Luminol: Innovation in Light-Based Diagnostic Platforms
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Technology Overview and Objectives
Luminol, a chemiluminescent compound, has emerged as a pivotal element in the development of innovative light-based diagnostic platforms. This technology harnesses the unique properties of luminol to create highly sensitive and specific detection systems for a wide range of applications, particularly in medical diagnostics, forensic science, and environmental monitoring.
The evolution of luminol-based technologies can be traced back to its discovery in the early 20th century. Initially utilized in forensic investigations for blood detection, luminol's potential in broader diagnostic applications has been increasingly recognized over the past few decades. The compound's ability to emit light when oxidized, especially in the presence of certain catalysts, forms the foundation of its diagnostic capabilities.
Recent advancements in luminol technology have focused on enhancing its sensitivity, specificity, and versatility. Researchers have made significant strides in optimizing the chemical composition and reaction conditions to improve light emission intensity and duration. These improvements have led to the development of more robust and reliable diagnostic platforms capable of detecting minute quantities of target substances.
The primary objective of current luminol-based diagnostic research is to create highly sensitive, rapid, and cost-effective detection systems. These platforms aim to provide real-time results in various settings, from clinical laboratories to point-of-care facilities and field environments. The ultimate goal is to develop portable, user-friendly devices that can deliver accurate diagnostic results with minimal sample preparation and processing time.
Another key focus area is the integration of luminol technology with other cutting-edge fields such as nanotechnology and microfluidics. This convergence has opened up new possibilities for miniaturization and automation of diagnostic processes, potentially revolutionizing on-site testing capabilities across multiple industries.
The ongoing research in luminol-based diagnostics also aims to expand its application spectrum. While traditionally associated with blood detection and certain medical tests, efforts are underway to adapt this technology for detecting a broader range of biological markers, environmental pollutants, and chemical compounds. This expansion could significantly impact fields such as personalized medicine, environmental monitoring, and food safety.
As the technology continues to evolve, researchers are exploring ways to overcome existing limitations, such as potential interference from other substances and the need for specialized equipment for result interpretation. The development of more selective luminol derivatives and advanced detection systems is at the forefront of these efforts, promising to further enhance the reliability and applicability of luminol-based diagnostic platforms.
The evolution of luminol-based technologies can be traced back to its discovery in the early 20th century. Initially utilized in forensic investigations for blood detection, luminol's potential in broader diagnostic applications has been increasingly recognized over the past few decades. The compound's ability to emit light when oxidized, especially in the presence of certain catalysts, forms the foundation of its diagnostic capabilities.
Recent advancements in luminol technology have focused on enhancing its sensitivity, specificity, and versatility. Researchers have made significant strides in optimizing the chemical composition and reaction conditions to improve light emission intensity and duration. These improvements have led to the development of more robust and reliable diagnostic platforms capable of detecting minute quantities of target substances.
The primary objective of current luminol-based diagnostic research is to create highly sensitive, rapid, and cost-effective detection systems. These platforms aim to provide real-time results in various settings, from clinical laboratories to point-of-care facilities and field environments. The ultimate goal is to develop portable, user-friendly devices that can deliver accurate diagnostic results with minimal sample preparation and processing time.
Another key focus area is the integration of luminol technology with other cutting-edge fields such as nanotechnology and microfluidics. This convergence has opened up new possibilities for miniaturization and automation of diagnostic processes, potentially revolutionizing on-site testing capabilities across multiple industries.
The ongoing research in luminol-based diagnostics also aims to expand its application spectrum. While traditionally associated with blood detection and certain medical tests, efforts are underway to adapt this technology for detecting a broader range of biological markers, environmental pollutants, and chemical compounds. This expansion could significantly impact fields such as personalized medicine, environmental monitoring, and food safety.
As the technology continues to evolve, researchers are exploring ways to overcome existing limitations, such as potential interference from other substances and the need for specialized equipment for result interpretation. The development of more selective luminol derivatives and advanced detection systems is at the forefront of these efforts, promising to further enhance the reliability and applicability of luminol-based diagnostic platforms.
