary issues is the high energy consumption associated with traditional production methods. The current petrochemical-based processes, such as the oxo synthesis from ethylene, require elevated temperatures and pressures, leading to substantial energy costs and environmental concerns.
Another major challenge lies in the limited selectivity of existing catalytic systems. The oxidation of propionaldehyde, a common route for propionic acid production, often results in unwanted by-products, reducing overall yield and necessitating complex separation processes. This lack of selectivity not only impacts the economics of production but also poses environmental challenges due to waste generation.
The reliance on fossil fuel-derived feedstocks presents a sustainability issue for propionic acid synthesis. As global efforts intensify to reduce carbon footprints, there is growing pressure to develop alternative, renewable-based production routes. However, bio-based methods currently struggle with low productivity and high production costs, making them less competitive compared to conventional petrochemical processes.
Catalyst deactivation and stability remain persistent problems in propionic acid synthesis. Many catalytic systems suffer from rapid degradation under reaction conditions, leading to decreased efficiency over time and frequent catalyst replacement. This issue is particularly pronounced in bio-based fermentation processes, where maintaining optimal microbial activity over extended periods is challenging.
Scale-up difficulties present another significant hurdle in propionic acid production. While laboratory-scale syntheses may show promise, translating these processes to industrial scales often encounters unforeseen complications. Issues such as heat and mass transfer limitations, reactor design challenges, and maintaining consistent product quality at larger scales impede the commercialization of new synthetic routes.
The corrosive nature of propionic acid poses materials compatibility challenges in production and storage. This necessitates the use of specialized, often expensive, corrosion-resistant materials throughout the production chain, adding to overall costs and complicating process design.
Lastly, the volatility of raw material prices, particularly for petrochemical feedstocks, introduces economic uncertainties in propionic acid production. This price instability affects the long-term viability and competitiveness of different synthetic routes, making it difficult for manufacturers to commit to significant process innovations or capital investments.
Another major challenge lies in the limited selectivity of existing catalytic systems. The oxidation of propionaldehyde, a common route for propionic acid production, often results in unwanted by-products, reducing overall yield and necessitating complex separation processes. This lack of selectivity not only impacts the economics of production but also poses environmental challenges due to waste generation.
The reliance on fossil fuel-derived feedstocks presents a sustainability issue for propionic acid synthesis. As global efforts intensify to reduce carbon footprints, there is growing pressure to develop alternative, renewable-based production routes. However, bio-based methods currently struggle with low productivity and high production costs, making them less competitive compared to conventional petrochemical processes.
Catalyst deactivation and stability remain persistent problems in propionic acid synthesis. Many catalytic systems suffer from rapid degradation under reaction conditions, leading to decreased efficiency over time and frequent catalyst replacement. This issue is particularly pronounced in bio-based fermentation processes, where maintaining optimal microbial activity over extended periods is challenging.
Scale-up difficulties present another significant hurdle in propionic acid production. While laboratory-scale syntheses may show promise, translating these processes to industrial scales often encounters unforeseen complications. Issues such as heat and mass transfer limitations, reactor design challenges, and maintaining consistent product quality at larger scales impede the commercialization of new synthetic routes.
The corrosive nature of propionic acid poses materials compatibility challenges in production and storage. This necessitates the use of specialized, often expensive, corrosion-resistant materials throughout the production chain, adding to overall costs and complicating process design.
Lastly, the volatility of raw material prices, particularly for petrochemical feedstocks, introduces economic uncertainties in propionic acid production. This price instability affects the long-term viability and competitiveness of different synthetic routes, making it difficult for manufacturers to commit to significant process innovations or capital investments.
Existing Synthetic Routes for Propionic Acid
01 Oxidation of propionaldehyde
One synthetic route for propionic acid involves the oxidation of propionaldehyde. This process typically uses catalysts and oxidizing agents to convert the aldehyde group to a carboxylic acid group. The reaction conditions and catalysts can be optimized to improve yield and selectivity.- Oxidation of propionaldehyde: One synthetic route for propionic acid involves the oxidation of propionaldehyde. This process typically uses catalysts and oxidizing agents to convert the aldehyde group to a carboxylic acid group. The reaction conditions and catalysts can be optimized to improve yield and selectivity.
- Carbonylation of ethylene: Propionic acid can be synthesized through the carbonylation of ethylene. This process involves the reaction of ethylene with carbon monoxide and water in the presence of a catalyst, typically a metal complex. The reaction conditions, such as temperature, pressure, and catalyst composition, can be adjusted to enhance the production of propionic acid.
