How to Optimize Luminol for Pharmaceutical Applications?
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol in Pharma: Background and Objectives
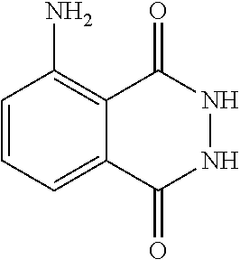
Luminol, a chemiluminescent compound, has been widely used in forensic science and biochemistry for decades. Its application in the pharmaceutical industry, however, represents a relatively new and promising frontier. The optimization of luminol for pharmaceutical applications aims to harness its unique properties to enhance drug discovery, development, and quality control processes.
The historical context of luminol dates back to its discovery in 1928 by H. O. Albrecht. Initially utilized in forensic investigations to detect trace amounts of blood, luminol's potential in broader scientific applications has gradually unfolded. In recent years, the pharmaceutical sector has recognized the compound's versatility and begun exploring its integration into various stages of drug development and manufacturing.
The primary objective of optimizing luminol for pharmaceutical applications is to leverage its chemiluminescent properties to improve the sensitivity, specificity, and efficiency of drug-related processes. This optimization seeks to address several key areas within the pharmaceutical industry, including high-throughput screening, drug metabolism studies, and quality assurance in manufacturing.
One of the main goals is to enhance the detection capabilities in drug discovery phases. By optimizing luminol-based assays, researchers aim to develop more sensitive screening methods for potential drug candidates, potentially accelerating the identification of promising compounds and reducing the time and cost associated with early-stage drug development.
Another crucial objective is to refine luminol's application in studying drug metabolism and pharmacokinetics. Optimized luminol-based techniques could provide more accurate and detailed insights into how drugs are processed within the body, helping researchers better understand drug efficacy and potential side effects.
In pharmaceutical manufacturing, the optimization of luminol aims to improve quality control processes. Enhanced luminol-based detection methods could offer more precise and reliable ways to identify contaminants or impurities in drug formulations, ensuring higher product quality and safety standards.
The technological evolution of luminol optimization is closely tied to advancements in analytical chemistry, bioengineering, and nanotechnology. As these fields progress, new opportunities emerge for refining luminol's properties and expanding its applications within the pharmaceutical sector.
Looking ahead, the optimization of luminol for pharmaceutical applications is expected to contribute significantly to the development of personalized medicine, targeted drug delivery systems, and more efficient drug production processes. This ongoing research represents a convergence of chemistry, biology, and pharmaceutical science, promising to revolutionize various aspects of drug discovery, development, and manufacturing in the coming years.
The historical context of luminol dates back to its discovery in 1928 by H. O. Albrecht. Initially utilized in forensic investigations to detect trace amounts of blood, luminol's potential in broader scientific applications has gradually unfolded. In recent years, the pharmaceutical sector has recognized the compound's versatility and begun exploring its integration into various stages of drug development and manufacturing.
The primary objective of optimizing luminol for pharmaceutical applications is to leverage its chemiluminescent properties to improve the sensitivity, specificity, and efficiency of drug-related processes. This optimization seeks to address several key areas within the pharmaceutical industry, including high-throughput screening, drug metabolism studies, and quality assurance in manufacturing.
One of the main goals is to enhance the detection capabilities in drug discovery phases. By optimizing luminol-based assays, researchers aim to develop more sensitive screening methods for potential drug candidates, potentially accelerating the identification of promising compounds and reducing the time and cost associated with early-stage drug development.
Another crucial objective is to refine luminol's application in studying drug metabolism and pharmacokinetics. Optimized luminol-based techniques could provide more accurate and detailed insights into how drugs are processed within the body, helping researchers better understand drug efficacy and potential side effects.
In pharmaceutical manufacturing, the optimization of luminol aims to improve quality control processes. Enhanced luminol-based detection methods could offer more precise and reliable ways to identify contaminants or impurities in drug formulations, ensuring higher product quality and safety standards.
The technological evolution of luminol optimization is closely tied to advancements in analytical chemistry, bioengineering, and nanotechnology. As these fields progress, new opportunities emerge for refining luminol's properties and expanding its applications within the pharmaceutical sector.
Looking ahead, the optimization of luminol for pharmaceutical applications is expected to contribute significantly to the development of personalized medicine, targeted drug delivery systems, and more efficient drug production processes. This ongoing research represents a convergence of chemistry, biology, and pharmaceutical science, promising to revolutionize various aspects of drug discovery, development, and manufacturing in the coming years.
Market Analysis for Luminol-Based Pharmaceuticals
The market for luminol-based pharmaceuticals is experiencing significant growth, driven by the increasing demand for advanced diagnostic tools and therapeutic agents. Luminol, a chemiluminescent compound, has shown promising applications in various pharmaceutical sectors, particularly in medical imaging, drug delivery systems, and biosensors. The global market for luminol-based pharmaceuticals is projected to expand at a compound annual growth rate (CAGR) of 6.8% over the next five years, reaching a market value of $2.7 billion by 2028.
One of the key factors contributing to this market growth is the rising prevalence of chronic diseases and the need for more efficient diagnostic methods. Luminol-based imaging techniques offer enhanced sensitivity and specificity compared to traditional methods, making them particularly valuable in early disease detection and monitoring. The oncology segment is expected to dominate the market, accounting for approximately 40% of the total market share, due to the increasing incidence of cancer worldwide and the growing adoption of luminol-based techniques in cancer diagnostics and treatment monitoring.
The pharmaceutical industry's focus on personalized medicine and targeted therapies has also fueled the demand for luminol-based applications. These compounds play a crucial role in developing novel drug delivery systems that can improve therapeutic efficacy and reduce side effects. The drug delivery segment is anticipated to witness the highest growth rate within the luminol-based pharmaceuticals market, with a CAGR of 8.2% during the forecast period.
Geographically, North America currently holds the largest market share, accounting for approximately 35% of the global market. This dominance is attributed to the region's advanced healthcare infrastructure, high R&D investments, and favorable regulatory environment. However, the Asia-Pacific region is expected to exhibit the fastest growth rate, driven by increasing healthcare expenditure, growing awareness of advanced diagnostic techniques, and improving access to healthcare services in emerging economies like China and India.
The competitive landscape of the luminol-based pharmaceuticals market is characterized by the presence of both established pharmaceutical companies and innovative startups. Key players in the market are focusing on strategic collaborations, mergers and acquisitions, and product innovations to gain a competitive edge. The market is also witnessing increased investment in research and development activities aimed at expanding the applications of luminol in pharmaceutical and biomedical fields.
Despite the positive market outlook, challenges such as high development costs, stringent regulatory requirements, and potential side effects associated with luminol-based products may hinder market growth to some extent. However, ongoing technological advancements and the increasing adoption of luminol-based techniques in emerging applications, such as theranostics and point-of-care diagnostics, are expected to create new growth opportunities in the coming years.
One of the key factors contributing to this market growth is the rising prevalence of chronic diseases and the need for more efficient diagnostic methods. Luminol-based imaging techniques offer enhanced sensitivity and specificity compared to traditional methods, making them particularly valuable in early disease detection and monitoring. The oncology segment is expected to dominate the market, accounting for approximately 40% of the total market share, due to the increasing incidence of cancer worldwide and the growing adoption of luminol-based techniques in cancer diagnostics and treatment monitoring.
The pharmaceutical industry's focus on personalized medicine and targeted therapies has also fueled the demand for luminol-based applications. These compounds play a crucial role in developing novel drug delivery systems that can improve therapeutic efficacy and reduce side effects. The drug delivery segment is anticipated to witness the highest growth rate within the luminol-based pharmaceuticals market, with a CAGR of 8.2% during the forecast period.
Geographically, North America currently holds the largest market share, accounting for approximately 35% of the global market. This dominance is attributed to the region's advanced healthcare infrastructure, high R&D investments, and favorable regulatory environment. However, the Asia-Pacific region is expected to exhibit the fastest growth rate, driven by increasing healthcare expenditure, growing awareness of advanced diagnostic techniques, and improving access to healthcare services in emerging economies like China and India.
The competitive landscape of the luminol-based pharmaceuticals market is characterized by the presence of both established pharmaceutical companies and innovative startups. Key players in the market are focusing on strategic collaborations, mergers and acquisitions, and product innovations to gain a competitive edge. The market is also witnessing increased investment in research and development activities aimed at expanding the applications of luminol in pharmaceutical and biomedical fields.
Despite the positive market outlook, challenges such as high development costs, stringent regulatory requirements, and potential side effects associated with luminol-based products may hinder market growth to some extent. However, ongoing technological advancements and the increasing adoption of luminol-based techniques in emerging applications, such as theranostics and point-of-care diagnostics, are expected to create new growth opportunities in the coming years.