Market Analysis for Light-Based Diagnostics
The light-based diagnostics market has experienced significant growth in recent years, driven by advancements in technology and increasing demand for rapid, non-invasive diagnostic solutions. This market segment encompasses a wide range of applications, including medical diagnostics, environmental monitoring, and food safety testing. The global market for light-based diagnostics is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to exceed 7% over the next five years.
One of the key factors fueling market growth is the rising prevalence of chronic diseases and the need for early detection and monitoring. Light-based diagnostic platforms offer advantages such as quick results, minimal invasiveness, and high sensitivity, making them particularly attractive for point-of-care testing and personalized medicine applications. Additionally, the ongoing COVID-19 pandemic has further accelerated the adoption of rapid diagnostic technologies, including those based on light detection principles.
In the medical field, light-based diagnostics are finding applications in various areas, including cancer detection, cardiovascular disease assessment, and infectious disease diagnosis. The oncology segment, in particular, is expected to witness substantial growth due to the increasing incidence of cancer worldwide and the demand for more accurate and less invasive diagnostic tools.
The environmental monitoring sector is another significant driver of market growth. Light-based sensors and detection systems are being increasingly employed for air and water quality monitoring, as well as for detecting pollutants and hazardous substances. This trend is supported by growing environmental concerns and stricter regulations on emissions and pollution control.
Geographically, North America currently holds the largest market share in light-based diagnostics, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is anticipated to exhibit the highest growth rate in the coming years, driven by improving healthcare infrastructure, rising healthcare expenditure, and increasing awareness about early disease detection.
Key players in the light-based diagnostics market include established medical device manufacturers, as well as innovative startups focusing on specific applications or technologies. These companies are investing heavily in research and development to improve the sensitivity, specificity, and portability of light-based diagnostic platforms. Collaborations between industry players and academic institutions are also contributing to technological advancements in this field.
Despite the positive outlook, challenges remain in the widespread adoption of light-based diagnostics. These include the need for standardization of testing protocols, regulatory hurdles in certain regions, and the initial cost of implementing new diagnostic systems. However, the potential benefits in terms of improved patient outcomes, reduced healthcare costs, and enhanced disease management are expected to drive continued innovation and market expansion in the coming years.
One of the key factors fueling market growth is the rising prevalence of chronic diseases and the need for early detection and monitoring. Light-based diagnostic platforms offer advantages such as quick results, minimal invasiveness, and high sensitivity, making them particularly attractive for point-of-care testing and personalized medicine applications. Additionally, the ongoing COVID-19 pandemic has further accelerated the adoption of rapid diagnostic technologies, including those based on light detection principles.
In the medical field, light-based diagnostics are finding applications in various areas, including cancer detection, cardiovascular disease assessment, and infectious disease diagnosis. The oncology segment, in particular, is expected to witness substantial growth due to the increasing incidence of cancer worldwide and the demand for more accurate and less invasive diagnostic tools.
The environmental monitoring sector is another significant driver of market growth. Light-based sensors and detection systems are being increasingly employed for air and water quality monitoring, as well as for detecting pollutants and hazardous substances. This trend is supported by growing environmental concerns and stricter regulations on emissions and pollution control.
Geographically, North America currently holds the largest market share in light-based diagnostics, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is anticipated to exhibit the highest growth rate in the coming years, driven by improving healthcare infrastructure, rising healthcare expenditure, and increasing awareness about early disease detection.
Key players in the light-based diagnostics market include established medical device manufacturers, as well as innovative startups focusing on specific applications or technologies. These companies are investing heavily in research and development to improve the sensitivity, specificity, and portability of light-based diagnostic platforms. Collaborations between industry players and academic institutions are also contributing to technological advancements in this field.
Despite the positive outlook, challenges remain in the widespread adoption of light-based diagnostics. These include the need for standardization of testing protocols, regulatory hurdles in certain regions, and the initial cost of implementing new diagnostic systems. However, the potential benefits in terms of improved patient outcomes, reduced healthcare costs, and enhanced disease management are expected to drive continued innovation and market expansion in the coming years.