- Fermentation processes: Propionic acid can be produced through fermentation processes using various microorganisms. These biological routes often involve the conversion of renewable feedstocks, such as glucose or lactose, into propionic acid. The selection of appropriate microorganisms and optimization of fermentation conditions are crucial for improving yield and productivity.
- Hydrocarboxylation of ethylene: Another synthetic route for propionic acid is the hydrocarboxylation of ethylene. This process involves the reaction of ethylene with carbon monoxide and water under high pressure and temperature conditions. Catalysts, often based on transition metals, are used to facilitate the reaction and improve selectivity towards propionic acid.
- Hydrolysis of propionitrile: Propionic acid can be synthesized through the hydrolysis of propionitrile. This process involves the reaction of propionitrile with water, often in the presence of an acid or base catalyst. The reaction conditions, such as temperature and catalyst concentration, can be optimized to improve the yield and purity of the resulting propionic acid.
02 Fermentation processes
Propionic acid can be produced through fermentation processes using various microorganisms. These biological routes often involve the conversion of renewable feedstocks or waste materials into propionic acid. The process can be optimized by selecting appropriate strains, controlling fermentation conditions, and developing efficient separation techniques.Expand Specific Solutions03 Carbonylation of ethylene
Another synthetic route for propionic acid production is the carbonylation of ethylene. This process typically involves the reaction of ethylene with carbon monoxide and water in the presence of a catalyst. The reaction conditions, such as temperature, pressure, and catalyst composition, can be adjusted to improve the yield and selectivity of propionic acid formation.Expand Specific Solutions04 Hydrocarboxylation of ethylene
Propionic acid can be synthesized through the hydrocarboxylation of ethylene. This process involves the reaction of ethylene with carbon monoxide and water or methanol in the presence of a catalyst. The reaction conditions and catalyst systems can be optimized to enhance the conversion and selectivity towards propionic acid.Expand Specific Solutions05 Catalytic dehydrogenation of propanol
The catalytic dehydrogenation of propanol is another synthetic route for propionic acid production. This process involves the removal of hydrogen from propanol using a suitable catalyst, typically under high temperature conditions. The catalyst composition and reaction parameters can be optimized to improve the yield and selectivity of propionic acid formation.Expand Specific Solutions
Key Players in Propionic Acid Industry
The innovation of propionic acid-based synthetic routes is in a mature stage, with a significant market size due to its wide applications in various industries. The technology has reached a high level of maturity, as evidenced by the involvement of major chemical companies like BASF Corp. and Wanhua Chemical Group Co., Ltd. These industry leaders have established production processes and continue to invest in research and development. However, there is still room for improvement in efficiency and sustainability, driving ongoing innovation efforts. Smaller specialized firms and research institutions, such as Shanghai Institute of Pharmaceutical Industry and Sichuan University, are also contributing to advancements in this field, focusing on niche applications and novel synthesis methods.
Wanhua Chemical Group Co., Ltd.
Technical Solution: Wanhua Chemical has focused on developing a green and sustainable route for propionic acid production. Their approach involves the direct oxidation of propanol using a heterogeneous catalyst system based on mixed metal oxides. The process operates in the liquid phase under moderate temperatures (150-200°C) and pressures (20-30 bar), utilizing air or oxygen as the oxidant[13]. Wanhua has reported achieving propanol conversion rates of up to 90% with selectivity towards propionic acid exceeding 95%[14]. The company has also implemented advanced process intensification techniques, such as structured reactors and membrane separation technologies, to enhance productivity and reduce energy consumption. Additionally, Wanhua has explored the use of bio-based propanol as a feedstock, further improving the sustainability profile of their process[15].
Strengths: Green chemistry approach, high selectivity, potential for bio-based feedstock utilization. Weaknesses: Moderate conversion rates, potential catalyst deactivation due to over-oxidation.
CJ CheilJedang Corp.
Technical Solution: CJ CheilJedang has pioneered a novel approach to propionic acid production using metabolically engineered Escherichia coli strains. Their process involves the introduction of heterologous pathways for propionic acid biosynthesis, coupled with the optimization of native metabolic pathways to enhance carbon flux towards the desired product[7]. The company has reported achieving propionic acid titers of up to 40 g/L in fed-batch fermentations using glucose as the primary carbon source[8]. Additionally, CJ CheilJedang has developed continuous fermentation processes to improve productivity and reduce operational costs. Their technology also includes innovative in situ product recovery methods to mitigate product inhibition and simplify downstream processing[9].
Strengths: High-titer production, potential for continuous operation, innovative product recovery. Weaknesses: Genetic stability of engineered strains, potential regulatory hurdles for GMO-derived products.
