Luminol in Innovative Diagnostic Protocols
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Research Background and Objectives
Luminol, a versatile chemiluminescent compound, has been a subject of scientific interest for over a century. Initially discovered in the late 1800s, its unique properties have led to diverse applications, particularly in forensic science and medical diagnostics. The evolution of luminol research has been marked by significant milestones, from its early use in crime scene investigations to its current role in cutting-edge diagnostic protocols.
The primary objective of luminol research in innovative diagnostic protocols is to harness its exceptional sensitivity and specificity for detecting various biological molecules and cellular components. This aim aligns with the growing demand for rapid, non-invasive, and highly accurate diagnostic tools in healthcare. The potential of luminol to revolutionize early disease detection, monitor treatment efficacy, and enable point-of-care diagnostics has driven intense research efforts in recent years.
Luminol's unique chemiluminescent properties stem from its ability to emit light when oxidized, typically in the presence of an appropriate catalyst. This reaction has been extensively studied and optimized for various applications. In the context of diagnostic protocols, researchers are exploring ways to enhance luminol's sensitivity, improve its selectivity for specific biomarkers, and develop novel detection systems that can capitalize on its light-emitting capabilities.
The technological trajectory of luminol research has seen a shift from traditional forensic applications to more sophisticated biomedical uses. This transition has been facilitated by advancements in related fields such as nanotechnology, microfluidics, and artificial intelligence. These interdisciplinary collaborations have opened new avenues for luminol-based diagnostics, including the development of highly sensitive biosensors, lab-on-a-chip devices, and automated diagnostic platforms.
Current research goals in luminol-based diagnostic protocols include enhancing the signal-to-noise ratio, developing multiplexed detection systems, and creating user-friendly platforms for clinical use. Additionally, there is a focus on expanding the range of detectable biomarkers and integrating luminol-based assays with emerging technologies like smartphone-based diagnostics and wearable sensors.
As the field progresses, researchers are also addressing challenges such as improving the stability of luminol reagents, reducing interference from biological matrices, and developing standardized protocols for clinical validation. The ultimate aim is to translate luminol-based diagnostic technologies from laboratory settings to practical, widely accessible clinical tools that can significantly impact patient care and public health.
The primary objective of luminol research in innovative diagnostic protocols is to harness its exceptional sensitivity and specificity for detecting various biological molecules and cellular components. This aim aligns with the growing demand for rapid, non-invasive, and highly accurate diagnostic tools in healthcare. The potential of luminol to revolutionize early disease detection, monitor treatment efficacy, and enable point-of-care diagnostics has driven intense research efforts in recent years.
Luminol's unique chemiluminescent properties stem from its ability to emit light when oxidized, typically in the presence of an appropriate catalyst. This reaction has been extensively studied and optimized for various applications. In the context of diagnostic protocols, researchers are exploring ways to enhance luminol's sensitivity, improve its selectivity for specific biomarkers, and develop novel detection systems that can capitalize on its light-emitting capabilities.
The technological trajectory of luminol research has seen a shift from traditional forensic applications to more sophisticated biomedical uses. This transition has been facilitated by advancements in related fields such as nanotechnology, microfluidics, and artificial intelligence. These interdisciplinary collaborations have opened new avenues for luminol-based diagnostics, including the development of highly sensitive biosensors, lab-on-a-chip devices, and automated diagnostic platforms.
Current research goals in luminol-based diagnostic protocols include enhancing the signal-to-noise ratio, developing multiplexed detection systems, and creating user-friendly platforms for clinical use. Additionally, there is a focus on expanding the range of detectable biomarkers and integrating luminol-based assays with emerging technologies like smartphone-based diagnostics and wearable sensors.
As the field progresses, researchers are also addressing challenges such as improving the stability of luminol reagents, reducing interference from biological matrices, and developing standardized protocols for clinical validation. The ultimate aim is to translate luminol-based diagnostic technologies from laboratory settings to practical, widely accessible clinical tools that can significantly impact patient care and public health.
Market Analysis for Luminol-Based Diagnostics
The market for luminol-based diagnostics has shown significant growth potential in recent years, driven by increasing demand for sensitive and cost-effective detection methods in various fields. Luminol, a chemiluminescent compound, has found applications in forensic science, medical diagnostics, and environmental monitoring due to its ability to produce a blue light when oxidized.
In the forensic science sector, luminol-based products have become essential tools for crime scene investigators. The global forensic technologies market, which includes luminol-based products, is expected to grow steadily. This growth is attributed to the rising crime rates, advancements in forensic techniques, and increasing government investments in forensic research.
The medical diagnostics sector represents another significant market for luminol-based technologies. Luminol's high sensitivity makes it valuable in detecting trace amounts of blood or other biological materials. This property has led to its incorporation in various diagnostic tests, including those for detecting certain types of cancer, infectious diseases, and autoimmune disorders. The global in vitro diagnostics market, which encompasses luminol-based tests, has been expanding rapidly, driven by the increasing prevalence of chronic and infectious diseases and the growing emphasis on early disease detection.
Environmental monitoring is an emerging application area for luminol-based diagnostics. The compound's ability to detect minute quantities of certain metals and oxidizing agents makes it useful in water quality testing and pollution monitoring. As environmental regulations become more stringent worldwide, the demand for sensitive and reliable detection methods is expected to increase, potentially expanding the market for luminol-based products in this sector.
The market for luminol and its derivatives is also influenced by ongoing research and development activities. Scientists are continually exploring new applications and improving existing techniques, which could open up new market opportunities. For instance, recent studies have investigated the use of luminol in detecting food contaminants and in developing more sensitive biosensors.
However, the market faces certain challenges. The availability of alternative detection methods, such as fluorescence-based techniques, poses competition. Additionally, concerns about false positives in some applications, particularly in forensic settings, have led to debates about the reliability of luminol-based tests in certain contexts.
Despite these challenges, the overall market outlook for luminol-based diagnostics remains positive. The compound's versatility, sensitivity, and relatively low cost continue to make it an attractive option in various fields. As research progresses and new applications emerge, the market is likely to see further expansion and diversification in the coming years.
In the forensic science sector, luminol-based products have become essential tools for crime scene investigators. The global forensic technologies market, which includes luminol-based products, is expected to grow steadily. This growth is attributed to the rising crime rates, advancements in forensic techniques, and increasing government investments in forensic research.
The medical diagnostics sector represents another significant market for luminol-based technologies. Luminol's high sensitivity makes it valuable in detecting trace amounts of blood or other biological materials. This property has led to its incorporation in various diagnostic tests, including those for detecting certain types of cancer, infectious diseases, and autoimmune disorders. The global in vitro diagnostics market, which encompasses luminol-based tests, has been expanding rapidly, driven by the increasing prevalence of chronic and infectious diseases and the growing emphasis on early disease detection.
Environmental monitoring is an emerging application area for luminol-based diagnostics. The compound's ability to detect minute quantities of certain metals and oxidizing agents makes it useful in water quality testing and pollution monitoring. As environmental regulations become more stringent worldwide, the demand for sensitive and reliable detection methods is expected to increase, potentially expanding the market for luminol-based products in this sector.
The market for luminol and its derivatives is also influenced by ongoing research and development activities. Scientists are continually exploring new applications and improving existing techniques, which could open up new market opportunities. For instance, recent studies have investigated the use of luminol in detecting food contaminants and in developing more sensitive biosensors.
However, the market faces certain challenges. The availability of alternative detection methods, such as fluorescence-based techniques, poses competition. Additionally, concerns about false positives in some applications, particularly in forensic settings, have led to debates about the reliability of luminol-based tests in certain contexts.
Despite these challenges, the overall market outlook for luminol-based diagnostics remains positive. The compound's versatility, sensitivity, and relatively low cost continue to make it an attractive option in various fields. As research progresses and new applications emerge, the market is likely to see further expansion and diversification in the coming years.
Current Challenges in Luminol Applications
Despite the widespread use of luminol in forensic science and medical diagnostics, several challenges persist in its application, hindering its full potential in innovative diagnostic protocols. One of the primary issues is the lack of specificity in luminol reactions. While luminol is highly sensitive to blood, it can also react with other substances, such as certain plant materials, cleaning agents, and metal ions, leading to false-positive results. This non-specificity can compromise the accuracy of diagnostic tests and forensic investigations, necessitating additional confirmatory tests.
Another significant challenge is the potential interference of luminol with subsequent DNA analysis. In forensic applications, preserving DNA evidence is crucial, but the use of luminol may affect the quality and quantity of recoverable DNA. This interference can be particularly problematic in cases where limited biological samples are available, potentially compromising the evidentiary value of the crime scene or diagnostic sample.
The stability of luminol solutions presents another hurdle in its application. Luminol solutions tend to degrade over time, especially when exposed to light or heat. This instability can lead to inconsistent results and reduced sensitivity, particularly in field applications where controlled storage conditions may not be feasible. The need for fresh preparation of luminol solutions adds complexity to its use in point-of-care diagnostics or on-site forensic investigations.
Furthermore, the interpretation of luminol-based test results often requires specialized training and experience. The subjective nature of visual assessment of chemiluminescence can lead to variability in results interpretation, especially in low-light conditions or when dealing with trace amounts of target substances. This subjectivity introduces a potential for human error and inconsistency in diagnostic and forensic outcomes.
The environmental and health impacts of luminol usage also pose challenges. While generally considered safe, prolonged exposure to luminol and its reaction products may have potential health risks that are not yet fully understood. Additionally, the disposal of luminol-containing solutions and contaminated materials requires careful consideration to minimize environmental impact, particularly in large-scale or frequent usage scenarios.
Lastly, the integration of luminol-based techniques with emerging technologies presents both opportunities and challenges. While there is potential for enhancing luminol's capabilities through combination with advanced imaging technologies or microfluidic systems, achieving seamless integration and maintaining the simplicity and cost-effectiveness of luminol-based tests remain significant hurdles. These integration efforts must balance the need for improved performance with practical considerations such as ease of use, portability, and affordability in diverse application settings.
Another significant challenge is the potential interference of luminol with subsequent DNA analysis. In forensic applications, preserving DNA evidence is crucial, but the use of luminol may affect the quality and quantity of recoverable DNA. This interference can be particularly problematic in cases where limited biological samples are available, potentially compromising the evidentiary value of the crime scene or diagnostic sample.
The stability of luminol solutions presents another hurdle in its application. Luminol solutions tend to degrade over time, especially when exposed to light or heat. This instability can lead to inconsistent results and reduced sensitivity, particularly in field applications where controlled storage conditions may not be feasible. The need for fresh preparation of luminol solutions adds complexity to its use in point-of-care diagnostics or on-site forensic investigations.
Furthermore, the interpretation of luminol-based test results often requires specialized training and experience. The subjective nature of visual assessment of chemiluminescence can lead to variability in results interpretation, especially in low-light conditions or when dealing with trace amounts of target substances. This subjectivity introduces a potential for human error and inconsistency in diagnostic and forensic outcomes.
The environmental and health impacts of luminol usage also pose challenges. While generally considered safe, prolonged exposure to luminol and its reaction products may have potential health risks that are not yet fully understood. Additionally, the disposal of luminol-containing solutions and contaminated materials requires careful consideration to minimize environmental impact, particularly in large-scale or frequent usage scenarios.
Lastly, the integration of luminol-based techniques with emerging technologies presents both opportunities and challenges. While there is potential for enhancing luminol's capabilities through combination with advanced imaging technologies or microfluidic systems, achieving seamless integration and maintaining the simplicity and cost-effectiveness of luminol-based tests remain significant hurdles. These integration efforts must balance the need for improved performance with practical considerations such as ease of use, portability, and affordability in diverse application settings.
Existing Luminol-Based Diagnostic Protocols
01 Luminol in forensic applications
Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When mixed with an oxidizing agent, it produces a blue chemiluminescence in the presence of iron from hemoglobin. This reaction is highly sensitive and can detect blood even after cleaning attempts.- Luminol in forensic applications: Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When mixed with an oxidizing agent, it produces a blue chemiluminescence in the presence of hemoglobin, allowing investigators to identify blood traces that are invisible to the naked eye. This technique is particularly useful for detecting blood that has been cleaned up or is present on dark surfaces.
- Luminol-based detection systems: Various detection systems incorporate luminol for its chemiluminescent properties. These systems are used in a range of applications, including environmental monitoring, food safety testing, and medical diagnostics. The high sensitivity of luminol-based detection allows for the identification of minute quantities of target substances, making it valuable in analytical chemistry and quality control processes.
- Luminol derivatives and modifications: Research has focused on developing luminol derivatives and modifications to enhance its performance or tailor its properties for specific applications. These modifications can include changes to the molecular structure to improve solubility, increase light output, or alter the emission wavelength. Such advancements have expanded the utility of luminol-based systems in various scientific and industrial fields.
- Luminol in biomedical research: Luminol plays a significant role in biomedical research, particularly in studying cellular processes and immune responses. It is used to detect and quantify reactive oxygen species and to investigate neutrophil function. The chemiluminescent properties of luminol allow researchers to visualize and measure biological processes in real-time, contributing to advancements in understanding disease mechanisms and developing new therapies.
- Industrial applications of luminol: Beyond its use in forensics and research, luminol has found applications in various industrial sectors. It is used in the development of glow sticks and other chemiluminescent products for emergency lighting and entertainment purposes. In the manufacturing industry, luminol-based systems are employed for quality control, leak detection, and monitoring of industrial processes, leveraging its high sensitivity to detect contaminants or process irregularities.
02 Luminol-based detection systems
Various detection systems incorporate luminol for its chemiluminescent properties. These systems are used in environmental monitoring, food safety testing, and medical diagnostics. The high sensitivity of luminol allows for the detection of minute quantities of target substances.Expand Specific Solutions03 Luminol derivatives and modifications
Research focuses on developing luminol derivatives and modifications to enhance its properties. These improvements aim to increase sensitivity, stability, and specificity for various applications. Modified luminol compounds can offer advantages such as longer-lasting luminescence or altered emission spectra.Expand Specific Solutions04 Luminol in analytical chemistry
Luminol is extensively used in analytical chemistry for quantitative and qualitative analysis. It serves as a reagent in flow injection analysis, high-performance liquid chromatography, and other analytical techniques. The chemiluminescent reaction of luminol allows for highly sensitive detection of various analytes.Expand Specific Solutions05 Industrial and environmental applications of luminol
Luminol finds applications in industrial processes and environmental monitoring. It is used in the detection of metal ions, oxidizing agents, and other substances in industrial effluents and natural water bodies. Luminol-based systems can also be employed for monitoring air quality and detecting pollutants.Expand Specific Solutions
Key Players in Luminol Research and Development
The research on luminol in innovative diagnostic protocols is in a dynamic phase, with the market showing significant growth potential. The technology is advancing rapidly, driven by increasing demand for sensitive and specific diagnostic tools. Key players like Roche Diagnostics, PerkinElmer Health Sciences, and FUJIFILM Corp are leading the field with their established expertise in diagnostic technologies. Emerging companies such as Alverix and EpimAb Biotherapeutics are also making notable contributions, particularly in developing portable and cost-effective solutions. Academic institutions like Washington University in St. Louis and New York University are fostering innovation through collaborative research efforts. The competitive landscape is characterized by a mix of established pharmaceutical giants and agile biotech startups, indicating a mature yet evolving market with ample room for technological advancements and market expansion.
Washington University in St. Louis
Technical Solution: Researchers at Washington University in St. Louis have been investigating the use of luminol in novel diagnostic protocols for neurodegenerative diseases. Their approach involves detecting specific protein aggregates associated with conditions like Alzheimer's and Parkinson's disease using luminol-based probes. The team has developed a method that combines luminol chemiluminescence with aptamer technology, achieving a detection sensitivity of 10 pM for amyloid-beta oligomers [5]. They have also explored the use of luminol in conjunction with nanoparticles for enhanced signal amplification, potentially allowing for earlier disease detection [6].
Strengths: High sensitivity for specific protein targets, potential for early disease detection. Weaknesses: Currently limited to research settings, may require further validation for clinical use.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences has focused on developing luminol-based diagnostic protocols for point-of-care testing. Their approach utilizes microfluidic chips with integrated luminol reagents for rapid detection of various analytes. The company has reported a detection limit in the femtomolar range for certain biomarkers using this technology [2]. They have also explored the use of luminol in conjunction with aptamer-based recognition elements, allowing for highly specific detection of pathogens and biomarkers. Revvity's research has shown promising results in detecting foodborne pathogens with a turnaround time of less than 30 minutes [4].
Strengths: Rapid results, potential for field use, and high specificity. Weaknesses: May have limitations in detecting multiple analytes simultaneously.
Breakthrough Luminol Innovations for Diagnostics
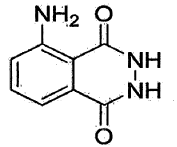
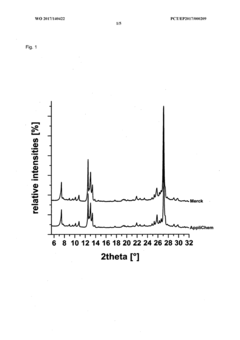
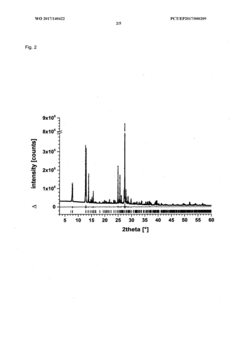
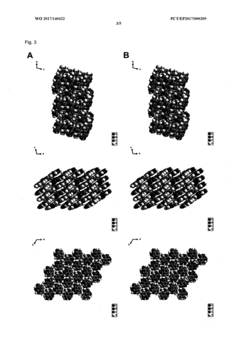
Method for producing a crystalline form of 5-amino-2,3-dihydrophthalazine-1,4-dione
PatentWO2017140422A1
Innovation
- A method involving dissolving 5-amino-2,3-dihydrophthalazine-1,4-dione in a refluxing ethanol-water solution, cooling, separating the precipitated crystals, and drying to produce a phase-pure crystalline form of luminol, which can be resuspended and washed for enhanced purity.
Photodynamic therapy using chemiluminescence and a ligand-photosensitiser conjugate
PatentInactiveUS20100297762A1
Innovation
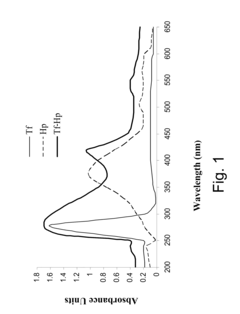
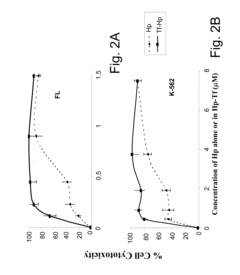
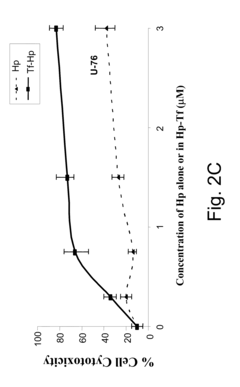
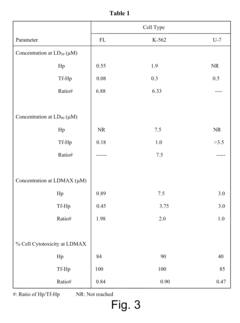
- A method involving a ligand-toxin conjugate (LTC) comprising a photosensitizer like hematoporphyrin conjugated with transferrin, combined with a chemiluminescent agent such as luminol, which activates the photosensitizer intracellularly to produce reactive oxygen species, thereby enhancing target cell destruction without requiring external light.
Regulatory Framework for Diagnostic Reagents
The regulatory framework for diagnostic reagents, including luminol-based systems, is a critical aspect of their development and implementation in innovative diagnostic protocols. In the United States, the Food and Drug Administration (FDA) oversees the regulation of diagnostic reagents through the Center for Devices and Radiological Health (CDRH). These reagents are typically classified as medical devices and are subject to premarket notification (510(k)) or premarket approval (PMA) processes, depending on their risk classification.
For luminol-based diagnostic protocols, the regulatory pathway often involves demonstrating substantial equivalence to predicate devices already on the market. This process requires extensive documentation of the reagent's safety, efficacy, and quality control measures. Manufacturers must adhere to Good Manufacturing Practices (GMP) and implement robust quality management systems to ensure consistent production and performance of the diagnostic reagents.
In the European Union, diagnostic reagents fall under the In Vitro Diagnostic Medical Devices Regulation (IVDR), which came into full effect in May 2022. This regulation introduces more stringent requirements for clinical evidence, post-market surveillance, and traceability. Luminol-based diagnostic protocols would likely be classified as Class B or C devices under the IVDR, necessitating conformity assessment by a notified body.
Japan's regulatory framework for diagnostic reagents is overseen by the Pharmaceuticals and Medical Devices Agency (PMDA). The classification and approval process for luminol-based diagnostics would depend on their intended use and risk level, with higher-risk devices requiring more extensive clinical data and review.
Globally, the International Medical Device Regulators Forum (IMDRF) plays a crucial role in harmonizing regulatory approaches across different jurisdictions. Their guidelines on Software as a Medical Device (SaMD) and cybersecurity are particularly relevant for innovative diagnostic protocols that may incorporate digital elements alongside luminol-based detection systems.
Regulatory bodies are increasingly focusing on the validation of novel biomarkers and the analytical performance of diagnostic tests. For luminol-based protocols, this may involve demonstrating the specificity and sensitivity of the chemiluminescent reaction in various biological matrices. Additionally, regulators are placing greater emphasis on real-world evidence and post-market surveillance data to ensure the ongoing safety and effectiveness of diagnostic reagents.
As research on luminol in innovative diagnostic protocols advances, developers must navigate these complex regulatory landscapes. Early engagement with regulatory authorities through pre-submission meetings can provide valuable guidance on study design, data requirements, and potential regulatory pathways. This proactive approach can help streamline the development process and increase the likelihood of successful market authorization for novel luminol-based diagnostic technologies.
For luminol-based diagnostic protocols, the regulatory pathway often involves demonstrating substantial equivalence to predicate devices already on the market. This process requires extensive documentation of the reagent's safety, efficacy, and quality control measures. Manufacturers must adhere to Good Manufacturing Practices (GMP) and implement robust quality management systems to ensure consistent production and performance of the diagnostic reagents.
In the European Union, diagnostic reagents fall under the In Vitro Diagnostic Medical Devices Regulation (IVDR), which came into full effect in May 2022. This regulation introduces more stringent requirements for clinical evidence, post-market surveillance, and traceability. Luminol-based diagnostic protocols would likely be classified as Class B or C devices under the IVDR, necessitating conformity assessment by a notified body.
Japan's regulatory framework for diagnostic reagents is overseen by the Pharmaceuticals and Medical Devices Agency (PMDA). The classification and approval process for luminol-based diagnostics would depend on their intended use and risk level, with higher-risk devices requiring more extensive clinical data and review.
Globally, the International Medical Device Regulators Forum (IMDRF) plays a crucial role in harmonizing regulatory approaches across different jurisdictions. Their guidelines on Software as a Medical Device (SaMD) and cybersecurity are particularly relevant for innovative diagnostic protocols that may incorporate digital elements alongside luminol-based detection systems.
Regulatory bodies are increasingly focusing on the validation of novel biomarkers and the analytical performance of diagnostic tests. For luminol-based protocols, this may involve demonstrating the specificity and sensitivity of the chemiluminescent reaction in various biological matrices. Additionally, regulators are placing greater emphasis on real-world evidence and post-market surveillance data to ensure the ongoing safety and effectiveness of diagnostic reagents.
As research on luminol in innovative diagnostic protocols advances, developers must navigate these complex regulatory landscapes. Early engagement with regulatory authorities through pre-submission meetings can provide valuable guidance on study design, data requirements, and potential regulatory pathways. This proactive approach can help streamline the development process and increase the likelihood of successful market authorization for novel luminol-based diagnostic technologies.
Biosafety Considerations in Luminol Usage
The use of luminol in innovative diagnostic protocols necessitates careful consideration of biosafety aspects to ensure the protection of researchers, laboratory personnel, and the environment. Luminol, while generally considered to have low toxicity, still requires proper handling and safety measures due to its chemical nature and potential reactivity.
One primary biosafety concern is the potential for skin and eye irritation upon direct contact with luminol. Laboratory personnel should wear appropriate personal protective equipment (PPE), including gloves, lab coats, and safety goggles, when handling luminol or its solutions. Proper ventilation in the laboratory is also crucial to minimize inhalation risks, especially when working with powdered luminol or preparing solutions.
The alkaline conditions often required for luminol reactions present another safety consideration. Many luminol-based diagnostic protocols involve the use of hydrogen peroxide and sodium hydroxide, which can be corrosive and harmful if mishandled. Strict adherence to proper storage, handling, and disposal protocols for these reagents is essential to maintain a safe working environment.
Environmental considerations are also paramount in luminol usage. While luminol itself is not considered highly toxic to aquatic life, the chemicals used in conjunction with it may pose environmental risks. Proper disposal of luminol-containing waste is necessary to prevent contamination of water systems or soil. Laboratories should follow local regulations and guidelines for the disposal of chemical waste.
The potential for false-positive results in forensic applications of luminol highlights another biosafety aspect. Misinterpretation of luminol reactions could lead to incorrect conclusions in criminal investigations, emphasizing the need for proper training and understanding of the limitations of luminol-based tests.
In the context of innovative diagnostic protocols, the integration of luminol into new testing platforms or devices may introduce additional biosafety considerations. For instance, the development of portable luminol-based diagnostic tools for field use would require careful design to prevent accidental exposure or release of reagents.
Lastly, the storage and transportation of luminol and associated chemicals for diagnostic purposes must adhere to safety regulations. Proper labeling, secure packaging, and compliance with transportation guidelines are essential to prevent accidents and ensure the integrity of the diagnostic materials.
One primary biosafety concern is the potential for skin and eye irritation upon direct contact with luminol. Laboratory personnel should wear appropriate personal protective equipment (PPE), including gloves, lab coats, and safety goggles, when handling luminol or its solutions. Proper ventilation in the laboratory is also crucial to minimize inhalation risks, especially when working with powdered luminol or preparing solutions.
The alkaline conditions often required for luminol reactions present another safety consideration. Many luminol-based diagnostic protocols involve the use of hydrogen peroxide and sodium hydroxide, which can be corrosive and harmful if mishandled. Strict adherence to proper storage, handling, and disposal protocols for these reagents is essential to maintain a safe working environment.
Environmental considerations are also paramount in luminol usage. While luminol itself is not considered highly toxic to aquatic life, the chemicals used in conjunction with it may pose environmental risks. Proper disposal of luminol-containing waste is necessary to prevent contamination of water systems or soil. Laboratories should follow local regulations and guidelines for the disposal of chemical waste.
The potential for false-positive results in forensic applications of luminol highlights another biosafety aspect. Misinterpretation of luminol reactions could lead to incorrect conclusions in criminal investigations, emphasizing the need for proper training and understanding of the limitations of luminol-based tests.
In the context of innovative diagnostic protocols, the integration of luminol into new testing platforms or devices may introduce additional biosafety considerations. For instance, the development of portable luminol-based diagnostic tools for field use would require careful design to prevent accidental exposure or release of reagents.
Lastly, the storage and transportation of luminol and associated chemicals for diagnostic purposes must adhere to safety regulations. Proper labeling, secure packaging, and compliance with transportation guidelines are essential to prevent accidents and ensure the integrity of the diagnostic materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!