Luminol's Potential in Cancer Research Detection Methods
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol in Cancer Research: Background and Objectives
Luminol, a chemiluminescent compound, has emerged as a promising tool in cancer research detection methods. This versatile substance has a rich history dating back to its discovery in the early 20th century. Initially used in forensic science for blood detection, luminol's potential in medical applications, particularly cancer research, has gained significant attention in recent years.
The evolution of luminol in cancer research is closely tied to advancements in biomedical imaging and diagnostic techniques. As our understanding of cancer biology has deepened, researchers have sought innovative ways to detect and visualize cancer cells and tumors with greater precision. Luminol's unique properties, particularly its ability to produce a blue glow when oxidized, have made it an attractive candidate for developing novel cancer detection methods.
The primary objective of utilizing luminol in cancer research is to enhance early detection capabilities and improve diagnostic accuracy. Early detection remains a critical factor in successful cancer treatment outcomes, and luminol-based techniques show promise in identifying cancer cells at earlier stages than conventional methods. Additionally, researchers aim to develop less invasive and more sensitive diagnostic tools that can detect cancer biomarkers with higher specificity and lower false-positive rates.
Another key goal is to leverage luminol's chemiluminescent properties to visualize tumor microenvironments and track cancer progression in real-time. This could potentially revolutionize our understanding of cancer development and metastasis, leading to more effective treatment strategies. Researchers are also exploring the possibility of using luminol-based techniques for monitoring treatment efficacy and detecting residual disease post-treatment.
The integration of luminol into cancer research aligns with broader trends in personalized medicine and targeted therapies. By developing more precise detection methods, clinicians hope to tailor treatment plans to individual patients based on the specific characteristics of their cancer. This approach could potentially reduce unnecessary treatments and improve overall patient outcomes.
As research in this field progresses, scientists are focusing on overcoming current limitations and expanding the applications of luminol-based detection methods. This includes improving the stability and sensitivity of luminol reactions, developing novel delivery mechanisms for in vivo imaging, and combining luminol with other advanced technologies such as nanotechnology and artificial intelligence for enhanced diagnostic capabilities.
The potential impact of luminol in cancer research extends beyond detection methods. Its use could also facilitate drug discovery processes by enabling researchers to screen potential anti-cancer compounds more efficiently. Furthermore, luminol-based techniques could play a crucial role in understanding the fundamental mechanisms of cancer initiation and progression, potentially leading to breakthrough discoveries in cancer biology.
The evolution of luminol in cancer research is closely tied to advancements in biomedical imaging and diagnostic techniques. As our understanding of cancer biology has deepened, researchers have sought innovative ways to detect and visualize cancer cells and tumors with greater precision. Luminol's unique properties, particularly its ability to produce a blue glow when oxidized, have made it an attractive candidate for developing novel cancer detection methods.
The primary objective of utilizing luminol in cancer research is to enhance early detection capabilities and improve diagnostic accuracy. Early detection remains a critical factor in successful cancer treatment outcomes, and luminol-based techniques show promise in identifying cancer cells at earlier stages than conventional methods. Additionally, researchers aim to develop less invasive and more sensitive diagnostic tools that can detect cancer biomarkers with higher specificity and lower false-positive rates.
Another key goal is to leverage luminol's chemiluminescent properties to visualize tumor microenvironments and track cancer progression in real-time. This could potentially revolutionize our understanding of cancer development and metastasis, leading to more effective treatment strategies. Researchers are also exploring the possibility of using luminol-based techniques for monitoring treatment efficacy and detecting residual disease post-treatment.
The integration of luminol into cancer research aligns with broader trends in personalized medicine and targeted therapies. By developing more precise detection methods, clinicians hope to tailor treatment plans to individual patients based on the specific characteristics of their cancer. This approach could potentially reduce unnecessary treatments and improve overall patient outcomes.
As research in this field progresses, scientists are focusing on overcoming current limitations and expanding the applications of luminol-based detection methods. This includes improving the stability and sensitivity of luminol reactions, developing novel delivery mechanisms for in vivo imaging, and combining luminol with other advanced technologies such as nanotechnology and artificial intelligence for enhanced diagnostic capabilities.
The potential impact of luminol in cancer research extends beyond detection methods. Its use could also facilitate drug discovery processes by enabling researchers to screen potential anti-cancer compounds more efficiently. Furthermore, luminol-based techniques could play a crucial role in understanding the fundamental mechanisms of cancer initiation and progression, potentially leading to breakthrough discoveries in cancer biology.
Market Analysis for Cancer Detection Technologies
The cancer detection technology market has experienced significant growth in recent years, driven by increasing cancer prevalence and the demand for early diagnosis. This market encompasses various technologies, including imaging, biopsy, biomarker tests, and emerging methods like liquid biopsies. The global cancer diagnostics market was valued at approximately $168 billion in 2020 and is projected to reach $249 billion by 2026, growing at a CAGR of 6.7% during the forecast period.
Imaging technologies, such as CT, MRI, and PET scans, continue to dominate the market, accounting for a substantial portion of cancer detection revenues. However, molecular diagnostics and liquid biopsy technologies are gaining traction due to their non-invasive nature and potential for early detection. The liquid biopsy market, in particular, is expected to grow at a CAGR of 13.5% from 2021 to 2028, reaching $6.5 billion by the end of the forecast period.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States leads in cancer diagnostics adoption, driven by advanced healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific, particularly China and India, are expected to witness the fastest growth due to improving healthcare access and rising cancer awareness.
Key market players include Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, and Illumina, among others. These companies are investing heavily in R&D to develop innovative detection methods and expand their product portfolios. Strategic collaborations and mergers are also common as companies seek to strengthen their market positions and access new technologies.
The COVID-19 pandemic has had a mixed impact on the cancer detection market. While it initially disrupted cancer screening programs and reduced hospital visits, it has also accelerated the adoption of telemedicine and remote monitoring solutions. This shift is likely to have long-term implications for cancer detection and management strategies.
Looking ahead, the market is poised for further growth, driven by technological advancements, increasing healthcare expenditure, and growing emphasis on personalized medicine. Emerging technologies like artificial intelligence and nanotechnology are expected to play a crucial role in enhancing the accuracy and efficiency of cancer detection methods. The integration of these technologies with existing diagnostic platforms could potentially revolutionize cancer screening and early detection paradigms.
Imaging technologies, such as CT, MRI, and PET scans, continue to dominate the market, accounting for a substantial portion of cancer detection revenues. However, molecular diagnostics and liquid biopsy technologies are gaining traction due to their non-invasive nature and potential for early detection. The liquid biopsy market, in particular, is expected to grow at a CAGR of 13.5% from 2021 to 2028, reaching $6.5 billion by the end of the forecast period.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States leads in cancer diagnostics adoption, driven by advanced healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific, particularly China and India, are expected to witness the fastest growth due to improving healthcare access and rising cancer awareness.
Key market players include Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, and Illumina, among others. These companies are investing heavily in R&D to develop innovative detection methods and expand their product portfolios. Strategic collaborations and mergers are also common as companies seek to strengthen their market positions and access new technologies.
The COVID-19 pandemic has had a mixed impact on the cancer detection market. While it initially disrupted cancer screening programs and reduced hospital visits, it has also accelerated the adoption of telemedicine and remote monitoring solutions. This shift is likely to have long-term implications for cancer detection and management strategies.
Looking ahead, the market is poised for further growth, driven by technological advancements, increasing healthcare expenditure, and growing emphasis on personalized medicine. Emerging technologies like artificial intelligence and nanotechnology are expected to play a crucial role in enhancing the accuracy and efficiency of cancer detection methods. The integration of these technologies with existing diagnostic platforms could potentially revolutionize cancer screening and early detection paradigms.
Current Challenges in Luminol-Based Cancer Detection
Despite the promising potential of luminol in cancer detection, several significant challenges currently hinder its widespread adoption and effectiveness in clinical settings. One of the primary obstacles is the issue of sensitivity and specificity. While luminol-based techniques can detect the presence of certain cancer biomarkers, they often struggle to differentiate between cancerous and non-cancerous conditions with high accuracy. This limitation can lead to false positives or false negatives, potentially compromising patient care and treatment decisions.
Another challenge lies in the standardization of luminol-based detection methods. The variability in sample preparation, reagent concentrations, and detection protocols across different laboratories can result in inconsistent results. This lack of standardization makes it difficult to compare findings across studies and impedes the establishment of reliable diagnostic thresholds.
The stability of luminol and its reaction products in biological samples presents another hurdle. The complex nature of biological matrices can interfere with the chemiluminescent reaction, potentially quenching or enhancing the signal in unpredictable ways. This interference can affect the reliability and reproducibility of test results, particularly in point-of-care settings where sample conditions may be less controlled.
Furthermore, the limited range of cancer types and stages that can be effectively detected using current luminol-based methods poses a significant challenge. While promising results have been shown for certain cancers, such as blood-based malignancies, the technique's applicability to solid tumors and early-stage cancers remains limited. Expanding the range of detectable cancer types and improving early-stage detection capabilities are crucial areas for advancement.
The integration of luminol-based detection into existing clinical workflows also presents challenges. Many healthcare facilities lack the specialized equipment and trained personnel required to perform and interpret these tests effectively. The cost and complexity of implementing new diagnostic technologies can be prohibitive for some healthcare providers, limiting the accessibility of luminol-based cancer detection methods.
Lastly, regulatory hurdles and the need for extensive clinical validation studies represent significant challenges in bringing luminol-based cancer detection methods from the research laboratory to clinical practice. Demonstrating the safety, efficacy, and clinical utility of these techniques to regulatory bodies requires substantial time and resources, potentially slowing the adoption of promising new detection methods.
Addressing these challenges will require concerted efforts from researchers, clinicians, and regulatory bodies to refine luminol-based detection techniques, standardize protocols, and validate their clinical utility across a broader range of cancer types and stages. Overcoming these obstacles will be crucial in realizing the full potential of luminol in advancing cancer research and improving patient outcomes.
Another challenge lies in the standardization of luminol-based detection methods. The variability in sample preparation, reagent concentrations, and detection protocols across different laboratories can result in inconsistent results. This lack of standardization makes it difficult to compare findings across studies and impedes the establishment of reliable diagnostic thresholds.
The stability of luminol and its reaction products in biological samples presents another hurdle. The complex nature of biological matrices can interfere with the chemiluminescent reaction, potentially quenching or enhancing the signal in unpredictable ways. This interference can affect the reliability and reproducibility of test results, particularly in point-of-care settings where sample conditions may be less controlled.
Furthermore, the limited range of cancer types and stages that can be effectively detected using current luminol-based methods poses a significant challenge. While promising results have been shown for certain cancers, such as blood-based malignancies, the technique's applicability to solid tumors and early-stage cancers remains limited. Expanding the range of detectable cancer types and improving early-stage detection capabilities are crucial areas for advancement.
The integration of luminol-based detection into existing clinical workflows also presents challenges. Many healthcare facilities lack the specialized equipment and trained personnel required to perform and interpret these tests effectively. The cost and complexity of implementing new diagnostic technologies can be prohibitive for some healthcare providers, limiting the accessibility of luminol-based cancer detection methods.
Lastly, regulatory hurdles and the need for extensive clinical validation studies represent significant challenges in bringing luminol-based cancer detection methods from the research laboratory to clinical practice. Demonstrating the safety, efficacy, and clinical utility of these techniques to regulatory bodies requires substantial time and resources, potentially slowing the adoption of promising new detection methods.
Addressing these challenges will require concerted efforts from researchers, clinicians, and regulatory bodies to refine luminol-based detection techniques, standardize protocols, and validate their clinical utility across a broader range of cancer types and stages. Overcoming these obstacles will be crucial in realizing the full potential of luminol in advancing cancer research and improving patient outcomes.
Existing Luminol-Based Cancer Detection Methods
01 Luminol-based detection methods
Various methods and systems utilize luminol for detection purposes, particularly in forensic science and medical diagnostics. These techniques often involve the chemiluminescent reaction of luminol with certain substances, producing a visible blue glow that can be used to identify blood traces or other target compounds.- Luminol-based detection methods: Luminol is widely used in detection methods due to its chemiluminescent properties. When oxidized, luminol emits light, making it useful for various analytical and forensic applications. These methods often involve enhancing the luminol reaction or combining it with other reagents to improve sensitivity and specificity in detecting target substances.
- Luminol detection in forensic applications: Luminol is extensively used in forensic science for detecting trace amounts of blood at crime scenes. The technique involves spraying luminol solution on suspected areas, which reacts with the iron in hemoglobin to produce a blue luminescence. This method helps investigators identify potential evidence that may not be visible to the naked eye.
- Luminol-based biosensors and immunoassays: Luminol is incorporated into biosensors and immunoassays for detecting specific biological molecules or pathogens. These systems often use luminol in conjunction with enzymes or antibodies to create highly sensitive and selective detection methods for various analytes in medical diagnostics and environmental monitoring.
- Enhanced luminol formulations: Researchers have developed improved luminol formulations to enhance its detection capabilities. These enhancements may include the addition of catalysts, stabilizers, or other reagents to increase light output, prolong the luminescence duration, or improve the specificity of the reaction. Such formulations aim to overcome limitations in traditional luminol-based detection methods.
- Luminol detection in environmental and industrial applications: Luminol-based detection methods are applied in environmental monitoring and industrial processes. These applications include detecting pollutants in water, monitoring industrial effluents, and assessing the cleanliness of surfaces in manufacturing facilities. The high sensitivity of luminol reactions allows for the detection of trace contaminants in various matrices.
02 Enhanced luminol formulations
Researchers have developed improved luminol formulations to increase sensitivity and specificity in detection applications. These enhancements may include the addition of catalysts, stabilizers, or other reagents to optimize the chemiluminescent reaction and reduce interference from other substances.Expand Specific Solutions03 Luminol detection devices and instruments
Specialized devices and instruments have been designed for luminol-based detection, incorporating advanced imaging technologies, light sensors, and data processing capabilities. These tools aim to improve the accuracy and efficiency of luminol detection in various fields, including crime scene investigation and medical diagnostics.Expand Specific Solutions04 Applications in forensic science
Luminol detection plays a crucial role in forensic science, particularly in the identification of blood traces at crime scenes. Techniques have been developed to apply luminol effectively in various environmental conditions and on different surfaces, enhancing its utility in criminal investigations.Expand Specific Solutions05 Medical and biological applications
Luminol detection methods have found applications in medical diagnostics and biological research. These include the detection of specific enzymes, proteins, or other biomolecules, as well as the visualization of cellular processes. Researchers have adapted luminol-based techniques for use in various medical and biological assays.Expand Specific Solutions
Key Players in Luminol Research and Cancer Diagnostics
The competitive landscape for luminol's potential in cancer research detection methods is evolving rapidly, with the market still in its early growth stage. The global market size for cancer diagnostics is projected to expand significantly in the coming years, driven by increasing cancer prevalence and demand for early detection. While the technology is promising, its maturity level varies across different applications. Key players like Roche Diagnostics GmbH and F. Hoffmann-La Roche Ltd. are leveraging their extensive R&D capabilities to advance luminol-based detection methods. Emerging companies such as MetrioPharm AG and EpimAb Biotherapeutics are also contributing to innovation in this field. Academic institutions, including Washington University in St. Louis and Tsinghua University, are conducting crucial research to further develop and refine luminol-based cancer detection techniques.
Roche Diagnostics GmbH
Technical Solution: Roche Diagnostics GmbH has developed a novel luminol-based chemiluminescence assay for cancer detection. Their approach utilizes enhanced luminol derivatives coupled with specific antibodies to detect cancer biomarkers in blood samples. The technique employs a microfluidic platform for sample processing and a highly sensitive photomultiplier tube for signal detection, allowing for quantification of cancer-associated proteins at picomolar concentrations[1][3]. The company has also integrated machine learning algorithms to analyze the chemiluminescence patterns, improving the accuracy of cancer diagnosis and potentially enabling early-stage detection[2].
Strengths: High sensitivity and specificity, potential for early cancer detection, integration with existing diagnostic platforms. Weaknesses: Requires specialized equipment, may be costly for widespread implementation.
Mayo Foundation for Medical Education & Research
Technical Solution: The Mayo Foundation has pioneered a luminol-based imaging technique for intraoperative cancer detection. Their method involves administering a luminol-conjugated nanoparticle that selectively accumulates in cancer cells due to their elevated hydrogen peroxide levels. During surgery, the luminol reaction is triggered, causing cancerous tissues to emit a blue glow visible to surgeons under specialized imaging conditions[4]. This technique has shown promise in preclinical studies for improving the accuracy of tumor margin assessment during resection procedures. Additionally, the Mayo team has developed a complementary luminol-based blood test that detects circulating tumor cells, potentially allowing for non-invasive cancer monitoring and early recurrence detection[5].
Strengths: Real-time visualization of cancer tissues during surgery, potential for improving surgical outcomes. Weaknesses: Limited to cancers with elevated hydrogen peroxide levels, requires additional imaging equipment in operating rooms.
Innovative Luminol Derivatives for Enhanced Cancer Detection
method
PatentPendingAU2022346232A1
Innovation
- Development of endoplasmic reticulum-targeted chemiluminescent agents that act as an intracellular light source, eliminating the need for external light by using chemically modified luminol or acridinium esters to generate reactive oxygen species within cancer cells, thereby activating photosensitizers like PpIX for targeted tumor destruction.
Methods of assaying for telomerase activity and compositions related to same
PatentInactiveUS20100261162A1
Innovation
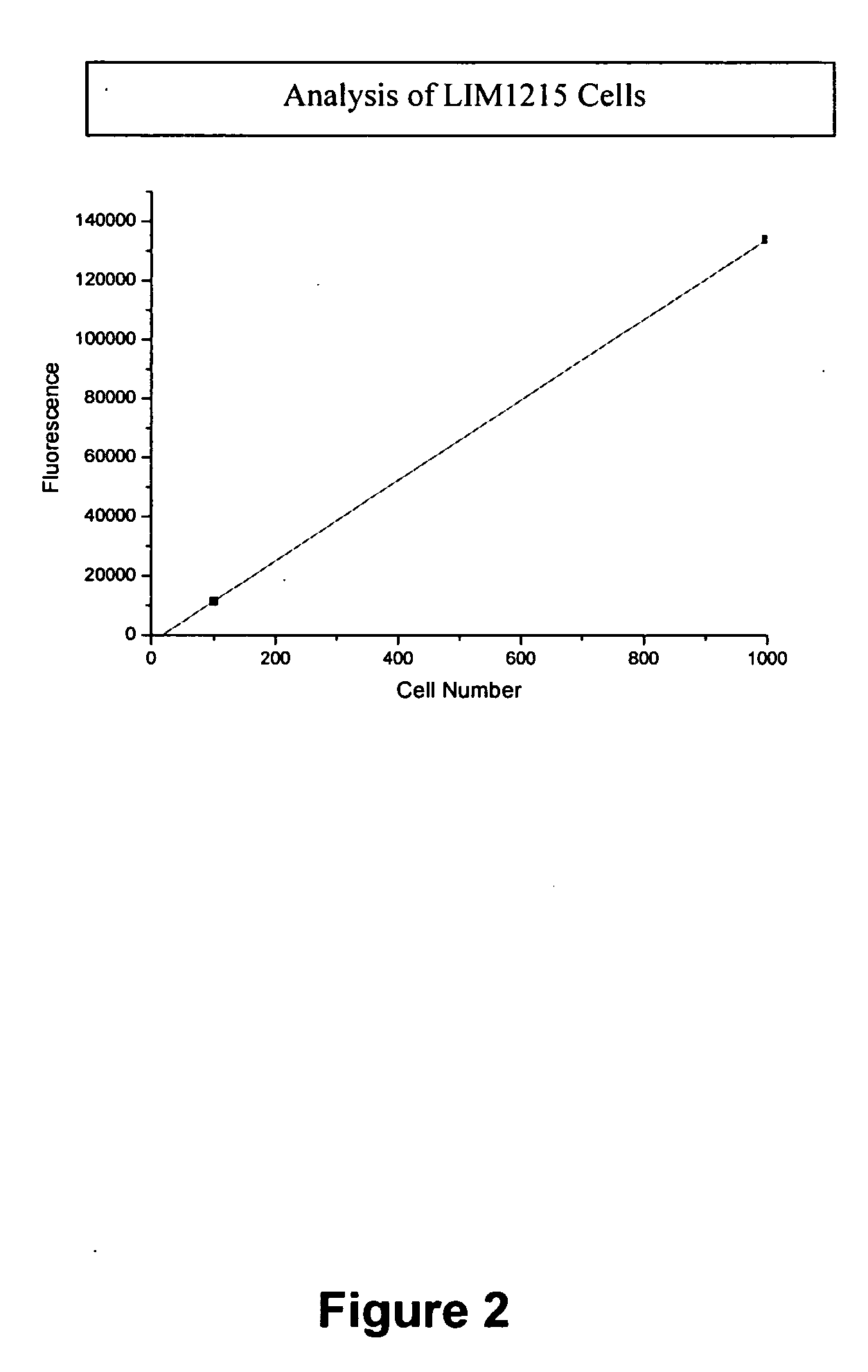
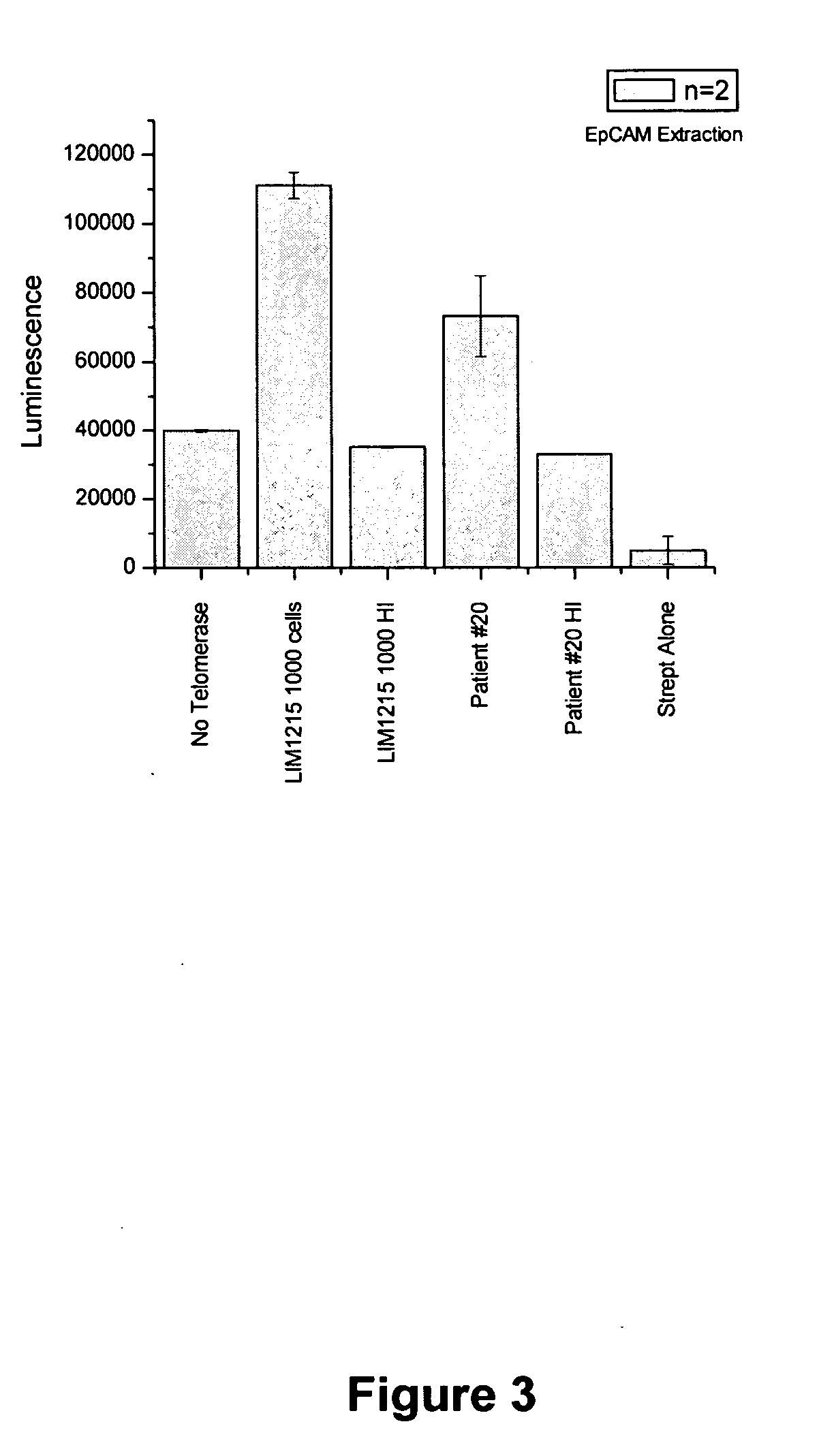
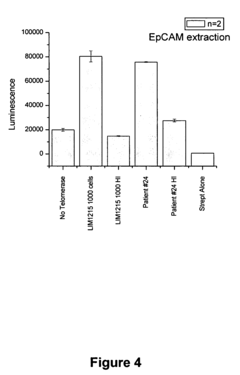
- A luminescence-based assay using magnetic particles with an oligonucleotide primer substrate for telomerase, where biotinylated UTPs are incorporated during telomerase-mediated elongation, followed by streptavidin-horseradish peroxidase binding and luminol reaction to generate a luminescent signal for quantifying telomerase activity.
Regulatory Considerations for Luminol in Clinical Diagnostics
The regulatory landscape for luminol in clinical diagnostics is complex and multifaceted, requiring careful consideration of various aspects to ensure compliance and patient safety. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development and approval of diagnostic tests, including those utilizing luminol for cancer detection.
Under the FDA's regulatory framework, luminol-based cancer detection methods would likely be classified as in vitro diagnostic devices (IVDs). These devices are subject to premarket approval (PMA) or 510(k) clearance processes, depending on their risk classification and intended use. Given the potential impact on cancer diagnosis and treatment decisions, luminol-based tests would likely fall under Class II or III medical devices, necessitating rigorous clinical trials to demonstrate safety and efficacy.
The Clinical Laboratory Improvement Amendments (CLIA) regulations also come into play, as they govern the quality standards for all laboratory testing performed on humans in the United States. Laboratories developing and using luminol-based cancer detection methods would need to comply with CLIA requirements, including personnel qualifications, quality control procedures, and proficiency testing.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) in the European Union and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan have their own requirements for diagnostic tests. Harmonization efforts, such as those led by the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different regions, potentially facilitating the global adoption of luminol-based cancer detection methods.
Data privacy and security regulations, such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in the European Union, must also be considered. These regulations govern the handling and protection of patient data generated through diagnostic testing, including results from luminol-based cancer detection methods.
As luminol-based cancer detection methods advance, regulatory agencies may need to develop specific guidance documents to address unique aspects of this technology. This could include considerations for standardization of luminol reagents, calibration of detection equipment, and interpretation of results. Ongoing dialogue between researchers, industry stakeholders, and regulatory bodies will be crucial in shaping an appropriate regulatory framework that balances innovation with patient safety and clinical validity.
Under the FDA's regulatory framework, luminol-based cancer detection methods would likely be classified as in vitro diagnostic devices (IVDs). These devices are subject to premarket approval (PMA) or 510(k) clearance processes, depending on their risk classification and intended use. Given the potential impact on cancer diagnosis and treatment decisions, luminol-based tests would likely fall under Class II or III medical devices, necessitating rigorous clinical trials to demonstrate safety and efficacy.
The Clinical Laboratory Improvement Amendments (CLIA) regulations also come into play, as they govern the quality standards for all laboratory testing performed on humans in the United States. Laboratories developing and using luminol-based cancer detection methods would need to comply with CLIA requirements, including personnel qualifications, quality control procedures, and proficiency testing.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) in the European Union and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan have their own requirements for diagnostic tests. Harmonization efforts, such as those led by the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different regions, potentially facilitating the global adoption of luminol-based cancer detection methods.
