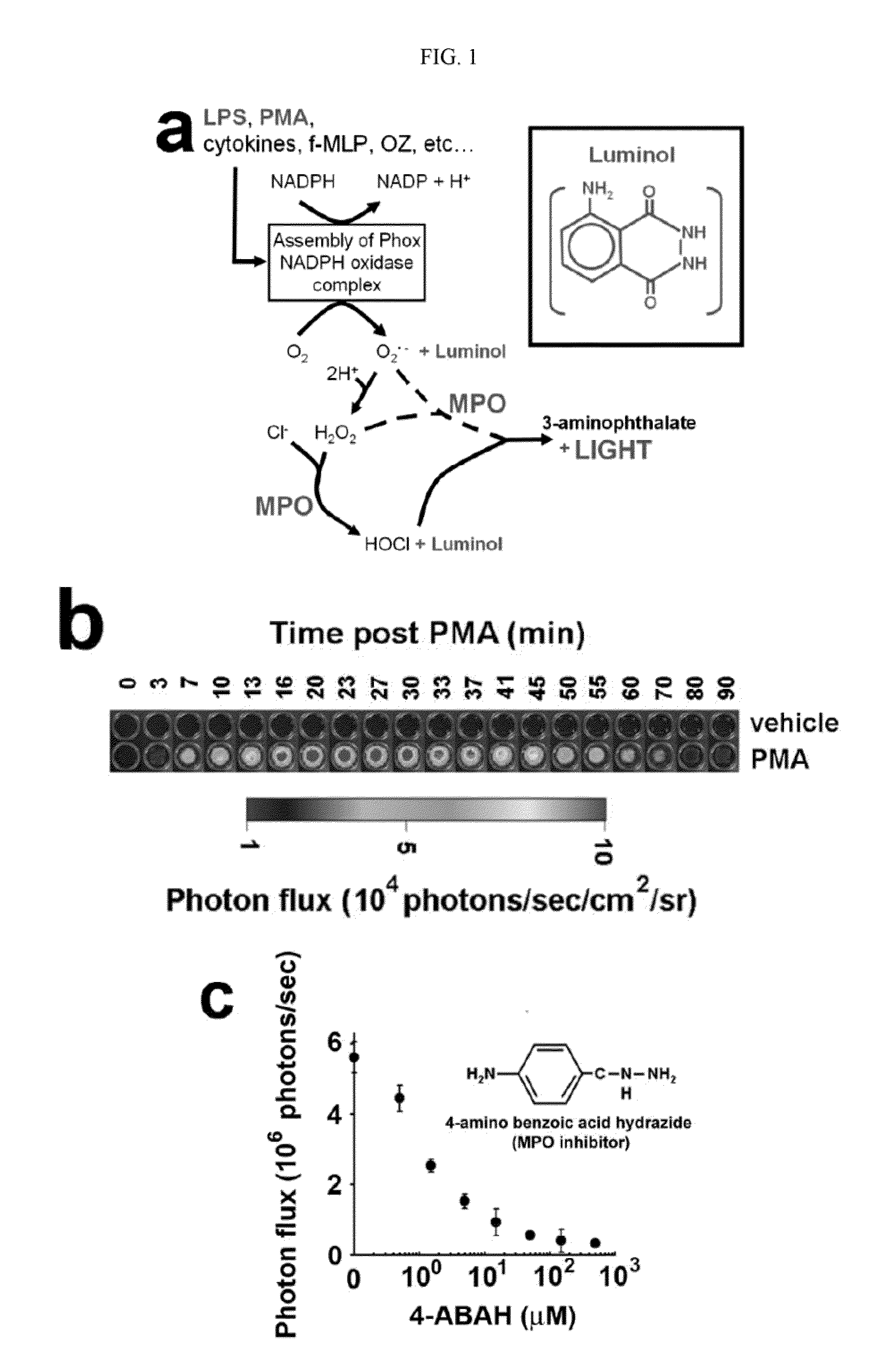
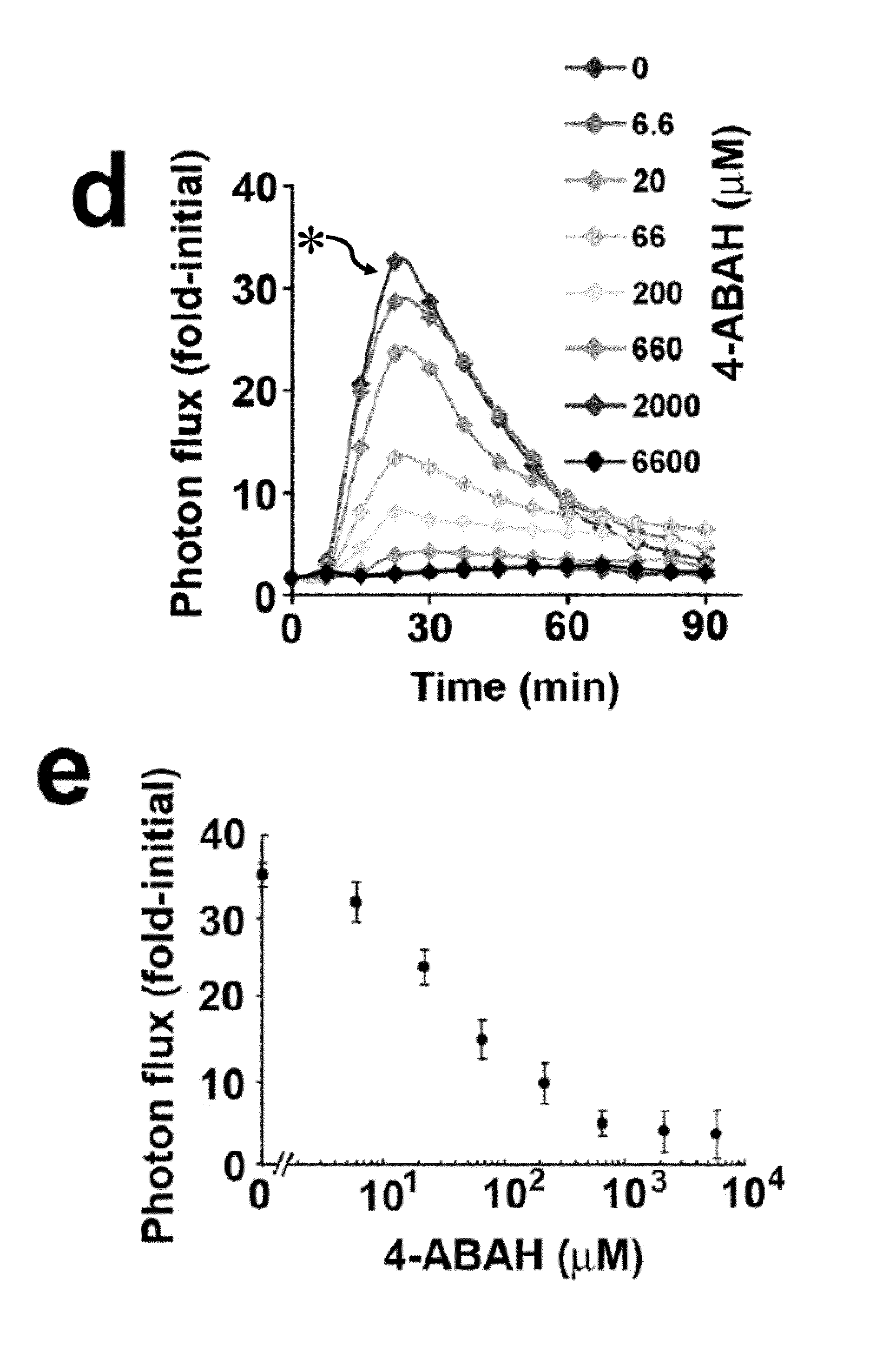
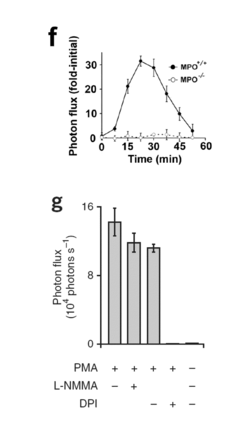
Luminol as a Diagnostic Tool in Medical Imaging
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol in Medical Imaging: Background and Objectives
Luminol, a chemiluminescent compound, has emerged as a promising diagnostic tool in medical imaging, revolutionizing the field of non-invasive diagnostic techniques. The journey of luminol in medical applications traces back to its initial discovery in the early 20th century, primarily used in forensic science for blood detection. Over the decades, researchers have recognized its potential in medical imaging due to its unique property of emitting light when oxidized.
The evolution of luminol in medical imaging has been driven by the increasing demand for more sensitive and specific diagnostic methods. As healthcare systems worldwide face challenges in early disease detection and monitoring, luminol-based imaging techniques have gained attention for their ability to visualize biological processes at the molecular level. This technology has shown particular promise in areas such as cancer detection, cardiovascular imaging, and neurological disorders.
The primary objective of research on luminol as a diagnostic tool in medical imaging is to develop highly sensitive, non-invasive imaging techniques that can detect and monitor various pathological conditions with greater accuracy than conventional methods. Researchers aim to exploit luminol's chemiluminescent properties to create imaging agents that can selectively bind to specific biomarkers or cellular processes, thereby enabling early detection of diseases and providing real-time monitoring of treatment efficacy.
Another crucial goal is to optimize luminol-based imaging techniques for clinical applications. This involves improving the stability and biocompatibility of luminol derivatives, enhancing signal intensity and duration, and developing advanced imaging systems capable of detecting and analyzing luminol-generated signals in vivo. Researchers are also focusing on creating multifunctional imaging probes that combine luminol with other imaging modalities, such as MRI or PET, to provide complementary diagnostic information.