Current Challenges in Luminol-Based Detection
Luminol-based detection systems face several significant challenges that hinder their widespread adoption and effectiveness in diagnostic applications. One of the primary issues is the limited sensitivity of luminol reactions, particularly in complex biological samples. The presence of interfering substances in these samples can lead to background noise, reducing the signal-to-noise ratio and compromising the accuracy of detection.
Another challenge is the relatively short duration of the luminol chemiluminescence reaction. The rapid decay of the light signal makes it difficult to capture and analyze, especially in point-of-care settings where sophisticated equipment may not be available. This temporal limitation also impacts the ability to perform quantitative measurements accurately over extended periods.
The stability of luminol reagents presents an additional hurdle. Luminol solutions can degrade over time, affecting the consistency and reliability of test results. This instability necessitates careful storage conditions and frequent preparation of fresh reagents, which can be impractical in resource-limited environments or for long-term field applications.
Specificity is another area of concern in luminol-based detection. While luminol is known for its broad reactivity with various oxidizing agents, this characteristic can also lead to false-positive results in the presence of non-target molecules. Improving the selectivity of luminol reactions without compromising sensitivity remains a significant challenge for researchers in this field.
The need for an alkaline environment to facilitate the luminol reaction poses challenges in certain biological applications. Maintaining the optimal pH for the reaction while ensuring compatibility with the sample and analytes of interest can be complex, particularly when dealing with pH-sensitive biomolecules or cellular systems.
Furthermore, the integration of luminol-based detection into miniaturized or portable devices faces technical difficulties. Scaling down the reaction volume while maintaining sensitivity and reproducibility requires innovative engineering solutions. Additionally, the development of user-friendly interfaces for result interpretation and data analysis in these compact systems presents ongoing challenges.
Lastly, the environmental and health concerns associated with some of the catalysts and enhancers used in luminol reactions, such as heavy metal ions, necessitate the exploration of safer alternatives. Finding eco-friendly and biocompatible components that can match or exceed the performance of traditional reagents is an active area of research in the field of luminol-based diagnostics.
Another challenge is the relatively short duration of the luminol chemiluminescence reaction. The rapid decay of the light signal makes it difficult to capture and analyze, especially in point-of-care settings where sophisticated equipment may not be available. This temporal limitation also impacts the ability to perform quantitative measurements accurately over extended periods.
The stability of luminol reagents presents an additional hurdle. Luminol solutions can degrade over time, affecting the consistency and reliability of test results. This instability necessitates careful storage conditions and frequent preparation of fresh reagents, which can be impractical in resource-limited environments or for long-term field applications.
Specificity is another area of concern in luminol-based detection. While luminol is known for its broad reactivity with various oxidizing agents, this characteristic can also lead to false-positive results in the presence of non-target molecules. Improving the selectivity of luminol reactions without compromising sensitivity remains a significant challenge for researchers in this field.
The need for an alkaline environment to facilitate the luminol reaction poses challenges in certain biological applications. Maintaining the optimal pH for the reaction while ensuring compatibility with the sample and analytes of interest can be complex, particularly when dealing with pH-sensitive biomolecules or cellular systems.
Furthermore, the integration of luminol-based detection into miniaturized or portable devices faces technical difficulties. Scaling down the reaction volume while maintaining sensitivity and reproducibility requires innovative engineering solutions. Additionally, the development of user-friendly interfaces for result interpretation and data analysis in these compact systems presents ongoing challenges.
Lastly, the environmental and health concerns associated with some of the catalysts and enhancers used in luminol reactions, such as heavy metal ions, necessitate the exploration of safer alternatives. Finding eco-friendly and biocompatible components that can match or exceed the performance of traditional reagents is an active area of research in the field of luminol-based diagnostics.
Existing Luminol-Based Diagnostic Solutions
01 Luminol-based detection systems for medical diagnostics
Luminol-based detection systems are utilized in medical diagnostics for various applications. These systems leverage the chemiluminescent properties of luminol to detect and analyze specific biomarkers or substances in biological samples. The light emitted during the luminol reaction can be measured and correlated with the presence or concentration of target analytes, enabling rapid and sensitive diagnostic tests.- Luminol-based detection systems for medical diagnostics: Luminol-based detection systems are utilized in medical diagnostics for various applications. These systems leverage the chemiluminescent properties of luminol to detect and analyze specific biomarkers or substances in biological samples. The light emitted during the luminol reaction can be measured and correlated with the presence or concentration of target analytes, enabling rapid and sensitive diagnostic tests.
- Luminol-enhanced blood detection in forensic applications: Luminol is widely used in forensic science for the detection of blood traces at crime scenes. When luminol comes into contact with the iron in hemoglobin, it produces a bright blue chemiluminescence. This reaction allows investigators to visualize blood patterns that are not visible to the naked eye, even in diluted or cleaned samples. Advanced techniques combine luminol with imaging systems for improved sensitivity and documentation.
- Luminol-based biosensors for environmental monitoring: Luminol-based biosensors are developed for environmental monitoring applications. These sensors utilize the chemiluminescent properties of luminol to detect and quantify various pollutants, heavy metals, or microorganisms in water, soil, or air samples. The intensity of the light emitted during the luminol reaction correlates with the concentration of the target analyte, providing a sensitive and rapid method for environmental analysis.
- Luminol-enhanced imaging techniques for research and diagnostics: Advanced imaging techniques incorporate luminol-based chemiluminescence for enhanced visualization in research and diagnostic applications. These methods combine luminol reactions with high-sensitivity cameras or microscopy systems to capture and analyze light emissions. This approach enables the study of cellular processes, protein interactions, or the detection of specific molecules in complex biological samples with high spatial and temporal resolution.
- Luminol-based assays for food safety and quality control: Luminol-based assays are developed for food safety and quality control applications. These tests utilize the chemiluminescent properties of luminol to detect contaminants, adulterants, or microbial presence in food products. The light emission from the luminol reaction can be measured to quantify the presence of specific compounds or microorganisms, providing a rapid and sensitive method for ensuring food safety and quality.
02 Luminol-enhanced blood detection in forensic applications
Luminol is widely used in forensic science for the detection of blood traces at crime scenes. When luminol comes into contact with the iron in hemoglobin, it produces a bright blue chemiluminescence. This reaction allows investigators to visualize blood patterns that are otherwise invisible to the naked eye, even in highly diluted samples or on surfaces that have been cleaned.Expand Specific Solutions03 Luminol-based biosensors and analytical devices
Luminol is incorporated into biosensors and analytical devices for detecting various substances in environmental, food safety, and clinical settings. These devices often combine luminol with specific enzymes or antibodies to create highly sensitive and selective detection systems. The chemiluminescent signal produced by luminol can be easily measured, allowing for quantitative analysis of target molecules.Expand Specific Solutions04 Enhanced luminol formulations for improved sensitivity
Researchers have developed enhanced luminol formulations to improve the sensitivity and specificity of luminol-based detection methods. These formulations may include additives or modifications to the luminol molecule itself, resulting in stronger chemiluminescent signals, longer-lasting light emission, or reduced interference from other substances. Such improvements enable the detection of lower concentrations of target analytes and expand the range of applications for luminol-based diagnostics.Expand Specific Solutions05 Integration of luminol-based detection in portable diagnostic devices
Luminol-based detection systems are being integrated into portable diagnostic devices for point-of-care testing and field applications. These devices combine miniaturized optics, microfluidics, and luminol chemistry to create compact, easy-to-use diagnostic tools. The portability and rapid results offered by these devices make them valuable for on-site testing in various settings, including healthcare facilities, environmental monitoring, and food safety inspections.Expand Specific Solutions
Key Players in Luminol Diagnostic Platforms
The "Luminol: Innovation in Light-Based Diagnostic Platforms" market is in a growth phase, with increasing demand for advanced diagnostic technologies. The global market for light-based diagnostics is expanding, driven by the need for rapid, accurate, and non-invasive testing methods. Companies like Alverix, Life Technologies, and FUJIFILM are leading innovation in this field, developing sophisticated platforms that integrate luminol-based detection systems. The technology's maturity varies, with established players refining existing solutions while newer entrants like Radisens Diagnostics and identifeye HEALTH introduce novel approaches, indicating a dynamic and competitive landscape with significant potential for further advancements and market expansion.
Alverix, Inc.
Technical Solution: Alverix has developed a portable, handheld diagnostic platform that utilizes luminol-based chemiluminescence for rapid and sensitive detection of various biomarkers. Their technology incorporates a proprietary photodetector system capable of measuring low-level light emissions from luminol reactions. The platform employs microfluidic cartridges for sample handling and reagent mixing, enabling point-of-care testing with minimal user intervention. Alverix's system can detect analytes at concentrations as low as femtomolar levels, rivaling laboratory-based methods[1]. The company has also implemented machine learning algorithms to improve signal processing and reduce background noise, enhancing overall assay sensitivity[3].
Strengths: High sensitivity, portability, and rapid results suitable for point-of-care diagnostics. Weaknesses: May require specialized reagents and cartridges, potentially increasing per-test costs.
Koninklijke Philips NV
Technical Solution: Philips has integrated luminol-based chemiluminescence into their advanced medical imaging systems, particularly for in vivo molecular imaging. Their approach combines luminol derivatives with targeted nanoparticles to visualize specific biological processes or disease markers in real-time. The company has developed a hybrid imaging modality that merges chemiluminescence with traditional radiological techniques, such as CT or MRI, for enhanced diagnostic accuracy[2]. Philips' platform includes sophisticated image processing software that can quantify and map chemiluminescent signals in three dimensions, allowing for precise localization of molecular events within the body[4].
Strengths: Integration with existing medical imaging modalities, potential for non-invasive molecular diagnostics. Weaknesses: Complexity of the system may limit widespread adoption in smaller healthcare settings.
Innovative Approaches in Luminol Chemistry
Construction and application of charge inversion type intelligent light diagnosis and treatment platform
PatentActiveCN113304261A
Innovation
- A charge-reversal intelligent light diagnosis and treatment platform is designed, which synthesizes near-infrared-excited photothermal/photodynamic integrated therapeutic agents and guanidine-based covalent organic nanosheets through electrostatic self-assembly to construct a bacterial-specific activation type that combines chemical and A multifunctional platform of photothermal, photodynamic and fluorescence, used for chemical/photothermal/photodynamic combined therapy guided by near-infrared fluorescence imaging.
Photodynamic therapy using chemiluminescence and a ligand-photosensitiser conjugate
PatentInactiveUS20100297762A1
Innovation
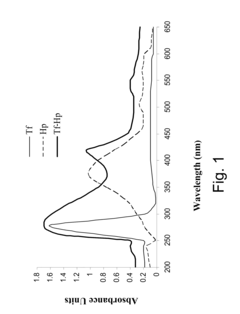
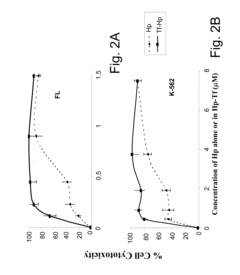
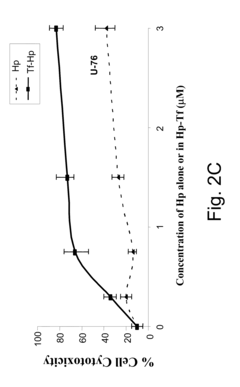
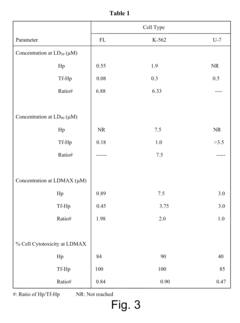
- A method involving a ligand-toxin conjugate (LTC) comprising a photosensitizer like hematoporphyrin conjugated with transferrin, combined with a chemiluminescent agent such as luminol, which activates the photosensitizer intracellularly to produce reactive oxygen species, thereby enhancing target cell destruction without requiring external light.