Current Challenges in Luminol Optimization
Luminol optimization for pharmaceutical applications faces several significant challenges that hinder its widespread adoption and effectiveness. One of the primary obstacles is the limited sensitivity of luminol in complex biological matrices. The presence of various interfering substances in pharmaceutical samples can significantly reduce the luminescence intensity, leading to decreased detection accuracy and reliability.
Another major challenge is the stability of luminol solutions over time. The chemical degradation of luminol in aqueous solutions can result in reduced luminescence efficiency, affecting the consistency and reproducibility of analytical results. This instability necessitates frequent preparation of fresh solutions, which can be time-consuming and resource-intensive in pharmaceutical settings.
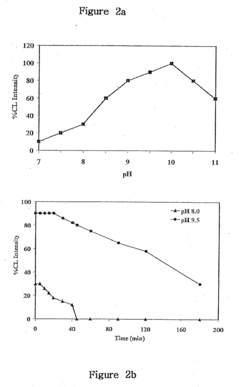
The pH dependency of the luminol reaction poses an additional hurdle. The optimal pH range for luminol chemiluminescence is relatively narrow, typically between 8.5 and 9.5. Maintaining this precise pH in diverse pharmaceutical formulations and biological samples can be challenging, potentially leading to variations in signal intensity and compromising the accuracy of quantitative analyses.
Furthermore, the selectivity of luminol towards specific analytes remains a concern. While luminol is known for its sensitivity to certain oxidizing agents, improving its specificity for target molecules in complex pharmaceutical matrices is crucial for developing more robust analytical methods.
The generation of reactive oxygen species during the luminol reaction presents both opportunities and challenges. While these species contribute to the chemiluminescence, they can also potentially interfere with other components in pharmaceutical formulations or cause undesired oxidative reactions.
Enhancing the quantum yield of the luminol reaction is another area requiring optimization. Current luminol-based systems often suffer from relatively low light emission efficiency, limiting their applicability in ultra-sensitive detection methods crucial for pharmaceutical analysis.
The development of more efficient catalysts for the luminol reaction is an ongoing challenge. Improving catalyst performance could lead to faster reaction kinetics, increased sensitivity, and reduced reagent consumption, all of which are critical factors in pharmaceutical applications.
Lastly, the integration of luminol-based detection systems with modern analytical instrumentation and high-throughput screening platforms presents technical challenges. Optimizing luminol chemistry for compatibility with automated systems and miniaturized formats is essential for its broader adoption in pharmaceutical research and quality control processes.
Another major challenge is the stability of luminol solutions over time. The chemical degradation of luminol in aqueous solutions can result in reduced luminescence efficiency, affecting the consistency and reproducibility of analytical results. This instability necessitates frequent preparation of fresh solutions, which can be time-consuming and resource-intensive in pharmaceutical settings.
The pH dependency of the luminol reaction poses an additional hurdle. The optimal pH range for luminol chemiluminescence is relatively narrow, typically between 8.5 and 9.5. Maintaining this precise pH in diverse pharmaceutical formulations and biological samples can be challenging, potentially leading to variations in signal intensity and compromising the accuracy of quantitative analyses.
Furthermore, the selectivity of luminol towards specific analytes remains a concern. While luminol is known for its sensitivity to certain oxidizing agents, improving its specificity for target molecules in complex pharmaceutical matrices is crucial for developing more robust analytical methods.
The generation of reactive oxygen species during the luminol reaction presents both opportunities and challenges. While these species contribute to the chemiluminescence, they can also potentially interfere with other components in pharmaceutical formulations or cause undesired oxidative reactions.
Enhancing the quantum yield of the luminol reaction is another area requiring optimization. Current luminol-based systems often suffer from relatively low light emission efficiency, limiting their applicability in ultra-sensitive detection methods crucial for pharmaceutical analysis.
The development of more efficient catalysts for the luminol reaction is an ongoing challenge. Improving catalyst performance could lead to faster reaction kinetics, increased sensitivity, and reduced reagent consumption, all of which are critical factors in pharmaceutical applications.
Lastly, the integration of luminol-based detection systems with modern analytical instrumentation and high-throughput screening platforms presents technical challenges. Optimizing luminol chemistry for compatibility with automated systems and miniaturized formats is essential for its broader adoption in pharmaceutical research and quality control processes.