The development of luminol as a diagnostic tool aligns with the broader trend in personalized medicine, where tailored diagnostic and therapeutic approaches are becoming increasingly important. By enabling more precise and earlier detection of diseases, luminol-based imaging could potentially lead to more effective treatment strategies and improved patient outcomes. Additionally, this technology holds promise for drug development and preclinical studies, offering new ways to visualize and understand drug efficacy and toxicity in living systems.
As research in this field progresses, the ultimate aim is to translate luminol-based imaging techniques from laboratory settings to clinical practice. This transition requires addressing challenges related to regulatory approval, cost-effectiveness, and integration into existing medical imaging workflows. The successful implementation of luminol as a diagnostic tool could significantly enhance the capabilities of medical imaging, offering new insights into disease processes and potentially transforming patient care across various medical specialties.
The evolution of luminol in medical imaging has been driven by the increasing demand for more sensitive and specific diagnostic methods. As healthcare systems worldwide face challenges in early disease detection and monitoring, luminol-based imaging techniques have gained attention for their ability to visualize biological processes at the molecular level. This technology has shown particular promise in areas such as cancer detection, cardiovascular imaging, and neurological disorders.
The primary objective of research on luminol as a diagnostic tool in medical imaging is to develop highly sensitive, non-invasive imaging techniques that can detect and monitor various pathological conditions with greater accuracy than conventional methods. Researchers aim to exploit luminol's chemiluminescent properties to create imaging agents that can selectively bind to specific biomarkers or cellular processes, thereby enabling early detection of diseases and providing real-time monitoring of treatment efficacy.
Another crucial goal is to optimize luminol-based imaging techniques for clinical applications. This involves improving the stability and biocompatibility of luminol derivatives, enhancing signal intensity and duration, and developing advanced imaging systems capable of detecting and analyzing luminol-generated signals in vivo. Researchers are also focusing on creating multifunctional imaging probes that combine luminol with other imaging modalities, such as MRI or PET, to provide complementary diagnostic information.
The development of luminol as a diagnostic tool aligns with the broader trend in personalized medicine, where tailored diagnostic and therapeutic approaches are becoming increasingly important. By enabling more precise and earlier detection of diseases, luminol-based imaging could potentially lead to more effective treatment strategies and improved patient outcomes. Additionally, this technology holds promise for drug development and preclinical studies, offering new ways to visualize and understand drug efficacy and toxicity in living systems.
As research in this field progresses, the ultimate aim is to translate luminol-based imaging techniques from laboratory settings to clinical practice. This transition requires addressing challenges related to regulatory approval, cost-effectiveness, and integration into existing medical imaging workflows. The successful implementation of luminol as a diagnostic tool could significantly enhance the capabilities of medical imaging, offering new insights into disease processes and potentially transforming patient care across various medical specialties.
Market Analysis for Luminol-Based Diagnostic Tools
The market for luminol-based diagnostic tools in medical imaging is experiencing significant growth, driven by the increasing demand for advanced diagnostic technologies and the rising prevalence of chronic diseases. Luminol, a chemiluminescent compound, has shown promising applications in various medical imaging techniques, particularly in the detection of blood and certain types of cancer cells.
The global medical imaging market, which encompasses luminol-based diagnostic tools, is projected to reach substantial value in the coming years. This growth is attributed to factors such as the aging population, technological advancements in imaging modalities, and the growing emphasis on early disease detection and personalized medicine.
Luminol-based diagnostic tools offer several advantages over traditional imaging methods, including high sensitivity, specificity, and the ability to detect minute traces of blood or specific cellular markers. These characteristics make them particularly valuable in oncology, forensic medicine, and cardiovascular diagnostics.
In the oncology sector, luminol-based tools are being explored for their potential in detecting and monitoring various types of cancer. The ability of luminol to react with certain enzymes overexpressed in cancer cells provides a unique opportunity for non-invasive cancer screening and treatment monitoring.
The forensic medicine field has also shown significant interest in luminol-based diagnostic tools. Law enforcement agencies and forensic laboratories are increasingly adopting these technologies for crime scene investigations, particularly in cases involving blood evidence detection.
Cardiovascular diagnostics represent another promising market segment for luminol-based tools. The compound's ability to detect trace amounts of blood can be utilized in identifying micro-bleeds or assessing the integrity of blood vessels, potentially revolutionizing the diagnosis and monitoring of cardiovascular conditions.
Geographically, North America and Europe currently dominate the market for luminol-based diagnostic tools, owing to their advanced healthcare infrastructure and higher adoption rates of innovative medical technologies. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in this market, driven by improving healthcare systems and increasing investments in medical research and development.
