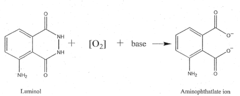
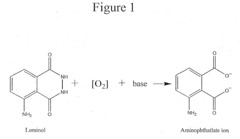
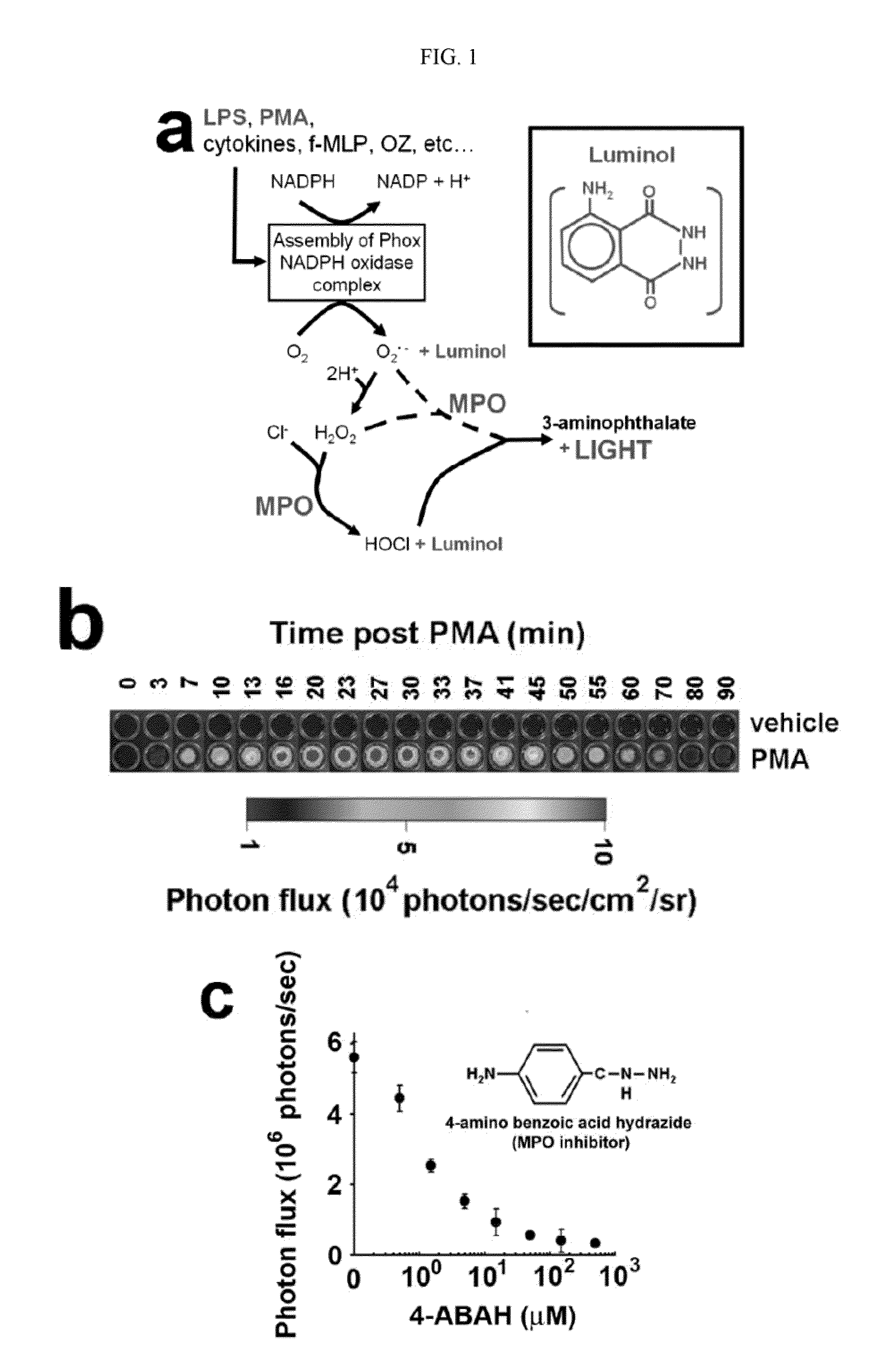