Innovative Catalysts for Propionic Acid Synthesis
Synthesis of 2-aryl propenoic acid esters for the production of non-steroidal anti-inflammatory drugs
PatentInactiveUS6683205B2
Innovation
- A cost-effective and environmentally friendly process is developed for producing 2-aryl-3-hydroxy-propenoic acid esters, which can be converted into 2-aryl propenoic acid esters and subsequently into naproxen, using an aryl aldehyde and alkyldiazoacetate in the presence of fluoroboric acid or an iron Lewis acid, followed by reduction and hydrolysis, allowing for the production of various NSAIDs like ibuprofen and flurbiprofen.
Processes and intermediates for synthesis of MRTX1133
PatentWO2025024447A2
Innovation
- The method involves a series of steps including reacting Boc-protected compounds with bases and aprotic solvents, using activating agents and organic bases, and subsequent reactions with ligands and palladium catalysts, culminating in the production of MRTX1133 with improved yield and purity.
Environmental Impact of Propionic Acid Manufacturing
The environmental impact of propionic acid manufacturing is a critical consideration in the development of innovative synthetic routes. Traditional production methods, primarily petrochemical-based processes, have been associated with significant environmental concerns. These include high energy consumption, greenhouse gas emissions, and the generation of hazardous waste streams. The oxidation of propanol or propionaldehyde, common industrial routes, often involves the use of heavy metal catalysts and strong oxidizing agents, leading to potential environmental contamination if not properly managed.
Recent advancements in biotechnological processes for propionic acid production have shown promise in reducing the environmental footprint. Fermentation-based methods utilizing renewable feedstocks, such as glucose or glycerol, offer a more sustainable alternative. These bio-based routes typically operate under milder conditions, consume less energy, and produce fewer toxic by-products. However, challenges remain in scaling up these processes to meet industrial demand while maintaining economic viability.
Water usage and wastewater management are significant environmental factors in propionic acid production. Both chemical and biological processes generate aqueous waste streams that require treatment before discharge. Implementing closed-loop systems and water recycling technologies can substantially reduce the water footprint of manufacturing operations. Additionally, the recovery and purification of propionic acid from reaction mixtures often involve energy-intensive distillation processes, contributing to the overall environmental impact.
The choice of raw materials plays a crucial role in the environmental profile of propionic acid synthesis. Shifting towards renewable carbon sources, such as biomass-derived feedstocks, can significantly reduce the reliance on fossil fuels and decrease the carbon footprint of production. However, careful consideration must be given to the sustainability of these alternative feedstocks, including land use changes and potential competition with food crops.
Innovative approaches to propionic acid synthesis are increasingly focusing on green chemistry principles. These include the development of catalytic systems that operate efficiently under ambient conditions, the use of non-toxic solvents or solvent-free reactions, and the design of atom-economical processes that minimize waste generation. Electrochemical methods for propionic acid production are also emerging as environmentally friendly alternatives, offering the potential for direct synthesis from simple precursors using renewable electricity sources.
Life cycle assessment (LCA) studies have become essential tools in evaluating the environmental impact of different propionic acid production routes. These comprehensive analyses consider all stages of the product lifecycle, from raw material extraction to end-of-life disposal, providing valuable insights into the overall sustainability of various synthetic pathways. Such assessments guide research efforts towards the most promising environmentally benign technologies and inform policy decisions aimed at promoting cleaner production practices in the chemical industry.
Recent advancements in biotechnological processes for propionic acid production have shown promise in reducing the environmental footprint. Fermentation-based methods utilizing renewable feedstocks, such as glucose or glycerol, offer a more sustainable alternative. These bio-based routes typically operate under milder conditions, consume less energy, and produce fewer toxic by-products. However, challenges remain in scaling up these processes to meet industrial demand while maintaining economic viability.
Water usage and wastewater management are significant environmental factors in propionic acid production. Both chemical and biological processes generate aqueous waste streams that require treatment before discharge. Implementing closed-loop systems and water recycling technologies can substantially reduce the water footprint of manufacturing operations. Additionally, the recovery and purification of propionic acid from reaction mixtures often involve energy-intensive distillation processes, contributing to the overall environmental impact.
The choice of raw materials plays a crucial role in the environmental profile of propionic acid synthesis. Shifting towards renewable carbon sources, such as biomass-derived feedstocks, can significantly reduce the reliance on fossil fuels and decrease the carbon footprint of production. However, careful consideration must be given to the sustainability of these alternative feedstocks, including land use changes and potential competition with food crops.