Despite the promising outlook, the market for luminol-based diagnostic tools faces certain challenges. These include regulatory hurdles, the need for extensive clinical validation, and competition from established imaging modalities. Additionally, concerns regarding the potential interference of luminol with other diagnostic tests and its stability under various environmental conditions need to be addressed to ensure widespread adoption.
The global medical imaging market, which encompasses luminol-based diagnostic tools, is projected to reach substantial value in the coming years. This growth is attributed to factors such as the aging population, technological advancements in imaging modalities, and the growing emphasis on early disease detection and personalized medicine.
Luminol-based diagnostic tools offer several advantages over traditional imaging methods, including high sensitivity, specificity, and the ability to detect minute traces of blood or specific cellular markers. These characteristics make them particularly valuable in oncology, forensic medicine, and cardiovascular diagnostics.
In the oncology sector, luminol-based tools are being explored for their potential in detecting and monitoring various types of cancer. The ability of luminol to react with certain enzymes overexpressed in cancer cells provides a unique opportunity for non-invasive cancer screening and treatment monitoring.
The forensic medicine field has also shown significant interest in luminol-based diagnostic tools. Law enforcement agencies and forensic laboratories are increasingly adopting these technologies for crime scene investigations, particularly in cases involving blood evidence detection.
Cardiovascular diagnostics represent another promising market segment for luminol-based tools. The compound's ability to detect trace amounts of blood can be utilized in identifying micro-bleeds or assessing the integrity of blood vessels, potentially revolutionizing the diagnosis and monitoring of cardiovascular conditions.
Geographically, North America and Europe currently dominate the market for luminol-based diagnostic tools, owing to their advanced healthcare infrastructure and higher adoption rates of innovative medical technologies. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in this market, driven by improving healthcare systems and increasing investments in medical research and development.
Despite the promising outlook, the market for luminol-based diagnostic tools faces certain challenges. These include regulatory hurdles, the need for extensive clinical validation, and competition from established imaging modalities. Additionally, concerns regarding the potential interference of luminol with other diagnostic tests and its stability under various environmental conditions need to be addressed to ensure widespread adoption.
Current Challenges in Luminol-Based Imaging Techniques
Despite the promising potential of luminol as a diagnostic tool in medical imaging, several significant challenges currently hinder its widespread adoption and effectiveness. One of the primary obstacles is the limited sensitivity of luminol-based techniques, particularly in detecting low concentrations of target molecules or cells. This sensitivity issue can lead to false negatives, potentially missing crucial diagnostic information in early-stage diseases or conditions with minimal biomarkers.
Another major challenge lies in the specificity of luminol-based imaging. While luminol is known for its chemiluminescent properties when reacting with certain substances, achieving high specificity for particular biomarkers or cellular components remains difficult. This lack of specificity can result in background noise and false positives, complicating the interpretation of imaging results and potentially leading to misdiagnosis.
The stability and longevity of the luminol signal pose additional challenges. The chemiluminescent reaction is typically short-lived, which can limit the time window for image acquisition and analysis. This temporal constraint may prove problematic in clinical settings where extended observation periods are necessary or when dealing with complex biological systems that require prolonged imaging sessions.
Furthermore, the penetration depth of luminol-based imaging techniques is currently limited, restricting their application to superficial tissues or ex vivo samples. This limitation significantly hampers the potential for non-invasive in vivo imaging of deep-seated tissues or organs, which is crucial for comprehensive diagnostic capabilities.
The standardization of luminol-based imaging protocols presents another hurdle. Variations in sample preparation, reagent concentrations, and imaging conditions can lead to inconsistent results across different laboratories or clinical settings. This lack of standardization impedes the reproducibility of findings and complicates the establishment of reliable diagnostic criteria.
Additionally, the integration of luminol-based imaging with existing medical imaging modalities remains a challenge. Developing hybrid systems that can combine the molecular specificity of luminol with the anatomical resolution of techniques like MRI or CT is an ongoing area of research, but technical and practical obstacles persist in achieving seamless integration.
Lastly, concerns regarding the potential toxicity or long-term effects of luminol and its byproducts in biological systems need to be thoroughly addressed. While luminol is generally considered safe for in vitro applications, its use in in vivo diagnostics requires extensive safety studies to ensure no adverse effects on patient health or the accuracy of diagnostic outcomes.
Another major challenge lies in the specificity of luminol-based imaging. While luminol is known for its chemiluminescent properties when reacting with certain substances, achieving high specificity for particular biomarkers or cellular components remains difficult. This lack of specificity can result in background noise and false positives, complicating the interpretation of imaging results and potentially leading to misdiagnosis.
The stability and longevity of the luminol signal pose additional challenges. The chemiluminescent reaction is typically short-lived, which can limit the time window for image acquisition and analysis. This temporal constraint may prove problematic in clinical settings where extended observation periods are necessary or when dealing with complex biological systems that require prolonged imaging sessions.