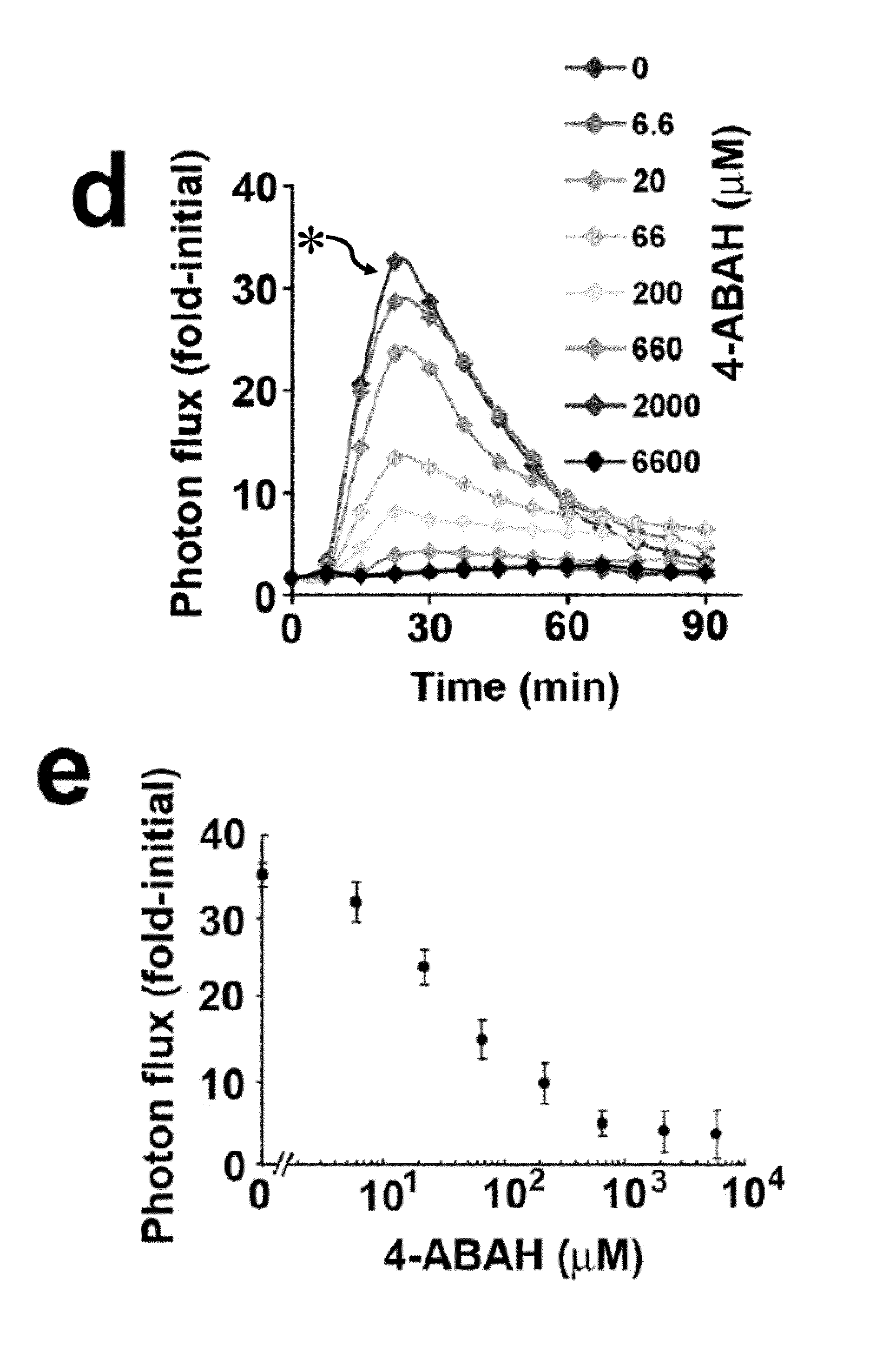
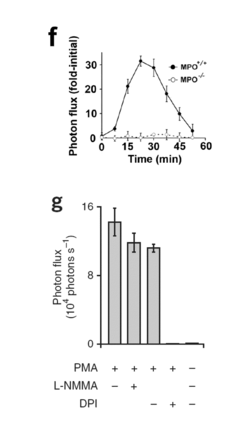
How Luminol Fosters Growth in Analytical Chemistry?