Regulatory Framework for Diagnostic Platforms
The regulatory framework for diagnostic platforms, particularly those utilizing innovative technologies like Luminol-based light detection, is a complex and evolving landscape. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development, approval, and marketing of diagnostic devices. The FDA categorizes these devices into different classes based on their intended use and risk level, with light-based diagnostic platforms typically falling under Class II or III.
For Luminol-based diagnostic platforms, manufacturers must navigate the premarket notification (510(k)) process or, in some cases, the more rigorous premarket approval (PMA) pathway. The 510(k) submission requires demonstrating that the new device is substantially equivalent to a legally marketed predicate device. However, given the innovative nature of Luminol technology, some aspects may require de novo classification or PMA.
The regulatory process also encompasses quality system regulations (QSRs), which mandate good manufacturing practices and quality control measures. Manufacturers of Luminol-based diagnostic platforms must establish and maintain a quality management system that complies with 21 CFR Part 820, ensuring consistent production of safe and effective devices.
Clinical performance data is crucial for regulatory approval. Manufacturers must conduct clinical studies to validate the accuracy, precision, and clinical utility of their Luminol-based diagnostic platforms. These studies should adhere to good clinical practice (GCP) guidelines and may require institutional review board (IRB) approval.
Post-market surveillance is another critical component of the regulatory framework. Once approved, manufacturers must implement systems for monitoring device performance, reporting adverse events, and conducting recalls if necessary. The FDA's Medical Device Reporting (MDR) regulation requires timely reporting of device-related adverse events and product problems.
Internationally, regulatory requirements vary, but many countries align with or reference FDA standards. The European Union's In Vitro Diagnostic Regulation (IVDR) provides a comprehensive framework for diagnostic devices, including performance evaluation, clinical evidence, and post-market surveillance requirements. Manufacturers seeking global market access must navigate these diverse regulatory landscapes, often requiring separate submissions and approvals for different regions.
As Luminol technology continues to advance, regulatory bodies may need to adapt their frameworks to address novel aspects of light-based diagnostics. This could involve developing new guidance documents, establishing specialized review pathways, or updating existing regulations to accommodate emerging technologies in the diagnostic field.
For Luminol-based diagnostic platforms, manufacturers must navigate the premarket notification (510(k)) process or, in some cases, the more rigorous premarket approval (PMA) pathway. The 510(k) submission requires demonstrating that the new device is substantially equivalent to a legally marketed predicate device. However, given the innovative nature of Luminol technology, some aspects may require de novo classification or PMA.
The regulatory process also encompasses quality system regulations (QSRs), which mandate good manufacturing practices and quality control measures. Manufacturers of Luminol-based diagnostic platforms must establish and maintain a quality management system that complies with 21 CFR Part 820, ensuring consistent production of safe and effective devices.
Clinical performance data is crucial for regulatory approval. Manufacturers must conduct clinical studies to validate the accuracy, precision, and clinical utility of their Luminol-based diagnostic platforms. These studies should adhere to good clinical practice (GCP) guidelines and may require institutional review board (IRB) approval.
Post-market surveillance is another critical component of the regulatory framework. Once approved, manufacturers must implement systems for monitoring device performance, reporting adverse events, and conducting recalls if necessary. The FDA's Medical Device Reporting (MDR) regulation requires timely reporting of device-related adverse events and product problems.
Internationally, regulatory requirements vary, but many countries align with or reference FDA standards. The European Union's In Vitro Diagnostic Regulation (IVDR) provides a comprehensive framework for diagnostic devices, including performance evaluation, clinical evidence, and post-market surveillance requirements. Manufacturers seeking global market access must navigate these diverse regulatory landscapes, often requiring separate submissions and approvals for different regions.
As Luminol technology continues to advance, regulatory bodies may need to adapt their frameworks to address novel aspects of light-based diagnostics. This could involve developing new guidance documents, establishing specialized review pathways, or updating existing regulations to accommodate emerging technologies in the diagnostic field.