Furthermore, the penetration depth of luminol-based imaging techniques is currently limited, restricting their application to superficial tissues or ex vivo samples. This limitation significantly hampers the potential for non-invasive in vivo imaging of deep-seated tissues or organs, which is crucial for comprehensive diagnostic capabilities.
The standardization of luminol-based imaging protocols presents another hurdle. Variations in sample preparation, reagent concentrations, and imaging conditions can lead to inconsistent results across different laboratories or clinical settings. This lack of standardization impedes the reproducibility of findings and complicates the establishment of reliable diagnostic criteria.
Additionally, the integration of luminol-based imaging with existing medical imaging modalities remains a challenge. Developing hybrid systems that can combine the molecular specificity of luminol with the anatomical resolution of techniques like MRI or CT is an ongoing area of research, but technical and practical obstacles persist in achieving seamless integration.
Lastly, concerns regarding the potential toxicity or long-term effects of luminol and its byproducts in biological systems need to be thoroughly addressed. While luminol is generally considered safe for in vitro applications, its use in in vivo diagnostics requires extensive safety studies to ensure no adverse effects on patient health or the accuracy of diagnostic outcomes.
Existing Luminol Applications in Medical Imaging
01 Luminol-based detection systems
Luminol is widely used in diagnostic systems due to its chemiluminescent properties. When oxidized, luminol emits light, making it useful for detecting various substances in forensic, medical, and environmental applications. These systems can be optimized for sensitivity and specificity in different diagnostic contexts.- Luminol-based detection systems: Luminol is used in diagnostic systems for its chemiluminescent properties. When oxidized, luminol emits light, making it useful for detecting various substances in forensic and medical applications. These systems can be optimized for sensitivity and specificity in different diagnostic contexts.
- Imaging and visualization techniques: Luminol's light-emitting properties are utilized in imaging and visualization techniques. These methods can be applied in medical diagnostics, such as detecting blood traces or visualizing cellular processes. Advanced imaging systems may incorporate luminol-based reactions for enhanced sensitivity and resolution.
- Biosensors and analytical devices: Luminol is incorporated into biosensors and analytical devices for detecting specific biological or chemical compounds. These devices may use luminol's chemiluminescence in combination with other reagents or enzymes to achieve high sensitivity and selectivity in diagnostics.
- Luminol in forensic applications: Luminol's diagnostic capability is extensively used in forensic science, particularly for detecting trace amounts of blood at crime scenes. Forensic kits and methods have been developed to optimize luminol's performance in various environmental conditions and surfaces.
- Enhancement of luminol reactions: Research focuses on enhancing luminol's diagnostic capabilities through various methods. This includes developing new formulations, combining luminol with other compounds, or using catalysts to increase the intensity and duration of the chemiluminescent reaction, thereby improving its diagnostic sensitivity and reliability.
02 Luminol in forensic blood detection
Luminol is particularly valuable in forensic science for detecting trace amounts of blood at crime scenes. When it reacts with the iron in hemoglobin, it produces a blue glow, allowing investigators to identify blood stains that are not visible to the naked eye, even after cleaning attempts.Expand Specific Solutions03 Luminol in medical diagnostics
In medical applications, luminol-based assays are used for various diagnostic purposes, including detecting specific proteins, enzymes, or cellular activities. These tests can be used to diagnose diseases, monitor treatment efficacy, or study biological processes at the molecular level.Expand Specific Solutions04 Enhanced luminol formulations
Researchers have developed enhanced luminol formulations to improve its diagnostic capabilities. These may include modifications to the luminol molecule, addition of catalysts or enhancers, or incorporation into nanoparticles or other delivery systems to increase sensitivity, stability, or specificity of the diagnostic tests.Expand Specific Solutions05 Luminol in environmental monitoring
Luminol-based systems are also used in environmental monitoring to detect pollutants, heavy metals, or other contaminants in water, soil, or air samples. These applications leverage luminol's sensitivity to various oxidizing agents and can provide rapid, on-site testing capabilities for environmental assessment and management.Expand Specific Solutions
Key Players in Luminol Research and Development
The research on luminol as a diagnostic tool in medical imaging is in an early developmental stage, with the market still emerging. The global medical imaging market, valued at over $30 billion, provides a substantial backdrop for this niche technology. While luminol has been used in forensics for decades, its application in medical imaging is relatively new, indicating low technological maturity. Companies like Olympus Corp., Canon Inc., and Koninklijke Philips NV are leveraging their expertise in medical imaging to explore luminol's potential. Research institutions such as Washington University in St. Louis and Institut National de la Santé et de la Recherche Médicale are also contributing to the field, suggesting a collaborative approach between industry and academia to advance this technology.
Olympus Corp.
Technical Solution: Olympus Corp. has developed advanced luminol-based imaging systems for medical diagnostics. Their technology utilizes enhanced chemiluminescence reactions to produce high-sensitivity images for detecting various pathological conditions. The system incorporates specialized optical sensors and image processing algorithms to capture and analyze the light emitted from luminol reactions in biological samples[1]. This allows for real-time visualization of specific biomarkers or cellular processes, enabling early disease detection and monitoring of treatment efficacy[3]. Olympus has also integrated this technology into minimally invasive endoscopic devices, expanding its applications in internal organ imaging and guided surgical procedures[5].