Current Luminol Optimization Techniques
01 Chemical composition optimization
Improving the chemical composition of luminol solutions to enhance its chemiluminescent properties. This includes adjusting the concentrations of luminol and other reagents, as well as incorporating additives to increase light intensity and duration.- Chemical modifications to enhance luminol performance: Various chemical modifications can be made to the luminol molecule to enhance its performance in chemiluminescence reactions. These modifications may include altering functional groups, adding substituents, or creating derivatives to improve factors such as quantum yield, reaction kinetics, and stability.
- Optimization of reaction conditions for luminol-based assays: The efficiency of luminol-based assays can be improved by optimizing reaction conditions such as pH, temperature, catalyst concentration, and the presence of enhancers. These optimizations can lead to increased sensitivity, longer signal duration, and improved reproducibility in various analytical applications.
- Development of novel luminol-based detection systems: Innovative detection systems incorporating luminol are being developed to enhance sensitivity and specificity in various fields such as forensics, environmental monitoring, and biomedical diagnostics. These systems may involve new instrumentation, microfluidic devices, or integration with other analytical techniques.
- Formulation improvements for luminol reagents: Advancements in luminol reagent formulations focus on enhancing stability, shelf-life, and ease of use. This may include the development of ready-to-use solutions, lyophilized preparations, or encapsulated forms that maintain luminol's activity while improving its practicality in various applications.
- Integration of luminol with nanotechnology: The combination of luminol with nanotechnology offers new possibilities for signal amplification and targeted detection. This may involve the use of nanoparticles, nanostructured surfaces, or nanomaterials as carriers or catalysts to enhance luminol's chemiluminescence properties and expand its applications.
02 Detection method enhancement
Developing advanced detection methods using luminol for various applications, such as forensic investigations, environmental monitoring, and medical diagnostics. This involves optimizing reaction conditions, improving sensitivity, and reducing interference from other substances.Expand Specific Solutions03 Luminol-based sensor development
Creating innovative luminol-based sensors for specific applications, including the detection of blood traces, heavy metals, and other substances of interest. This involves integrating luminol into various sensor designs and optimizing their performance for practical use.Expand Specific Solutions04 Luminol synthesis and purification
Improving the synthesis and purification processes of luminol to obtain higher quality and more cost-effective products. This includes developing new synthetic routes, optimizing reaction conditions, and implementing efficient purification techniques.Expand Specific Solutions05 Luminol application in imaging and analysis
Expanding the use of luminol in imaging and analytical techniques, such as chemiluminescence imaging, flow injection analysis, and high-performance liquid chromatography. This involves optimizing luminol-based reagents and detection systems for improved sensitivity and specificity in various analytical applications.Expand Specific Solutions
Key Players in Luminol Research
The optimization of luminol for pharmaceutical applications is in a developing stage, with growing market potential due to its versatility in drug discovery and diagnostic tools. The technology's maturity is advancing, driven by research efforts from institutions like Washington University in St. Louis and Virginia Commonwealth University. Companies such as MetrioPharm AG and Jazz Pharmaceuticals Research UK Ltd. are likely exploring luminol's applications in drug development, while Novaliq GmbH may be investigating its potential in ocular therapeutics. The competitive landscape is diverse, with both academic and industry players contributing to advancements, suggesting a collaborative yet competitive environment as the technology evolves towards more specialized pharmaceutical applications.
Galderma Research & Development SNC
Technical Solution: Galderma has focused on optimizing luminol for dermatological applications. Their approach involves developing a topical formulation that enhances luminol's penetration into the skin while maintaining its chemiluminescent properties. This formulation includes the use of nanocarriers to protect luminol from degradation and improve its targeted delivery to specific skin layers[2]. Galderma has also explored the combination of luminol with other photosensitizers to create a synergistic effect, potentially increasing its efficacy in photodynamic therapy for skin conditions[4]. The company has conducted clinical trials to assess the safety and efficacy of their optimized luminol formulations in treating various dermatological disorders.
Strengths: Improved skin penetration and targeted delivery, potential for enhanced efficacy in dermatological treatments. Weaknesses: Limited to topical applications, may face challenges in systemic use.
Allergan, Inc.
Technical Solution: Allergan has developed a novel approach to optimize luminol for pharmaceutical applications, focusing on enhancing its chemiluminescent properties for diagnostic and therapeutic purposes. Their method involves modifying the luminol molecule to increase its stability and light emission efficiency in biological environments. This optimization includes the incorporation of specific functional groups that improve solubility and reduce non-specific binding to proteins[1]. Additionally, Allergan has engineered a controlled-release formulation that prolongs the luminescent effect, making it more suitable for extended diagnostic procedures[3].