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Background and Objectives
Luminol, a chemiluminescent compound, has played a pivotal role in the evolution of analytical chemistry since its discovery in the early 20th century. This versatile molecule has become a cornerstone in various analytical techniques, particularly in forensic science, biochemistry, and environmental monitoring. The journey of luminol from a curious chemical phenomenon to a powerful analytical tool exemplifies the dynamic nature of scientific progress and its impact on practical applications.
The primary objective in exploring luminol's potential in analytical chemistry is to enhance detection sensitivity, specificity, and reliability across a wide range of applications. Researchers aim to leverage luminol's unique chemiluminescent properties to develop more sophisticated analytical methods, pushing the boundaries of what can be detected and measured in complex chemical and biological systems.
As analytical chemistry continues to advance, luminol serves as a model compound for understanding and exploiting chemiluminescence reactions. The ongoing research focuses on optimizing reaction conditions, improving signal intensity, and expanding the scope of detectable analytes. These efforts are driven by the need for more sensitive and accurate analytical techniques in fields such as medical diagnostics, environmental monitoring, and industrial quality control.
The evolution of luminol-based analytical methods has been marked by significant milestones. From its initial use in blood detection at crime scenes to its application in immunoassays and enzyme activity measurements, luminol has consistently demonstrated its versatility. The development of enhanced luminol derivatives and novel reaction systems has further expanded its analytical capabilities, enabling the detection of trace amounts of various substances with unprecedented sensitivity.
Looking ahead, the objectives for luminol research in analytical chemistry are multifaceted. Scientists are exploring ways to integrate luminol-based detection systems with advanced technologies such as microfluidics and nanotechnology. These efforts aim to create more compact, efficient, and sensitive analytical devices. Additionally, there is a growing interest in developing environmentally friendly and sustainable luminol-based analytical methods, aligning with the broader trend towards green chemistry.
The pursuit of these objectives not only drives innovation in analytical chemistry but also fosters interdisciplinary collaboration. As luminol continues to bridge gaps between chemistry, biology, and materials science, it catalyzes the growth of analytical chemistry as a field, inspiring new research directions and technological advancements that promise to revolutionize chemical analysis across various sectors.
The primary objective in exploring luminol's potential in analytical chemistry is to enhance detection sensitivity, specificity, and reliability across a wide range of applications. Researchers aim to leverage luminol's unique chemiluminescent properties to develop more sophisticated analytical methods, pushing the boundaries of what can be detected and measured in complex chemical and biological systems.
As analytical chemistry continues to advance, luminol serves as a model compound for understanding and exploiting chemiluminescence reactions. The ongoing research focuses on optimizing reaction conditions, improving signal intensity, and expanding the scope of detectable analytes. These efforts are driven by the need for more sensitive and accurate analytical techniques in fields such as medical diagnostics, environmental monitoring, and industrial quality control.
The evolution of luminol-based analytical methods has been marked by significant milestones. From its initial use in blood detection at crime scenes to its application in immunoassays and enzyme activity measurements, luminol has consistently demonstrated its versatility. The development of enhanced luminol derivatives and novel reaction systems has further expanded its analytical capabilities, enabling the detection of trace amounts of various substances with unprecedented sensitivity.
Looking ahead, the objectives for luminol research in analytical chemistry are multifaceted. Scientists are exploring ways to integrate luminol-based detection systems with advanced technologies such as microfluidics and nanotechnology. These efforts aim to create more compact, efficient, and sensitive analytical devices. Additionally, there is a growing interest in developing environmentally friendly and sustainable luminol-based analytical methods, aligning with the broader trend towards green chemistry.
The pursuit of these objectives not only drives innovation in analytical chemistry but also fosters interdisciplinary collaboration. As luminol continues to bridge gaps between chemistry, biology, and materials science, it catalyzes the growth of analytical chemistry as a field, inspiring new research directions and technological advancements that promise to revolutionize chemical analysis across various sectors.
Market Analysis for Luminol Applications
The market for luminol applications in analytical chemistry has experienced significant growth in recent years, driven by increasing demand for sensitive and reliable detection methods across various industries. Luminol, a versatile chemiluminescent compound, has found widespread use in forensic science, environmental monitoring, and biomedical research, contributing to the expansion of its market potential.