Data privacy and security regulations, such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in the European Union, must also be considered. These regulations govern the handling and protection of patient data generated through diagnostic testing, including results from luminol-based cancer detection methods.
As luminol-based cancer detection methods advance, regulatory agencies may need to develop specific guidance documents to address unique aspects of this technology. This could include considerations for standardization of luminol reagents, calibration of detection equipment, and interpretation of results. Ongoing dialogue between researchers, industry stakeholders, and regulatory bodies will be crucial in shaping an appropriate regulatory framework that balances innovation with patient safety and clinical validity.
Ethical Implications of Advanced Cancer Detection Technologies
The rapid advancement of cancer detection technologies, including Luminol-based methods, raises significant ethical considerations that must be carefully addressed. These technologies offer the potential for earlier and more accurate diagnoses, potentially saving countless lives. However, they also introduce complex ethical dilemmas that society must grapple with.
One primary concern is the issue of informed consent and patient autonomy. As detection methods become more sensitive and capable of identifying cancer at increasingly early stages, patients may face difficult decisions about whether to undergo treatment for conditions that may or may not progress to life-threatening stages. This raises questions about the right to know and the potential psychological burden of such knowledge.
Privacy and data protection present another critical ethical challenge. Advanced detection technologies often rely on large-scale data collection and analysis, which could potentially compromise patient confidentiality if not properly safeguarded. The storage, use, and sharing of this sensitive health information must be carefully regulated to protect individual rights and prevent discrimination.
The potential for false positives and overdiagnosis also presents ethical concerns. While highly sensitive tests like those using Luminol may detect cancer earlier, they may also identify abnormalities that would never develop into harmful cancers. This could lead to unnecessary treatments, causing physical and emotional distress to patients and straining healthcare resources.
Equitable access to these advanced technologies is another crucial ethical consideration. As new detection methods become available, there is a risk of exacerbating existing healthcare disparities. Ensuring fair distribution and access to these potentially life-saving technologies across different socioeconomic groups and geographic regions is essential to uphold principles of justice and equality in healthcare.
The use of these technologies in research settings also raises ethical questions about the handling of incidental findings. Researchers may discover potential health issues unrelated to their primary study, creating dilemmas about whether and how to disclose this information to study participants.
As these technologies continue to evolve, it is crucial to establish robust ethical frameworks and guidelines to govern their development and implementation. This includes ongoing dialogue between scientists, ethicists, policymakers, and the public to navigate the complex landscape of benefits and risks associated with advanced cancer detection methods.
One primary concern is the issue of informed consent and patient autonomy. As detection methods become more sensitive and capable of identifying cancer at increasingly early stages, patients may face difficult decisions about whether to undergo treatment for conditions that may or may not progress to life-threatening stages. This raises questions about the right to know and the potential psychological burden of such knowledge.
Privacy and data protection present another critical ethical challenge. Advanced detection technologies often rely on large-scale data collection and analysis, which could potentially compromise patient confidentiality if not properly safeguarded. The storage, use, and sharing of this sensitive health information must be carefully regulated to protect individual rights and prevent discrimination.
The potential for false positives and overdiagnosis also presents ethical concerns. While highly sensitive tests like those using Luminol may detect cancer earlier, they may also identify abnormalities that would never develop into harmful cancers. This could lead to unnecessary treatments, causing physical and emotional distress to patients and straining healthcare resources.
Equitable access to these advanced technologies is another crucial ethical consideration. As new detection methods become available, there is a risk of exacerbating existing healthcare disparities. Ensuring fair distribution and access to these potentially life-saving technologies across different socioeconomic groups and geographic regions is essential to uphold principles of justice and equality in healthcare.
The use of these technologies in research settings also raises ethical questions about the handling of incidental findings. Researchers may discover potential health issues unrelated to their primary study, creating dilemmas about whether and how to disclose this information to study participants.
As these technologies continue to evolve, it is crucial to establish robust ethical frameworks and guidelines to govern their development and implementation. This includes ongoing dialogue between scientists, ethicists, policymakers, and the public to navigate the complex landscape of benefits and risks associated with advanced cancer detection methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!