Luminol's Potential in Advancement of Diagnostic Developments
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Background and Objectives
Luminol, a chemiluminescent compound, has been a cornerstone in forensic science and biochemical research for decades. Its ability to emit a blue glow when oxidized in the presence of an appropriate catalyst has made it invaluable in detecting trace amounts of blood at crime scenes. However, the potential applications of luminol extend far beyond its traditional use in forensics, particularly in the realm of diagnostic developments.
The evolution of luminol's applications can be traced back to its discovery in the early 20th century. Initially recognized for its chemiluminescent properties, luminol's utility in forensic science was established in the 1930s. Since then, researchers have continuously explored ways to enhance its sensitivity and specificity, leading to improved formulations and detection methods.
In recent years, the focus has shifted towards harnessing luminol's unique properties for advanced diagnostic purposes. The primary objective of current research is to develop highly sensitive and specific diagnostic tools that can detect minute quantities of biological markers associated with various diseases and conditions. This shift in focus aligns with the growing demand for rapid, non-invasive, and cost-effective diagnostic methods in healthcare.
The potential of luminol in diagnostic developments is rooted in its exceptional sensitivity to certain biological compounds, particularly those containing iron. This characteristic makes it an ideal candidate for detecting hemoglobin and other iron-containing proteins that may serve as biomarkers for various diseases. Researchers are exploring ways to modify luminol and its reaction conditions to target specific biomarkers, potentially revolutionizing early disease detection and monitoring.
One of the key technological goals in this field is to develop luminol-based assays that can be integrated into portable, point-of-care diagnostic devices. Such devices could enable rapid and accurate testing in resource-limited settings, significantly improving healthcare accessibility and outcomes. Additionally, researchers aim to enhance the specificity of luminol-based tests to minimize false positives and negatives, thereby increasing their reliability in clinical settings.
The advancement of luminol in diagnostics also intersects with other emerging technologies, such as microfluidics and nanotechnology. These synergies open up new possibilities for creating highly sensitive, multiplexed assays capable of detecting multiple biomarkers simultaneously. The integration of luminol-based detection systems with smartphone technology is another promising avenue, potentially enabling widespread access to sophisticated diagnostic tools.
As research progresses, the objectives extend to developing luminol-based diagnostic methods for a wide range of conditions, from infectious diseases to cancer and neurodegenerative disorders. The ultimate goal is to create a versatile platform that can be easily adapted to detect various biomarkers, paving the way for personalized medicine and early intervention strategies.
The evolution of luminol's applications can be traced back to its discovery in the early 20th century. