How to Leverage Luminol in Diagnostic Device Creation?
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol in Diagnostics: Background and Objectives
Luminol, a chemiluminescent compound, has been a subject of interest in the field of diagnostics for several decades. This versatile chemical has the unique property of emitting light when oxidized, making it an invaluable tool in various scientific and forensic applications. In the context of diagnostic device creation, luminol presents exciting opportunities for developing innovative and sensitive detection methods.
The history of luminol in diagnostics can be traced back to its discovery in the early 20th century. Initially used in forensic science for blood detection, its potential in medical diagnostics became apparent as researchers explored its chemical properties and reaction mechanisms. Over time, the understanding of luminol's chemiluminescence has evolved, leading to its integration into various diagnostic platforms.
The primary objective of leveraging luminol in diagnostic device creation is to harness its chemiluminescent properties for detecting specific biomarkers or analytes. This approach aims to develop highly sensitive, rapid, and cost-effective diagnostic tools that can revolutionize healthcare delivery, particularly in resource-limited settings. The ultimate goal is to create point-of-care devices that can provide accurate results with minimal sample preparation and equipment requirements.
Current technological trends in luminol-based diagnostics focus on enhancing sensitivity, specificity, and multiplexing capabilities. Researchers are exploring novel ways to amplify the luminol signal, develop more stable formulations, and integrate it with other detection technologies. The convergence of luminol chemistry with nanotechnology, microfluidics, and advanced imaging techniques is opening up new avenues for diagnostic device innovation.
The market demand for luminol-based diagnostic devices is driven by the growing need for rapid, accurate, and portable diagnostic solutions. With the increasing prevalence of infectious diseases, cancer, and chronic conditions, there is a pressing need for diagnostic tools that can provide timely results at the point of care. Luminol-based devices have the potential to address these needs by offering sensitive detection methods that can be miniaturized and integrated into portable platforms.
As we look towards the future, the evolution of luminol in diagnostics is likely to be shaped by advancements in materials science, bioengineering, and data analytics. The integration of artificial intelligence and machine learning algorithms with luminol-based detection systems could lead to more sophisticated diagnostic devices capable of interpreting complex chemiluminescent signals and providing more accurate diagnoses.
In conclusion, leveraging luminol in diagnostic device creation represents a promising frontier in medical technology. By building upon its well-established chemiluminescent properties and addressing current technological challenges, researchers and innovators have the opportunity to develop next-generation diagnostic tools that could significantly impact healthcare delivery and patient outcomes.
The history of luminol in diagnostics can be traced back to its discovery in the early 20th century. Initially used in forensic science for blood detection, its potential in medical diagnostics became apparent as researchers explored its chemical properties and reaction mechanisms. Over time, the understanding of luminol's chemiluminescence has evolved, leading to its integration into various diagnostic platforms.
The primary objective of leveraging luminol in diagnostic device creation is to harness its chemiluminescent properties for detecting specific biomarkers or analytes. This approach aims to develop highly sensitive, rapid, and cost-effective diagnostic tools that can revolutionize healthcare delivery, particularly in resource-limited settings. The ultimate goal is to create point-of-care devices that can provide accurate results with minimal sample preparation and equipment requirements.
Current technological trends in luminol-based diagnostics focus on enhancing sensitivity, specificity, and multiplexing capabilities. Researchers are exploring novel ways to amplify the luminol signal, develop more stable formulations, and integrate it with other detection technologies. The convergence of luminol chemistry with nanotechnology, microfluidics, and advanced imaging techniques is opening up new avenues for diagnostic device innovation.
The market demand for luminol-based diagnostic devices is driven by the growing need for rapid, accurate, and portable diagnostic solutions. With the increasing prevalence of infectious diseases, cancer, and chronic conditions, there is a pressing need for diagnostic tools that can provide timely results at the point of care. Luminol-based devices have the potential to address these needs by offering sensitive detection methods that can be miniaturized and integrated into portable platforms.
As we look towards the future, the evolution of luminol in diagnostics is likely to be shaped by advancements in materials science, bioengineering, and data analytics. The integration of artificial intelligence and machine learning algorithms with luminol-based detection systems could lead to more sophisticated diagnostic devices capable of interpreting complex chemiluminescent signals and providing more accurate diagnoses.
In conclusion, leveraging luminol in diagnostic device creation represents a promising frontier in medical technology. By building upon its well-established chemiluminescent properties and addressing current technological challenges, researchers and innovators have the opportunity to develop next-generation diagnostic tools that could significantly impact healthcare delivery and patient outcomes.
Market Analysis for Luminol-Based Diagnostic Devices
The market for luminol-based diagnostic devices is experiencing significant growth, driven by increasing demand for rapid and sensitive detection methods in various fields. Luminol, a chemiluminescent compound, has found widespread applications in forensic science, medical diagnostics, and environmental monitoring due to its ability to produce a bright blue light when oxidized.
In the medical diagnostics sector, luminol-based devices are gaining traction for their potential in early disease detection and point-of-care testing. The global market for point-of-care diagnostics is projected to reach substantial figures in the coming years, with luminol-based technologies playing a crucial role in this expansion. These devices offer advantages such as high sensitivity, rapid results, and cost-effectiveness, making them attractive for both clinical and home-use settings.
The forensic science market is another key driver for luminol-based diagnostic devices. Law enforcement agencies and forensic laboratories worldwide are adopting these technologies for crime scene investigations, particularly in the detection of blood traces. The increasing focus on advanced forensic techniques and the need for more accurate evidence collection methods are fueling the demand for luminol-based solutions in this sector.
Environmental monitoring represents an emerging market for luminol-based diagnostic devices. These tools are being utilized for detecting pollutants in water bodies, soil contamination, and air quality assessment. As environmental regulations become more stringent globally, the demand for sensitive and reliable detection methods is expected to boost the adoption of luminol-based technologies in this field.
The pharmaceutical and biotechnology industries are also contributing to the market growth of luminol-based diagnostic devices. These sectors are leveraging luminol's properties for drug discovery processes, enzyme activity assays, and protein detection methods. The increasing investment in research and development activities in these industries is likely to create new opportunities for luminol-based diagnostic technologies.
Geographically, North America and Europe currently dominate the market for luminol-based diagnostic devices, owing to advanced healthcare infrastructure, robust research activities, and stringent regulatory standards. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare facilities, increasing awareness about advanced diagnostic techniques, and rising investments in forensic and environmental sciences.
Despite the positive market outlook, challenges such as the need for skilled personnel to operate these devices and potential interference from other substances in complex samples need to be addressed. Ongoing research and development efforts are focused on enhancing the specificity and sensitivity of luminol-based detection methods to overcome these limitations and expand their applications across various industries.
In the medical diagnostics sector, luminol-based devices are gaining traction for their potential in early disease detection and point-of-care testing. The global market for point-of-care diagnostics is projected to reach substantial figures in the coming years, with luminol-based technologies playing a crucial role in this expansion. These devices offer advantages such as high sensitivity, rapid results, and cost-effectiveness, making them attractive for both clinical and home-use settings.
The forensic science market is another key driver for luminol-based diagnostic devices. Law enforcement agencies and forensic laboratories worldwide are adopting these technologies for crime scene investigations, particularly in the detection of blood traces. The increasing focus on advanced forensic techniques and the need for more accurate evidence collection methods are fueling the demand for luminol-based solutions in this sector.
Environmental monitoring represents an emerging market for luminol-based diagnostic devices. These tools are being utilized for detecting pollutants in water bodies, soil contamination, and air quality assessment. As environmental regulations become more stringent globally, the demand for sensitive and reliable detection methods is expected to boost the adoption of luminol-based technologies in this field.
The pharmaceutical and biotechnology industries are also contributing to the market growth of luminol-based diagnostic devices. These sectors are leveraging luminol's properties for drug discovery processes, enzyme activity assays, and protein detection methods. The increasing investment in research and development activities in these industries is likely to create new opportunities for luminol-based diagnostic technologies.
Geographically, North America and Europe currently dominate the market for luminol-based diagnostic devices, owing to advanced healthcare infrastructure, robust research activities, and stringent regulatory standards. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare facilities, increasing awareness about advanced diagnostic techniques, and rising investments in forensic and environmental sciences.
Despite the positive market outlook, challenges such as the need for skilled personnel to operate these devices and potential interference from other substances in complex samples need to be addressed. Ongoing research and development efforts are focused on enhancing the specificity and sensitivity of luminol-based detection methods to overcome these limitations and expand their applications across various industries.
Current Challenges in Luminol Application
Despite the widespread use of luminol in forensic science and diagnostic applications, several challenges persist in its application for diagnostic device creation. One of the primary obstacles is the sensitivity and specificity of luminol-based tests. While luminol is known for its ability to detect trace amounts of blood, it can also produce false positives when reacting with other substances, such as certain plant materials or cleaning agents. This lack of specificity can lead to inaccurate results in diagnostic devices, potentially compromising their reliability in clinical settings.
Another significant challenge is the stability of luminol solutions. The compound tends to degrade over time, especially when exposed to light or heat. This instability can affect the shelf life of diagnostic devices incorporating luminol, making it difficult to maintain consistent performance over extended periods. Manufacturers must develop innovative packaging and storage solutions to preserve the integrity of luminol-based reagents in diagnostic kits.
The intensity and duration of the chemiluminescent reaction pose additional challenges in device design. The luminol reaction, while visually striking, can be relatively short-lived and may not provide a sustained signal for accurate measurement in some diagnostic applications. Engineers must devise ways to either prolong the reaction or develop highly sensitive detection systems capable of capturing and analyzing brief luminescent signals.
Environmental factors also present hurdles in luminol application. The pH sensitivity of the luminol reaction means that slight variations in sample acidity or alkalinity can significantly affect test results. This sensitivity necessitates careful calibration and control of reaction conditions within diagnostic devices, adding complexity to their design and operation.
Furthermore, the integration of luminol-based detection systems with other components of diagnostic devices presents technical challenges. Miniaturization of the reaction chamber, precise fluid handling, and the incorporation of sensitive light detection systems all require sophisticated engineering solutions. These challenges are particularly pronounced in the development of point-of-care devices, where compactness and ease of use are paramount.
Lastly, regulatory compliance and standardization pose significant hurdles in the commercialization of luminol-based diagnostic devices. Ensuring consistent quality, establishing reliable manufacturing processes, and meeting stringent regulatory requirements for medical devices add layers of complexity to the development process. Manufacturers must navigate these regulatory landscapes while addressing the technical challenges inherent in luminol application.
Another significant challenge is the stability of luminol solutions. The compound tends to degrade over time, especially when exposed to light or heat. This instability can affect the shelf life of diagnostic devices incorporating luminol, making it difficult to maintain consistent performance over extended periods. Manufacturers must develop innovative packaging and storage solutions to preserve the integrity of luminol-based reagents in diagnostic kits.
The intensity and duration of the chemiluminescent reaction pose additional challenges in device design. The luminol reaction, while visually striking, can be relatively short-lived and may not provide a sustained signal for accurate measurement in some diagnostic applications. Engineers must devise ways to either prolong the reaction or develop highly sensitive detection systems capable of capturing and analyzing brief luminescent signals.
Environmental factors also present hurdles in luminol application. The pH sensitivity of the luminol reaction means that slight variations in sample acidity or alkalinity can significantly affect test results. This sensitivity necessitates careful calibration and control of reaction conditions within diagnostic devices, adding complexity to their design and operation.
Furthermore, the integration of luminol-based detection systems with other components of diagnostic devices presents technical challenges. Miniaturization of the reaction chamber, precise fluid handling, and the incorporation of sensitive light detection systems all require sophisticated engineering solutions. These challenges are particularly pronounced in the development of point-of-care devices, where compactness and ease of use are paramount.
Lastly, regulatory compliance and standardization pose significant hurdles in the commercialization of luminol-based diagnostic devices. Ensuring consistent quality, establishing reliable manufacturing processes, and meeting stringent regulatory requirements for medical devices add layers of complexity to the development process. Manufacturers must navigate these regulatory landscapes while addressing the technical challenges inherent in luminol application.
Existing Luminol Integration Methods
01 Luminol in forensic applications
Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When mixed with an oxidizing agent, it produces a blue chemiluminescence in the presence of iron in hemoglobin. This reaction is highly sensitive and can detect blood even after cleaning attempts.- Luminol in chemiluminescence detection: Luminol is widely used in chemiluminescence detection methods for various applications. It produces light when oxidized, making it useful for detecting trace amounts of blood, metal ions, or other substances in forensic science, environmental monitoring, and medical diagnostics.
- Luminol-based biosensors and immunoassays: Luminol is incorporated into biosensors and immunoassays for highly sensitive detection of specific biomolecules. These systems often combine luminol with enzymes or nanoparticles to enhance sensitivity and specificity in medical diagnostics and research applications.
- Luminol in environmental monitoring: Luminol-based systems are developed for environmental monitoring, including the detection of pollutants in water and air. These methods often involve coupling luminol with specific catalysts or reagents to detect target compounds with high sensitivity.
- Enhanced luminol formulations: Research focuses on improving luminol formulations to enhance chemiluminescence intensity and duration. This includes developing new derivatives, optimizing reaction conditions, and incorporating additives to increase light output and stability for various applications.
- Luminol in imaging and visualization techniques: Luminol is utilized in imaging and visualization techniques for both scientific research and practical applications. This includes developing luminol-based sprays or solutions for crime scene investigation, as well as incorporating luminol into advanced imaging systems for biological and materials research.
02 Luminol-based detection systems
Various detection systems incorporate luminol for its chemiluminescent properties. These systems are used in environmental monitoring, food safety testing, and medical diagnostics. The high sensitivity of luminol allows for the detection of minute quantities of target substances.Expand Specific Solutions03 Luminol derivatives and modifications
Research focuses on developing luminol derivatives and modifications to enhance its properties. These improvements aim to increase sensitivity, stability, and specificity for various applications. Modified luminol compounds can offer advantages such as longer-lasting luminescence or better solubility.Expand Specific Solutions04 Luminol in analytical chemistry
Luminol is extensively used in analytical chemistry for quantitative and qualitative analysis. It serves as a reagent in flow injection analysis, high-performance liquid chromatography, and other analytical techniques. The chemiluminescent reaction of luminol allows for highly sensitive detection of various analytes.Expand Specific Solutions05 Luminol in biomedical research
In biomedical research, luminol is used to study cellular processes, particularly those involving reactive oxygen species. It helps in investigating oxidative stress, immune cell function, and enzyme activity. The chemiluminescent properties of luminol make it valuable for imaging and quantifying biological processes in real-time.Expand Specific Solutions
Key Players in Luminol-Based Diagnostics
The development of luminol-based diagnostic devices is in a growth phase, with increasing market size and technological advancements. The competitive landscape is characterized by a mix of established healthcare companies and specialized biotech firms. Key players like Siemens Healthineers AG and FUJIFILM Corp. are leveraging their extensive resources and expertise to develop sophisticated diagnostic solutions. Smaller, innovative companies such as Alverix, Inc. and Cyanagen Srl are focusing on niche applications and novel approaches to luminol-based diagnostics. The technology's maturity is progressing, with ongoing research at institutions like Washington University in St. Louis and Peking University contributing to its evolution and potential applications in various medical fields.
Alverix, Inc.
Technical Solution: Alverix, Inc. has developed a unique approach to leveraging luminol in diagnostic device creation through their advanced reader technology. Their platform combines luminol-based chemiluminescence with sophisticated optoelectronic detection systems to create highly sensitive point-of-care diagnostic devices. Alverix's technology utilizes a proprietary light collection and signal processing algorithm that enhances the detection of low-level luminol signals. This allows for the development of rapid, quantitative tests with improved sensitivity compared to traditional lateral flow assays. The company has applied this technology to create portable readers for various diagnostic applications, including infectious disease testing and cardiac marker detection[13][14]. Alverix's approach also involves optimizing the luminol reaction chemistry and integrating it with microfluidic cartridges to enable precise fluid control and enhance assay performance[15].
Strengths: High sensitivity in portable format, quantitative results, versatility across multiple analytes. Weaknesses: Reliance on proprietary reader technology, potential for higher cost per test compared to traditional lateral flow assays.
Siemens Healthineers AG
Technical Solution: Siemens Healthineers has incorporated luminol-based chemiluminescence into their ADVIA Centaur® XP Immunoassay System. This system utilizes acridinium ester-labeled reagents and luminol-based detection to achieve high-sensitivity immunoassays. The technology involves a unique "flash" chemiluminescence reaction, where the acridinium ester is rapidly oxidized in the presence of hydrogen peroxide and sodium hydroxide, producing light. This light is then amplified by the luminol-peroxidase system, significantly enhancing the signal intensity. The ADVIA Centaur system employs magnetic particle separation and washing steps to minimize background noise, resulting in improved assay precision and sensitivity[4][5]. Siemens has applied this technology to develop a wide range of diagnostic tests, including those for thyroid function, fertility hormones, and cardiac markers[6].
Strengths: Rapid test results, high throughput capability, wide dynamic range. Weaknesses: Requires specialized instrumentation, potential for light signal decay over time.
Innovative Luminol Formulations and Techniques
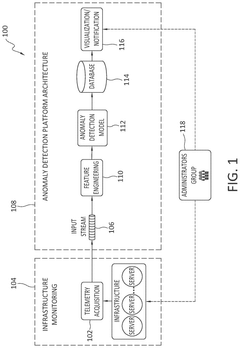
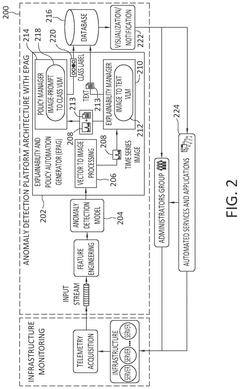
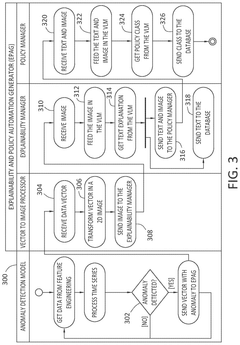
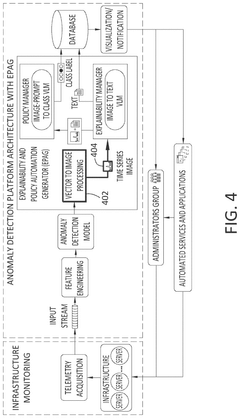
Enhancing anomaly detection pipeline with a post-hoc generative ai model to support human understanding and policy automation
PatentPendingUS20250118066A1
Innovation
- The implementation of an EPAG module that includes a Vector to Image Processor, an Explainability Manager, and a Policy Manager, which generates human-readable explanations and automated action recommendations by transforming input vectors into visual representations, using fine-tuned VLM models for explanation and policy classification.
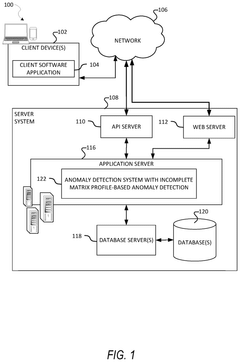
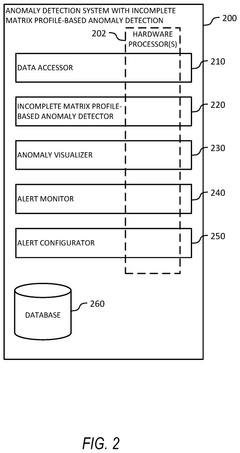
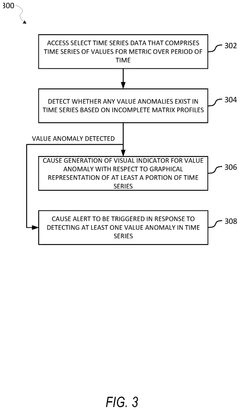
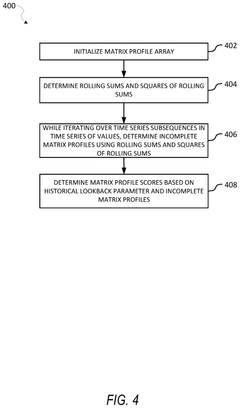
Incomplete matrix profile-based anomaly detection in time series data
PatentPendingUS20250106131A1
Innovation
- The use of incomplete matrix profiles for anomaly detection, which iterates over time series data, determining scores for each time point based on a historical lookback parameter, allowing for online detection and interactive simulation of anomalies at low granularity, such as minutes or seconds, thereby reducing computational complexity from O(n*h*h) to O(n) time.
Regulatory Framework for Chemiluminescent Diagnostics
The regulatory framework for chemiluminescent diagnostics plays a crucial role in ensuring the safety, efficacy, and quality of diagnostic devices utilizing luminol and other chemiluminescent compounds. In the United States, the Food and Drug Administration (FDA) oversees the regulation of these devices under the Medical Device Regulations. The FDA classifies chemiluminescent diagnostic devices based on their intended use and risk level, typically falling under Class II or Class III categories.
For Class II devices, manufacturers must submit a 510(k) premarket notification, demonstrating substantial equivalence to a legally marketed predicate device. This process involves providing detailed information on the device's design, performance data, and risk assessment. Class III devices, considered high-risk, require a more rigorous Premarket Approval (PMA) application, including extensive clinical trial data to prove safety and effectiveness.
In the European Union, chemiluminescent diagnostic devices are regulated under the In Vitro Diagnostic Regulation (IVDR), which replaced the previous In Vitro Diagnostic Directive (IVDD) in May 2022. The IVDR introduces a risk-based classification system, with most chemiluminescent diagnostics falling under Class B or C. Manufacturers must comply with stricter requirements for clinical evidence, post-market surveillance, and traceability.
The regulatory framework also addresses the chemical safety aspects of luminol and other reagents used in these devices. Manufacturers must comply with regulations such as REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) in the EU and the Toxic Substances Control Act (TSCA) in the US. These regulations ensure proper handling, storage, and disposal of potentially hazardous chemicals used in diagnostic devices.
Quality management systems are an integral part of the regulatory framework. Manufacturers must adhere to Good Manufacturing Practices (GMP) and implement ISO 13485 standards for medical devices. These systems ensure consistent production, quality control, and continuous improvement of chemiluminescent diagnostic devices.
Regulatory bodies also focus on the analytical performance of chemiluminescent diagnostics. Manufacturers must provide data on sensitivity, specificity, accuracy, and precision of their devices. Additionally, they must demonstrate the stability and shelf-life of the chemiluminescent reagents used in the diagnostic process.
As the field of chemiluminescent diagnostics continues to evolve, regulatory frameworks are adapting to address emerging technologies and applications. This includes considerations for point-of-care testing, multiplexed assays, and integration with digital health platforms. Manufacturers developing innovative luminol-based diagnostic devices must stay informed about these evolving regulations to ensure compliance and successful market entry.
For Class II devices, manufacturers must submit a 510(k) premarket notification, demonstrating substantial equivalence to a legally marketed predicate device. This process involves providing detailed information on the device's design, performance data, and risk assessment. Class III devices, considered high-risk, require a more rigorous Premarket Approval (PMA) application, including extensive clinical trial data to prove safety and effectiveness.
In the European Union, chemiluminescent diagnostic devices are regulated under the In Vitro Diagnostic Regulation (IVDR), which replaced the previous In Vitro Diagnostic Directive (IVDD) in May 2022. The IVDR introduces a risk-based classification system, with most chemiluminescent diagnostics falling under Class B or C. Manufacturers must comply with stricter requirements for clinical evidence, post-market surveillance, and traceability.
The regulatory framework also addresses the chemical safety aspects of luminol and other reagents used in these devices. Manufacturers must comply with regulations such as REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) in the EU and the Toxic Substances Control Act (TSCA) in the US. These regulations ensure proper handling, storage, and disposal of potentially hazardous chemicals used in diagnostic devices.
Quality management systems are an integral part of the regulatory framework. Manufacturers must adhere to Good Manufacturing Practices (GMP) and implement ISO 13485 standards for medical devices. These systems ensure consistent production, quality control, and continuous improvement of chemiluminescent diagnostic devices.
Regulatory bodies also focus on the analytical performance of chemiluminescent diagnostics. Manufacturers must provide data on sensitivity, specificity, accuracy, and precision of their devices. Additionally, they must demonstrate the stability and shelf-life of the chemiluminescent reagents used in the diagnostic process.
As the field of chemiluminescent diagnostics continues to evolve, regulatory frameworks are adapting to address emerging technologies and applications. This includes considerations for point-of-care testing, multiplexed assays, and integration with digital health platforms. Manufacturers developing innovative luminol-based diagnostic devices must stay informed about these evolving regulations to ensure compliance and successful market entry.
Biosafety Considerations in Luminol Device Development
When developing diagnostic devices utilizing luminol, biosafety considerations are paramount to ensure the protection of both users and the environment. The primary concern revolves around the potential toxicity of luminol and its reaction products. While luminol itself is generally considered to have low toxicity, prolonged exposure or ingestion can cause irritation to the skin, eyes, and respiratory system. Therefore, proper handling protocols and protective equipment are essential during device manufacturing and usage.
The luminol reaction typically involves the use of hydrogen peroxide as an oxidizing agent, which can be corrosive and harmful if mishandled. Careful consideration must be given to the storage, handling, and disposal of this reagent. Additionally, the alkaline conditions required for the luminol reaction necessitate the use of strong bases, such as sodium hydroxide, which can pose safety risks if not properly managed.
In the context of diagnostic devices, it is crucial to design containment systems that prevent direct contact between the user and the reagents. This may involve the development of sealed cartridges or microfluidic systems that isolate the chemical components. Furthermore, the integration of fail-safe mechanisms to prevent leakage or accidental exposure is essential for maintaining biosafety standards.
The disposal of used diagnostic devices containing luminol and its reaction products must also be carefully considered. Proper waste management protocols should be established to prevent environmental contamination and ensure compliance with local regulations. This may include the development of biodegradable materials for device components or the implementation of recycling programs for certain parts of the device.
Another important aspect of biosafety in luminol-based diagnostic devices is the potential for false-positive results due to contamination. Strict quality control measures must be implemented during manufacturing to prevent the introduction of blood or other catalysts that could trigger the luminol reaction. This includes maintaining a sterile production environment and implementing rigorous testing procedures to ensure the reliability and safety of the final product.
Regulatory compliance is a critical component of biosafety considerations. Developers must adhere to guidelines set forth by agencies such as the FDA and EPA, which may require extensive safety testing and documentation. This includes conducting thorough risk assessments and implementing appropriate risk mitigation strategies throughout the device's lifecycle, from development to disposal.
The luminol reaction typically involves the use of hydrogen peroxide as an oxidizing agent, which can be corrosive and harmful if mishandled. Careful consideration must be given to the storage, handling, and disposal of this reagent. Additionally, the alkaline conditions required for the luminol reaction necessitate the use of strong bases, such as sodium hydroxide, which can pose safety risks if not properly managed.
In the context of diagnostic devices, it is crucial to design containment systems that prevent direct contact between the user and the reagents. This may involve the development of sealed cartridges or microfluidic systems that isolate the chemical components. Furthermore, the integration of fail-safe mechanisms to prevent leakage or accidental exposure is essential for maintaining biosafety standards.
The disposal of used diagnostic devices containing luminol and its reaction products must also be carefully considered. Proper waste management protocols should be established to prevent environmental contamination and ensure compliance with local regulations. This may include the development of biodegradable materials for device components or the implementation of recycling programs for certain parts of the device.
Another important aspect of biosafety in luminol-based diagnostic devices is the potential for false-positive results due to contamination. Strict quality control measures must be implemented during manufacturing to prevent the introduction of blood or other catalysts that could trigger the luminol reaction. This includes maintaining a sterile production environment and implementing rigorous testing procedures to ensure the reliability and safety of the final product.
Regulatory compliance is a critical component of biosafety considerations. Developers must adhere to guidelines set forth by agencies such as the FDA and EPA, which may require extensive safety testing and documentation. This includes conducting thorough risk assessments and implementing appropriate risk mitigation strategies throughout the device's lifecycle, from development to disposal.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!