In the forensic science sector, luminol remains a crucial tool for crime scene investigators, enabling the detection of trace amounts of blood even after attempts to clean or remove evidence. This application has led to a steady demand from law enforcement agencies and forensic laboratories worldwide. The market for luminol in this sector is expected to grow as advancements in forensic techniques continue to emphasize the importance of chemical analysis in criminal investigations.
Environmental monitoring represents another key market for luminol applications. The compound's ability to detect and quantify various pollutants, particularly heavy metals and oxidizing agents in water samples, has made it invaluable in assessing water quality and environmental contamination. As global concerns about environmental pollution intensify, the demand for luminol-based detection methods in this sector is projected to increase substantially.
In the biomedical research field, luminol has found applications in immunoassays, enzyme activity measurements, and the study of cellular processes involving reactive oxygen species. The growing focus on personalized medicine and the need for more sensitive diagnostic tools have further boosted the demand for luminol-based techniques in this sector. Research institutions, pharmaceutical companies, and diagnostic laboratories are key consumers driving market growth in this area.
The industrial sector also presents opportunities for luminol applications, particularly in quality control processes where the detection of contaminants or specific chemical compounds is critical. Food and beverage, pharmaceutical, and chemical industries are increasingly adopting luminol-based analytical methods to ensure product safety and compliance with regulatory standards.
Geographically, North America and Europe currently dominate the market for luminol applications, owing to their advanced research infrastructure and stringent regulatory environments. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing investments in forensic science, environmental protection, and biomedical research in countries like China, Japan, and India.
The market for luminol applications is characterized by a mix of established chemical suppliers and specialized analytical instrument manufacturers. Key players are focusing on developing more sensitive and specific luminol-based detection kits and instruments to maintain their competitive edge. Collaborations between academic institutions and industry partners are also contributing to the development of novel applications, further expanding the market potential of luminol in analytical chemistry.
In the forensic science sector, luminol remains a crucial tool for crime scene investigators, enabling the detection of trace amounts of blood even after attempts to clean or remove evidence. This application has led to a steady demand from law enforcement agencies and forensic laboratories worldwide. The market for luminol in this sector is expected to grow as advancements in forensic techniques continue to emphasize the importance of chemical analysis in criminal investigations.
Environmental monitoring represents another key market for luminol applications. The compound's ability to detect and quantify various pollutants, particularly heavy metals and oxidizing agents in water samples, has made it invaluable in assessing water quality and environmental contamination. As global concerns about environmental pollution intensify, the demand for luminol-based detection methods in this sector is projected to increase substantially.
In the biomedical research field, luminol has found applications in immunoassays, enzyme activity measurements, and the study of cellular processes involving reactive oxygen species. The growing focus on personalized medicine and the need for more sensitive diagnostic tools have further boosted the demand for luminol-based techniques in this sector. Research institutions, pharmaceutical companies, and diagnostic laboratories are key consumers driving market growth in this area.
The industrial sector also presents opportunities for luminol applications, particularly in quality control processes where the detection of contaminants or specific chemical compounds is critical. Food and beverage, pharmaceutical, and chemical industries are increasingly adopting luminol-based analytical methods to ensure product safety and compliance with regulatory standards.
Geographically, North America and Europe currently dominate the market for luminol applications, owing to their advanced research infrastructure and stringent regulatory environments. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing investments in forensic science, environmental protection, and biomedical research in countries like China, Japan, and India.
The market for luminol applications is characterized by a mix of established chemical suppliers and specialized analytical instrument manufacturers. Key players are focusing on developing more sensitive and specific luminol-based detection kits and instruments to maintain their competitive edge. Collaborations between academic institutions and industry partners are also contributing to the development of novel applications, further expanding the market potential of luminol in analytical chemistry.
Current Challenges in Luminol Chemistry
Despite its widespread use in analytical chemistry, luminol faces several significant challenges that hinder its full potential. One of the primary issues is the lack of specificity in luminol reactions. While luminol is highly sensitive to various oxidizing agents, it can react with a broad range of substances, leading to potential false positives in forensic and analytical applications. This non-specificity can complicate the interpretation of results, especially in complex matrices or environments with multiple interfering compounds.