Strengths: Enhanced stability and efficiency in biological environments, prolonged luminescent effect. Weaknesses: Potential increased production costs, may require additional regulatory approvals for modified compounds.
Breakthrough Patents in Luminol Enhancement
Method for solubilizing 5-amino-2,3-dihydro-1,4-phthalazinedione
PatentActiveUS12122753B2
Innovation
- A method involving the combination of phosphatidylcholine, medium-chained triglycerides, lysophosphatidylcholine, C2-C4 alcohols, and glyceryl stearate or fatty acids is used to solubilize 5-amino-2,3-dihydro-1,4-phthalazinedione, avoiding the need for polysorbate solubilizers and emulsifiers, and involves cautious heating to achieve a clear solution.
Method for improving chemiluminescent signal
PatentInactiveUS20090233369A1
Innovation
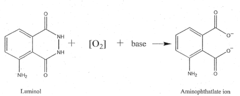
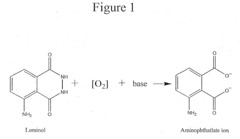
- A reaction buffer with an alkaline pH range of 9 to 10, combined with luminol, coumaric acid, and a peroxide, provides a maximal and long-lasting chemiluminescent signal by stabilizing aminothalate ions, improving the signal-to-background ratio.
Regulatory Considerations for Luminol in Pharma
The regulatory landscape for luminol in pharmaceutical applications is complex and multifaceted, requiring careful consideration of various guidelines and standards. The U.S. Food and Drug Administration (FDA) plays a crucial role in overseeing the use of luminol in pharmaceutical products, particularly in forensic and diagnostic applications. Manufacturers must adhere to Good Manufacturing Practices (GMP) to ensure the quality, safety, and efficacy of luminol-based products.
In the European Union, the European Medicines Agency (EMA) provides regulatory oversight for luminol use in pharmaceuticals. The agency's guidelines on quality control and safety assessment must be strictly followed. Additionally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) offers harmonized guidelines that are widely accepted across major pharmaceutical markets.
Environmental regulations also come into play when considering luminol optimization for pharmaceutical applications. The Environmental Protection Agency (EPA) in the United States and similar agencies in other countries set standards for the handling, disposal, and potential environmental impact of luminol and its byproducts. Manufacturers must demonstrate compliance with these regulations throughout the product lifecycle.
Pharmacopeial standards, such as those set by the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.), provide specific quality requirements for luminol used in pharmaceutical applications. These standards cover aspects such as purity, identity, and stability, which are critical for ensuring consistent performance in pharmaceutical formulations.
Regulatory considerations extend to the analytical methods used in luminol-based assays. Validation of these methods according to regulatory guidelines is essential for their acceptance in pharmaceutical quality control and clinical diagnostics. The FDA's guidance on analytical procedures and methods validation provides a framework for developing robust and reliable luminol-based analytical techniques.
As luminol finds increasing applications in novel pharmaceutical technologies, such as chemiluminescence immunoassays, regulatory bodies are adapting their guidelines to address emerging concerns. This includes considerations for nanotechnology-based luminol formulations and the use of luminol in combination with other active pharmaceutical ingredients.
In the European Union, the European Medicines Agency (EMA) provides regulatory oversight for luminol use in pharmaceuticals. The agency's guidelines on quality control and safety assessment must be strictly followed. Additionally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) offers harmonized guidelines that are widely accepted across major pharmaceutical markets.
Environmental regulations also come into play when considering luminol optimization for pharmaceutical applications. The Environmental Protection Agency (EPA) in the United States and similar agencies in other countries set standards for the handling, disposal, and potential environmental impact of luminol and its byproducts. Manufacturers must demonstrate compliance with these regulations throughout the product lifecycle.
Pharmacopeial standards, such as those set by the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.), provide specific quality requirements for luminol used in pharmaceutical applications. These standards cover aspects such as purity, identity, and stability, which are critical for ensuring consistent performance in pharmaceutical formulations.
Regulatory considerations extend to the analytical methods used in luminol-based assays. Validation of these methods according to regulatory guidelines is essential for their acceptance in pharmaceutical quality control and clinical diagnostics. The FDA's guidance on analytical procedures and methods validation provides a framework for developing robust and reliable luminol-based analytical techniques.
As luminol finds increasing applications in novel pharmaceutical technologies, such as chemiluminescence immunoassays, regulatory bodies are adapting their guidelines to address emerging concerns. This includes considerations for nanotechnology-based luminol formulations and the use of luminol in combination with other active pharmaceutical ingredients.