Strengths: High sensitivity and specificity in detecting biological markers; integration with existing medical imaging platforms. Weaknesses: Potential interference from other light-emitting biological processes; limited penetration depth in thick tissues.
Canon, Inc.
Technical Solution: Canon has pioneered the development of luminol-enhanced medical imaging systems that combine high-resolution optical technologies with advanced chemiluminescence detection. Their approach utilizes proprietary luminol derivatives and catalysts to amplify the light signal, resulting in improved sensitivity and spatial resolution[2]. Canon's systems incorporate specialized CCD sensors optimized for low-light imaging, coupled with sophisticated image processing algorithms to reduce noise and enhance contrast[4]. This technology has been successfully applied in various medical fields, including cancer diagnostics, wound healing assessment, and cardiovascular imaging[6]. Canon has also developed portable versions of their luminol-based imaging devices, enabling point-of-care diagnostics in resource-limited settings.
Strengths: High-resolution imaging capabilities; versatile applications across multiple medical fields. Weaknesses: Relatively high cost of equipment; requires specialized training for operation and interpretation.
Innovative Luminol Formulations and Enhancements
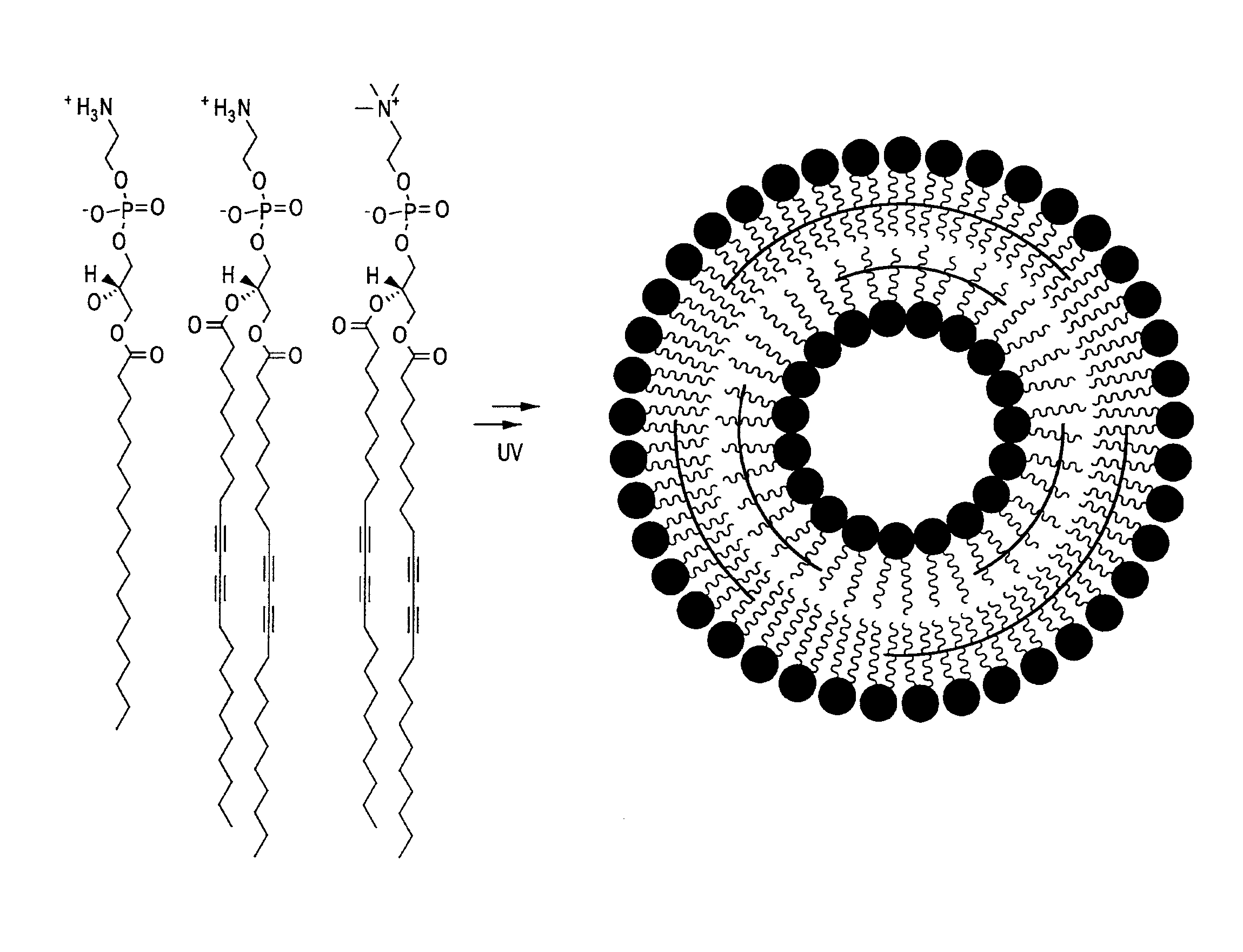
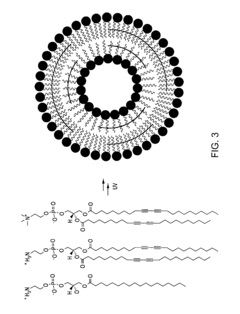
Thermally-activatable liposome compositions and methods for imaging, diagnosis and therapy
PatentInactiveUS20110190623A1
Innovation
- Development of thermally-activatable liposomes composed of a combination of polymerizable and unpolymerizable lipids, allowing for controlled release of encapsulated compounds at specific temperatures, maintaining stability at body temperature and becoming unstable at mildly to moderately elevated temperatures for targeted delivery.