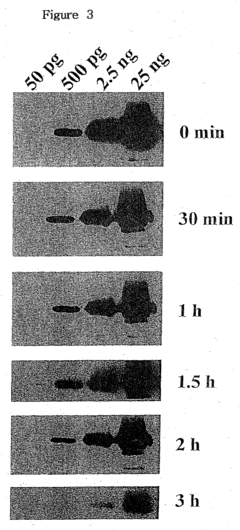
Another challenge is the relatively short duration of the luminol chemiluminescence reaction. The light emission, though intense, typically lasts only for a few seconds. This brief reaction time can make it difficult to capture and analyze the luminescence signal accurately, particularly in field applications or when dealing with large sample areas. Researchers are continually seeking ways to prolong the emission time without compromising the sensitivity of the reaction.
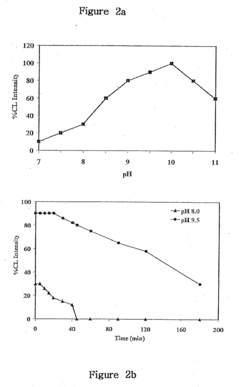
The pH dependency of the luminol reaction presents another hurdle. The optimal pH range for luminol chemiluminescence is typically between 10 and 11, which can be problematic when analyzing samples with different pH levels or when working in environments where maintaining a consistent pH is challenging. This pH sensitivity can affect the reliability and reproducibility of luminol-based assays across various analytical scenarios.
Stability issues also plague luminol chemistry. The luminol reagent and its working solutions can degrade over time, especially when exposed to light or elevated temperatures. This instability necessitates frequent preparation of fresh solutions and careful storage conditions, which can be impractical in some analytical settings, particularly for long-term or field-based studies.
Furthermore, the quantitative aspects of luminol chemistry remain a challenge. While luminol can provide excellent qualitative results, achieving accurate and reproducible quantitative measurements can be difficult. Factors such as reaction kinetics, substrate concentration, and the presence of catalysts or enhancers can all influence the intensity and duration of the chemiluminescence, making standardization and calibration complex tasks.
Lastly, the environmental and health concerns associated with luminol and its reaction products pose challenges in certain applications. Although luminol itself is not highly toxic, some of the oxidizing agents used in conjunction with it, such as hydrogen peroxide, can be hazardous. Additionally, the alkaline conditions required for the reaction can be corrosive. These safety considerations necessitate careful handling and disposal procedures, which can limit the widespread adoption of luminol-based techniques in some settings.
Another challenge is the relatively short duration of the luminol chemiluminescence reaction. The light emission, though intense, typically lasts only for a few seconds. This brief reaction time can make it difficult to capture and analyze the luminescence signal accurately, particularly in field applications or when dealing with large sample areas. Researchers are continually seeking ways to prolong the emission time without compromising the sensitivity of the reaction.
The pH dependency of the luminol reaction presents another hurdle. The optimal pH range for luminol chemiluminescence is typically between 10 and 11, which can be problematic when analyzing samples with different pH levels or when working in environments where maintaining a consistent pH is challenging. This pH sensitivity can affect the reliability and reproducibility of luminol-based assays across various analytical scenarios.
Stability issues also plague luminol chemistry. The luminol reagent and its working solutions can degrade over time, especially when exposed to light or elevated temperatures. This instability necessitates frequent preparation of fresh solutions and careful storage conditions, which can be impractical in some analytical settings, particularly for long-term or field-based studies.
Furthermore, the quantitative aspects of luminol chemistry remain a challenge. While luminol can provide excellent qualitative results, achieving accurate and reproducible quantitative measurements can be difficult. Factors such as reaction kinetics, substrate concentration, and the presence of catalysts or enhancers can all influence the intensity and duration of the chemiluminescence, making standardization and calibration complex tasks.
Lastly, the environmental and health concerns associated with luminol and its reaction products pose challenges in certain applications. Although luminol itself is not highly toxic, some of the oxidizing agents used in conjunction with it, such as hydrogen peroxide, can be hazardous. Additionally, the alkaline conditions required for the reaction can be corrosive. These safety considerations necessitate careful handling and disposal procedures, which can limit the widespread adoption of luminol-based techniques in some settings.
Current Luminol-based Analytical Methods
01 Luminol synthesis and production methods
Various methods for synthesizing and producing luminol are described, including chemical reactions, purification processes, and optimization techniques to improve yield and quality. These methods aim to enhance the efficiency of luminol production for various applications.- Luminol synthesis and production methods: Various methods for synthesizing and producing luminol are described, including chemical reactions, purification processes, and optimization techniques to improve yield and quality. These methods aim to enhance the efficiency of luminol production for use in chemiluminescence applications.
- Luminol-based detection systems: Development of detection systems utilizing luminol's chemiluminescent properties for various applications, such as forensic analysis, environmental monitoring, and medical diagnostics. These systems often involve integrating luminol with other reagents and detection equipment to enhance sensitivity and specificity.
- Luminol derivatives and modifications: Research into the development of luminol derivatives and modifications to enhance its properties, such as increased light output, stability, or specificity for certain applications. This includes chemical modifications to the luminol structure and the creation of novel compounds based on luminol.
- Luminol in biological and medical applications: Exploration of luminol's use in biological and medical fields, including its application in imaging techniques, cellular studies, and diagnostic assays. This involves developing methods to incorporate luminol into biological systems and optimize its performance in these contexts.
- Luminol-based analytical techniques: Development of analytical techniques and methodologies that utilize luminol's chemiluminescent properties for quantitative and qualitative analysis in various fields, such as environmental monitoring, food safety, and industrial quality control. These techniques often involve optimizing reaction conditions and detection methods to improve sensitivity and accuracy.
02 Luminol-based detection systems
Development of detection systems utilizing luminol's chemiluminescent properties for various applications, such as forensic analysis, environmental monitoring, and medical diagnostics. These systems often involve integrating luminol with other reagents and detection equipment to enhance sensitivity and specificity.Expand Specific Solutions03 Luminol derivatives and modifications
Research into the development of luminol derivatives and modifications to enhance its properties, such as increased luminescence intensity, improved stability, or altered emission spectra. These modifications aim to expand the range of applications for luminol-based compounds.Expand Specific Solutions04 Luminol in biological and medical applications
Exploration of luminol's use in biological and medical fields, including its application in imaging techniques, cellular studies, and diagnostic assays. This involves developing methods to incorporate luminol into biological systems and optimize its performance in these contexts.Expand Specific Solutions05 Luminol-based analytical techniques
Development of analytical techniques leveraging luminol's chemiluminescent properties for quantitative and qualitative analysis in various fields, such as environmental monitoring, food safety, and industrial quality control. These techniques often involve optimizing reaction conditions and detection methods to improve sensitivity and accuracy.Expand Specific Solutions
Key Players in Luminol Research
The luminol market in analytical chemistry is in a growth phase, driven by increasing demand for forensic applications and clinical diagnostics. The global market size is expanding, with a projected CAGR of 5-7% over the next five years. Technologically, luminol-based chemiluminescence is mature but still evolving, with companies like Promega Corp. and Cyanagen Srl leading in reagent development. Academic institutions such as Washington University in St. Louis and Fudan University are advancing fundamental research, while firms like Chemclin Diagnostics and Alverix, Inc. are innovating in instrument design and point-of-care applications, indicating a dynamic competitive landscape with opportunities for both established players and new entrants.
Promega Corp.
Technical Solution: Promega has developed advanced luminol-based chemiluminescence systems for analytical chemistry applications. Their technology utilizes enhanced luminol derivatives and optimized reaction conditions to achieve higher sensitivity and longer-lasting light emission. The company's luminol reagents are designed for use in various analytical techniques, including Western blotting, ELISA, and forensic blood detection. Promega's luminol products incorporate stabilizers to extend shelf life and reduce background noise, resulting in improved signal-to-noise ratios[1][3]. Their formulations also include enhancers that amplify the light output, allowing for detection of trace amounts of analytes in complex biological samples.
Strengths: High sensitivity, long-lasting emission, versatile applications. Weaknesses: May require specialized equipment, potential for interference in complex matrices.
Cyanagen Srl
Technical Solution: Cyanagen has innovated in the field of luminol-based chemiluminescence with their AURORA platform. This technology focuses on enhancing the quantum yield and kinetics of the luminol reaction for improved analytical performance. The AURORA system incorporates novel acridinium ester derivatives that offer superior light output compared to traditional luminol[2]. Cyanagen's approach includes the development of specialized triggers and catalysts that allow for precise control over the chemiluminescence reaction, enabling both flash and glow-type emission profiles. Their reagents are optimized for use in immunoassays, nucleic acid detection, and high-throughput screening applications, providing researchers with tools for ultra-sensitive detection in analytical chemistry[4].
Strengths: Ultra-high sensitivity, controllable reaction kinetics, broad application range. Weaknesses: Proprietary reagents may be more expensive, requires specific handling protocols.
Innovative Luminol Applications
Method for improving chemiluminescent signal
PatentInactiveUS20090233369A1
Innovation
- A reaction buffer with an alkaline pH range of 9 to 10, combined with luminol, coumaric acid, and a peroxide, provides a maximal and long-lasting chemiluminescent signal by stabilizing aminothalate ions, improving the signal-to-background ratio.
Bioluminescence imaging of myeloperoxidase activity in vivo, methods, compositions and apparatuses therefor
PatentInactiveUS20110250145A1
Innovation
- The development of methods for non-invasive imaging of MPO activity using luminogenic-optical probes that emit light upon contact with oxidizing agents, allowing for the visualization of MPO activity in vivo, particularly through bioluminescence imaging (BLI) techniques.
Environmental Impact of Luminol Use
The use of luminol in analytical chemistry has raised concerns about its potential environmental impact. While luminol has proven to be a valuable tool in forensic science and chemical analysis, its widespread application necessitates a careful examination of its ecological footprint.
One of the primary environmental considerations is the persistence of luminol in aquatic ecosystems. When luminol is used in crime scene investigations or laboratory settings, residual amounts may find their way into water systems through runoff or improper disposal. Studies have shown that luminol can remain stable in water for extended periods, potentially affecting aquatic organisms and disrupting local ecosystems.
The biodegradation of luminol in natural environments is another area of concern. Research indicates that luminol does not readily break down in soil and water, leading to potential accumulation over time. This persistence raises questions about long-term effects on soil microorganisms and plant life, which play crucial roles in maintaining ecological balance.
Furthermore, the production process of luminol involves the use of various chemicals and solvents, some of which may have their own environmental implications. The manufacturing and disposal of these precursor materials contribute to the overall environmental footprint of luminol use in analytical chemistry.
There is also growing interest in understanding the potential for bioaccumulation of luminol in food chains. While current evidence is limited, some researchers suggest that luminol could be absorbed by aquatic plants and animals, potentially leading to concentration in higher trophic levels. This aspect requires further investigation to assess any potential risks to wildlife and human health.
The chemiluminescent reaction of luminol, while useful for detection purposes, generates byproducts that may have environmental consequences. These reaction products, including oxidized forms of luminol and other intermediates, need to be studied for their potential toxicity and environmental fate.
To address these environmental concerns, efforts are being made to develop more eco-friendly alternatives to luminol or to improve its formulation for reduced environmental impact. Some researchers are exploring biodegradable variants or investigating ways to enhance the efficiency of luminol reactions, thereby reducing the amount needed for analytical purposes.
In conclusion, while luminol has significantly contributed to advancements in analytical chemistry, its environmental impact cannot be overlooked. Continued research is essential to fully understand and mitigate any potential negative effects, ensuring that the benefits of luminol use are balanced with responsible environmental stewardship.
One of the primary environmental considerations is the persistence of luminol in aquatic ecosystems. When luminol is used in crime scene investigations or laboratory settings, residual amounts may find their way into water systems through runoff or improper disposal. Studies have shown that luminol can remain stable in water for extended periods, potentially affecting aquatic organisms and disrupting local ecosystems.
The biodegradation of luminol in natural environments is another area of concern. Research indicates that luminol does not readily break down in soil and water, leading to potential accumulation over time. This persistence raises questions about long-term effects on soil microorganisms and plant life, which play crucial roles in maintaining ecological balance.
Furthermore, the production process of luminol involves the use of various chemicals and solvents, some of which may have their own environmental implications. The manufacturing and disposal of these precursor materials contribute to the overall environmental footprint of luminol use in analytical chemistry.
There is also growing interest in understanding the potential for bioaccumulation of luminol in food chains. While current evidence is limited, some researchers suggest that luminol could be absorbed by aquatic plants and animals, potentially leading to concentration in higher trophic levels. This aspect requires further investigation to assess any potential risks to wildlife and human health.
The chemiluminescent reaction of luminol, while useful for detection purposes, generates byproducts that may have environmental consequences. These reaction products, including oxidized forms of luminol and other intermediates, need to be studied for their potential toxicity and environmental fate.
To address these environmental concerns, efforts are being made to develop more eco-friendly alternatives to luminol or to improve its formulation for reduced environmental impact. Some researchers are exploring biodegradable variants or investigating ways to enhance the efficiency of luminol reactions, thereby reducing the amount needed for analytical purposes.