Environmental Impact of Luminol-Based Systems
The environmental impact of luminol-based diagnostic systems is a crucial consideration in the development and deployment of these innovative light-based platforms. These systems, while offering significant advantages in medical diagnostics and forensic investigations, also present potential environmental concerns that must be carefully evaluated and addressed.
Luminol-based systems typically involve the use of chemical reagents and substrates, which may have implications for waste management and disposal. The primary environmental concern stems from the potential release of these chemicals into water systems or soil. While luminol itself is generally considered to have low toxicity, other components of the diagnostic platform, such as oxidizing agents or catalysts, may pose more significant environmental risks if not properly handled.
The production and manufacturing processes of luminol-based diagnostic platforms also contribute to their overall environmental footprint. These processes may involve energy-intensive steps and the use of various chemicals, potentially leading to greenhouse gas emissions and the generation of industrial waste. As the demand for these diagnostic tools increases, the cumulative environmental impact of their production could become more pronounced.
However, it is important to note that luminol-based systems often require very small quantities of reagents, which can help minimize their environmental impact compared to traditional diagnostic methods. Additionally, the high sensitivity of these systems may reduce the need for multiple tests, potentially decreasing overall resource consumption and waste generation in diagnostic procedures.
The disposal of used luminol-based diagnostic devices presents another environmental challenge. Many of these devices are designed for single use, contributing to medical waste streams. Proper disposal protocols must be established to prevent contamination of landfills or water sources with potentially harmful chemicals or biological materials.
On the positive side, the development of more environmentally friendly luminol derivatives and improved system designs is an active area of research. Scientists are exploring biodegradable materials for device components and investigating ways to enhance the recyclability of these diagnostic platforms. Some researchers are also focusing on developing regenerable luminol-based systems, which could significantly reduce waste generation.
The environmental impact of luminol-based systems extends beyond their immediate use and disposal. These innovative diagnostic platforms have the potential to improve early disease detection and enhance forensic investigations, potentially leading to more efficient healthcare systems and reduced resource consumption in the long term. By enabling faster and more accurate diagnoses, these systems could contribute to reduced hospital stays, decreased use of broad-spectrum antibiotics, and more targeted treatments, all of which have positive environmental implications.
Luminol-based systems typically involve the use of chemical reagents and substrates, which may have implications for waste management and disposal. The primary environmental concern stems from the potential release of these chemicals into water systems or soil. While luminol itself is generally considered to have low toxicity, other components of the diagnostic platform, such as oxidizing agents or catalysts, may pose more significant environmental risks if not properly handled.
The production and manufacturing processes of luminol-based diagnostic platforms also contribute to their overall environmental footprint. These processes may involve energy-intensive steps and the use of various chemicals, potentially leading to greenhouse gas emissions and the generation of industrial waste. As the demand for these diagnostic tools increases, the cumulative environmental impact of their production could become more pronounced.
However, it is important to note that luminol-based systems often require very small quantities of reagents, which can help minimize their environmental impact compared to traditional diagnostic methods. Additionally, the high sensitivity of these systems may reduce the need for multiple tests, potentially decreasing overall resource consumption and waste generation in diagnostic procedures.
The disposal of used luminol-based diagnostic devices presents another environmental challenge. Many of these devices are designed for single use, contributing to medical waste streams. Proper disposal protocols must be established to prevent contamination of landfills or water sources with potentially harmful chemicals or biological materials.
On the positive side, the development of more environmentally friendly luminol derivatives and improved system designs is an active area of research. Scientists are exploring biodegradable materials for device components and investigating ways to enhance the recyclability of these diagnostic platforms. Some researchers are also focusing on developing regenerable luminol-based systems, which could significantly reduce waste generation.
The environmental impact of luminol-based systems extends beyond their immediate use and disposal. These innovative diagnostic platforms have the potential to improve early disease detection and enhance forensic investigations, potentially leading to more efficient healthcare systems and reduced resource consumption in the long term. By enabling faster and more accurate diagnoses, these systems could contribute to reduced hospital stays, decreased use of broad-spectrum antibiotics, and more targeted treatments, all of which have positive environmental implications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!