Bioluminescence imaging of myeloperoxidase activity in vivo, methods, compositions and apparatuses therefor
PatentInactiveUS20110250145A1
Innovation
- The development of methods for non-invasive imaging of MPO activity using luminogenic-optical probes that emit light upon contact with oxidizing agents, allowing for the visualization of MPO activity in vivo, particularly through bioluminescence imaging (BLI) techniques.
Regulatory Framework for Chemiluminescent Diagnostics
The regulatory framework for chemiluminescent diagnostics, including luminol-based medical imaging tools, is a complex and evolving landscape. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development, approval, and marketing of such diagnostic technologies. The FDA classifies medical devices into three categories based on their risk level and intended use, with most chemiluminescent diagnostic tools falling under Class II or III, requiring more stringent regulatory oversight.
For luminol-based imaging tools, manufacturers must navigate the premarket notification (510(k)) process or the more rigorous premarket approval (PMA) pathway, depending on the device's classification. This involves submitting comprehensive data on the device's safety and efficacy, including clinical trial results, risk assessments, and quality control measures. The FDA also mandates compliance with Good Manufacturing Practices (GMP) to ensure consistent product quality and safety.
In the European Union, the regulatory landscape is governed by the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation introduces a new risk-based classification system for in vitro diagnostic medical devices, including chemiluminescent diagnostics. Manufacturers must obtain CE marking to market their products in the EU, demonstrating compliance with essential requirements for safety and performance.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory standards across different countries. This effort aims to streamline the approval process for innovative diagnostic technologies while maintaining high safety standards. However, significant variations in regulatory requirements still exist between different regions, posing challenges for manufacturers seeking global market access.
Specific to luminol-based imaging tools, regulatory bodies focus on several key aspects: the chemical safety of luminol and its reagents, the accuracy and reliability of the imaging results, and the potential for false positives or negatives. Manufacturers must provide extensive documentation on the chemical properties of luminol, its interaction with biological tissues, and any potential toxicity concerns.
Additionally, regulatory frameworks increasingly emphasize post-market surveillance and vigilance. Manufacturers of luminol-based diagnostic tools are required to implement robust systems for monitoring device performance, reporting adverse events, and conducting necessary recalls or corrective actions. This ongoing commitment to safety and efficacy extends throughout the product lifecycle, ensuring continued regulatory compliance and patient protection.
As the field of chemiluminescent diagnostics advances, regulatory bodies are adapting their frameworks to keep pace with technological innovations. This includes developing new guidance documents, refining classification criteria, and exploring novel approaches to evaluating the safety and efficacy of emerging diagnostic technologies. The evolving regulatory landscape presents both challenges and opportunities for researchers and manufacturers working on luminol-based medical imaging tools, necessitating a proactive approach to regulatory compliance and engagement with relevant authorities.
For luminol-based imaging tools, manufacturers must navigate the premarket notification (510(k)) process or the more rigorous premarket approval (PMA) pathway, depending on the device's classification. This involves submitting comprehensive data on the device's safety and efficacy, including clinical trial results, risk assessments, and quality control measures. The FDA also mandates compliance with Good Manufacturing Practices (GMP) to ensure consistent product quality and safety.
In the European Union, the regulatory landscape is governed by the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation introduces a new risk-based classification system for in vitro diagnostic medical devices, including chemiluminescent diagnostics. Manufacturers must obtain CE marking to market their products in the EU, demonstrating compliance with essential requirements for safety and performance.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory standards across different countries. This effort aims to streamline the approval process for innovative diagnostic technologies while maintaining high safety standards. However, significant variations in regulatory requirements still exist between different regions, posing challenges for manufacturers seeking global market access.
Specific to luminol-based imaging tools, regulatory bodies focus on several key aspects: the chemical safety of luminol and its reagents, the accuracy and reliability of the imaging results, and the potential for false positives or negatives. Manufacturers must provide extensive documentation on the chemical properties of luminol, its interaction with biological tissues, and any potential toxicity concerns.