Innovative approaches to propionic acid synthesis are increasingly focusing on green chemistry principles. These include the development of catalytic systems that operate efficiently under ambient conditions, the use of non-toxic solvents or solvent-free reactions, and the design of atom-economical processes that minimize waste generation. Electrochemical methods for propionic acid production are also emerging as environmentally friendly alternatives, offering the potential for direct synthesis from simple precursors using renewable electricity sources.
Life cycle assessment (LCA) studies have become essential tools in evaluating the environmental impact of different propionic acid production routes. These comprehensive analyses consider all stages of the product lifecycle, from raw material extraction to end-of-life disposal, providing valuable insights into the overall sustainability of various synthetic pathways. Such assessments guide research efforts towards the most promising environmentally benign technologies and inform policy decisions aimed at promoting cleaner production practices in the chemical industry.
Economic Feasibility of Novel Synthetic Routes
The economic feasibility of novel synthetic routes for propionic acid production is a critical factor in determining their potential for industrial adoption. Traditional methods, such as the oxo process and the Reppe process, have long dominated the market due to their established infrastructure and optimized cost structures. However, the increasing demand for sustainable and cost-effective production methods has spurred research into alternative synthetic pathways.
One promising avenue is the development of bio-based production routes. These methods utilize renewable feedstocks and microbial fermentation processes, potentially offering significant cost advantages over petrochemical-based routes. The economic viability of these bio-based methods hinges on factors such as feedstock availability, conversion efficiency, and downstream processing costs. Recent advancements in metabolic engineering and synthetic biology have improved the yield and productivity of microbial strains, enhancing the economic competitiveness of these routes.
Another area of innovation lies in the realm of catalytic processes. Novel catalysts and reaction conditions can potentially reduce energy requirements and improve selectivity, leading to more economically favorable production routes. For instance, the development of heterogeneous catalysts for the direct oxidation of propane to propionic acid could significantly reduce production costs by simplifying the process and utilizing cheaper raw materials.
The economic feasibility of these novel routes is also influenced by market dynamics and regulatory environments. As sustainability becomes an increasingly important factor in industrial decision-making, processes that reduce carbon footprint or utilize waste streams may gain economic advantages through carbon credits or government incentives. Additionally, the volatility of petrochemical feedstock prices can impact the relative economic attractiveness of alternative synthetic routes.
Scale-up considerations play a crucial role in determining the economic viability of new production methods. While a novel route may show promise at the laboratory scale, the challenges and costs associated with industrial-scale implementation can be substantial. Factors such as equipment requirements, process control, and waste management must be carefully evaluated to assess the true economic potential of a new synthetic pathway.
In conclusion, the economic feasibility of novel synthetic routes for propionic acid production is a complex interplay of technological innovation, market forces, and regulatory landscapes. As research progresses, it is essential to conduct comprehensive techno-economic analyses that consider not only the direct production costs but also the broader economic implications of adopting new technologies in the propionic acid value chain.
One promising avenue is the development of bio-based production routes. These methods utilize renewable feedstocks and microbial fermentation processes, potentially offering significant cost advantages over petrochemical-based routes. The economic viability of these bio-based methods hinges on factors such as feedstock availability, conversion efficiency, and downstream processing costs. Recent advancements in metabolic engineering and synthetic biology have improved the yield and productivity of microbial strains, enhancing the economic competitiveness of these routes.
Another area of innovation lies in the realm of catalytic processes. Novel catalysts and reaction conditions can potentially reduce energy requirements and improve selectivity, leading to more economically favorable production routes. For instance, the development of heterogeneous catalysts for the direct oxidation of propane to propionic acid could significantly reduce production costs by simplifying the process and utilizing cheaper raw materials.
The economic feasibility of these novel routes is also influenced by market dynamics and regulatory environments. As sustainability becomes an increasingly important factor in industrial decision-making, processes that reduce carbon footprint or utilize waste streams may gain economic advantages through carbon credits or government incentives. Additionally, the volatility of petrochemical feedstock prices can impact the relative economic attractiveness of alternative synthetic routes.
Scale-up considerations play a crucial role in determining the economic viability of new production methods. While a novel route may show promise at the laboratory scale, the challenges and costs associated with industrial-scale implementation can be substantial. Factors such as equipment requirements, process control, and waste management must be carefully evaluated to assess the true economic potential of a new synthetic pathway.
In conclusion, the economic feasibility of novel synthetic routes for propionic acid production is a complex interplay of technological innovation, market forces, and regulatory landscapes. As research progresses, it is essential to conduct comprehensive techno-economic analyses that consider not only the direct production costs but also the broader economic implications of adopting new technologies in the propionic acid value chain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!