In conclusion, while luminol has significantly contributed to advancements in analytical chemistry, its environmental impact cannot be overlooked. Continued research is essential to fully understand and mitigate any potential negative effects, ensuring that the benefits of luminol use are balanced with responsible environmental stewardship.
Luminol in Forensic Science
Luminol has become an indispensable tool in forensic science, revolutionizing the field of crime scene investigation. This chemiluminescent compound has proven particularly effective in detecting and visualizing traces of blood, even in minute quantities or after attempts to clean or conceal evidence. The application of luminol in forensic contexts relies on its ability to react with the iron in hemoglobin, producing a distinctive blue glow that can be photographed and documented as evidence.
The use of luminol in forensic science extends beyond mere blood detection. It has been instrumental in reconstructing crime scenes, providing investigators with crucial information about the sequence of events and the potential movements of both victims and perpetrators. The compound's sensitivity allows for the detection of blood traces that may have been overlooked by visual inspection alone, thereby expanding the scope of evidence collection and analysis.
Forensic scientists have developed various techniques to optimize the use of luminol in crime scene investigations. These include methods to enhance the luminol reaction's specificity and sensitivity, as well as protocols for proper application and documentation. The development of specialized equipment, such as high-sensitivity cameras and light-filtering devices, has further improved the ability to capture and analyze luminol-induced chemiluminescence in challenging environments.
The integration of luminol into forensic workflows has also spurred advancements in related analytical techniques. For instance, the need for confirmatory tests following luminol reactions has led to improvements in DNA analysis methods, allowing for the extraction and amplification of genetic material from increasingly smaller samples. This synergy between luminol-based detection and subsequent analytical procedures has significantly enhanced the overall capabilities of forensic laboratories.
However, the use of luminol in forensic science is not without challenges. False positives can occur due to the compound's reactivity with substances other than blood, necessitating careful interpretation of results. Additionally, the potential for luminol to interfere with subsequent DNA analysis has prompted research into alternative formulations and application methods that minimize such risks while maintaining detection efficacy.
The ongoing research and development in luminol-based forensic techniques continue to push the boundaries of analytical chemistry. Scientists are exploring ways to increase the specificity of the luminol reaction, develop more stable and long-lasting formulations, and create portable, user-friendly detection systems for field use. These efforts not only enhance the capabilities of forensic science but also contribute to broader advancements in analytical chemistry, particularly in the areas of trace detection and chemiluminescence-based assays.
The use of luminol in forensic science extends beyond mere blood detection. It has been instrumental in reconstructing crime scenes, providing investigators with crucial information about the sequence of events and the potential movements of both victims and perpetrators. The compound's sensitivity allows for the detection of blood traces that may have been overlooked by visual inspection alone, thereby expanding the scope of evidence collection and analysis.
Forensic scientists have developed various techniques to optimize the use of luminol in crime scene investigations. These include methods to enhance the luminol reaction's specificity and sensitivity, as well as protocols for proper application and documentation. The development of specialized equipment, such as high-sensitivity cameras and light-filtering devices, has further improved the ability to capture and analyze luminol-induced chemiluminescence in challenging environments.
The integration of luminol into forensic workflows has also spurred advancements in related analytical techniques. For instance, the need for confirmatory tests following luminol reactions has led to improvements in DNA analysis methods, allowing for the extraction and amplification of genetic material from increasingly smaller samples. This synergy between luminol-based detection and subsequent analytical procedures has significantly enhanced the overall capabilities of forensic laboratories.
However, the use of luminol in forensic science is not without challenges. False positives can occur due to the compound's reactivity with substances other than blood, necessitating careful interpretation of results. Additionally, the potential for luminol to interfere with subsequent DNA analysis has prompted research into alternative formulations and application methods that minimize such risks while maintaining detection efficacy.
The ongoing research and development in luminol-based forensic techniques continue to push the boundaries of analytical chemistry. Scientists are exploring ways to increase the specificity of the luminol reaction, develop more stable and long-lasting formulations, and create portable, user-friendly detection systems for field use. These efforts not only enhance the capabilities of forensic science but also contribute to broader advancements in analytical chemistry, particularly in the areas of trace detection and chemiluminescence-based assays.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!