Additionally, regulatory frameworks increasingly emphasize post-market surveillance and vigilance. Manufacturers of luminol-based diagnostic tools are required to implement robust systems for monitoring device performance, reporting adverse events, and conducting necessary recalls or corrective actions. This ongoing commitment to safety and efficacy extends throughout the product lifecycle, ensuring continued regulatory compliance and patient protection.
As the field of chemiluminescent diagnostics advances, regulatory bodies are adapting their frameworks to keep pace with technological innovations. This includes developing new guidance documents, refining classification criteria, and exploring novel approaches to evaluating the safety and efficacy of emerging diagnostic technologies. The evolving regulatory landscape presents both challenges and opportunities for researchers and manufacturers working on luminol-based medical imaging tools, necessitating a proactive approach to regulatory compliance and engagement with relevant authorities.
Biosafety and Toxicology Considerations for Luminol Use
The use of luminol as a diagnostic tool in medical imaging presents significant biosafety and toxicology considerations that must be carefully evaluated. Luminol, while widely used in forensic science for blood detection, has potential applications in medical imaging due to its chemiluminescent properties. However, its introduction into the human body for diagnostic purposes requires thorough assessment of its safety profile.
One primary concern is the potential toxicity of luminol and its metabolites. Studies have shown that luminol can be metabolized in the body, producing various compounds that may have different toxicological profiles. The long-term effects of these metabolites on human health are not yet fully understood, necessitating comprehensive toxicological studies to determine safe dosage levels and exposure limits.
Another critical aspect is the potential for allergic reactions or hypersensitivity to luminol. As with any foreign substance introduced into the body, there is a risk of immune system responses. Researchers must conduct extensive allergenicity tests to identify potential risks and develop protocols for patient screening prior to luminol-based diagnostic procedures.
The interaction of luminol with other medications or existing medical conditions is another area of concern. Patients undergoing various treatments or with complex medical histories may experience unexpected reactions when exposed to luminol. Thorough drug interaction studies and patient risk assessments are essential to ensure the safe application of luminol-based diagnostic techniques.
Environmental impact is also a consideration in the use of luminol for medical imaging. The disposal of luminol and its byproducts must be carefully managed to prevent contamination of water systems or soil. Developing eco-friendly disposal methods and understanding the environmental fate of luminol compounds are crucial aspects of its implementation in medical settings.
Regulatory compliance is a significant challenge in the adoption of luminol for medical imaging. Stringent safety standards set by regulatory bodies such as the FDA and EMA must be met. This involves extensive clinical trials, toxicology reports, and long-term safety studies to demonstrate the efficacy and safety of luminol-based diagnostic methods.
To address these concerns, researchers are exploring various strategies. These include the development of targeted delivery systems to minimize systemic exposure, the use of modified luminol compounds with improved safety profiles, and the implementation of advanced imaging techniques that require lower doses of luminol. Additionally, the establishment of standardized protocols for luminol use in medical imaging is crucial to ensure consistent safety practices across different healthcare settings.
One primary concern is the potential toxicity of luminol and its metabolites. Studies have shown that luminol can be metabolized in the body, producing various compounds that may have different toxicological profiles. The long-term effects of these metabolites on human health are not yet fully understood, necessitating comprehensive toxicological studies to determine safe dosage levels and exposure limits.
Another critical aspect is the potential for allergic reactions or hypersensitivity to luminol. As with any foreign substance introduced into the body, there is a risk of immune system responses. Researchers must conduct extensive allergenicity tests to identify potential risks and develop protocols for patient screening prior to luminol-based diagnostic procedures.
The interaction of luminol with other medications or existing medical conditions is another area of concern. Patients undergoing various treatments or with complex medical histories may experience unexpected reactions when exposed to luminol. Thorough drug interaction studies and patient risk assessments are essential to ensure the safe application of luminol-based diagnostic techniques.
Environmental impact is also a consideration in the use of luminol for medical imaging. The disposal of luminol and its byproducts must be carefully managed to prevent contamination of water systems or soil. Developing eco-friendly disposal methods and understanding the environmental fate of luminol compounds are crucial aspects of its implementation in medical settings.
Regulatory compliance is a significant challenge in the adoption of luminol for medical imaging. Stringent safety standards set by regulatory bodies such as the FDA and EMA must be met. This involves extensive clinical trials, toxicology reports, and long-term safety studies to demonstrate the efficacy and safety of luminol-based diagnostic methods.
To address these concerns, researchers are exploring various strategies. These include the development of targeted delivery systems to minimize systemic exposure, the use of modified luminol compounds with improved safety profiles, and the implementation of advanced imaging techniques that require lower doses of luminol. Additionally, the establishment of standardized protocols for luminol use in medical imaging is crucial to ensure consistent safety practices across different healthcare settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!