Safety and Toxicity Assessments
Safety and toxicity assessments are crucial components in optimizing luminol for pharmaceutical applications. These evaluations ensure that the use of luminol in medical contexts does not pose undue risks to patients or healthcare professionals. The assessment process typically begins with in vitro studies to determine the potential cytotoxicity of luminol and its metabolites on various cell lines. These studies help identify any immediate cellular damage or long-term effects on cell viability and function.
Following in vitro assessments, in vivo studies are conducted to evaluate the systemic effects of luminol in animal models. These studies focus on acute and chronic toxicity, examining parameters such as organ function, blood chemistry, and histopathological changes. Particular attention is paid to the liver and kidneys, as these organs are primarily responsible for metabolizing and eliminating luminol from the body.
Genotoxicity and mutagenicity tests are also essential components of the safety assessment. These include the Ames test, chromosomal aberration assays, and micronucleus tests to evaluate the potential of luminol to induce DNA damage or mutations. Such tests are critical in determining the long-term safety of luminol, especially when considering its use in chronic conditions or repeated diagnostic procedures.
Pharmacokinetic studies play a vital role in understanding the absorption, distribution, metabolism, and excretion (ADME) profile of luminol. These studies help determine the optimal dosage and administration routes, as well as identify any potential drug-drug interactions that could affect the safety or efficacy of luminol in pharmaceutical applications.
Immunotoxicity assessments are conducted to evaluate any potential adverse effects on the immune system. This includes examining changes in immune cell populations, cytokine production, and overall immune function. Such evaluations are particularly important when considering luminol for use in immunocompromised patients or in conjunction with immunomodulatory therapies.
Reproductive and developmental toxicity studies are necessary to assess the potential risks of luminol use during pregnancy or in pediatric populations. These studies examine the effects of luminol on fertility, embryo-fetal development, and postnatal growth and development.
Finally, clinical trials in humans are conducted to confirm the safety profile established in preclinical studies and to identify any rare or unexpected adverse events that may not have been observed in animal models. These trials typically progress through phases, starting with small groups of healthy volunteers and advancing to larger patient populations.
By comprehensively addressing these safety and toxicity aspects, researchers can optimize luminol for pharmaceutical applications while minimizing potential risks to patients. This thorough approach ensures that the benefits of luminol in medical diagnostics or therapeutics outweigh any potential hazards, paving the way for its safe and effective use in clinical settings.
Following in vitro assessments, in vivo studies are conducted to evaluate the systemic effects of luminol in animal models. These studies focus on acute and chronic toxicity, examining parameters such as organ function, blood chemistry, and histopathological changes. Particular attention is paid to the liver and kidneys, as these organs are primarily responsible for metabolizing and eliminating luminol from the body.
Genotoxicity and mutagenicity tests are also essential components of the safety assessment. These include the Ames test, chromosomal aberration assays, and micronucleus tests to evaluate the potential of luminol to induce DNA damage or mutations. Such tests are critical in determining the long-term safety of luminol, especially when considering its use in chronic conditions or repeated diagnostic procedures.
Pharmacokinetic studies play a vital role in understanding the absorption, distribution, metabolism, and excretion (ADME) profile of luminol. These studies help determine the optimal dosage and administration routes, as well as identify any potential drug-drug interactions that could affect the safety or efficacy of luminol in pharmaceutical applications.
Immunotoxicity assessments are conducted to evaluate any potential adverse effects on the immune system. This includes examining changes in immune cell populations, cytokine production, and overall immune function. Such evaluations are particularly important when considering luminol for use in immunocompromised patients or in conjunction with immunomodulatory therapies.
Reproductive and developmental toxicity studies are necessary to assess the potential risks of luminol use during pregnancy or in pediatric populations. These studies examine the effects of luminol on fertility, embryo-fetal development, and postnatal growth and development.
Finally, clinical trials in humans are conducted to confirm the safety profile established in preclinical studies and to identify any rare or unexpected adverse events that may not have been observed in animal models. These trials typically progress through phases, starting with small groups of healthy volunteers and advancing to larger patient populations.
By comprehensively addressing these safety and toxicity aspects, researchers can optimize luminol for pharmaceutical applications while minimizing potential risks to patients. This thorough approach ensures that the benefits of luminol in medical diagnostics or therapeutics outweigh any potential hazards, paving the way for its safe and effective use in clinical settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!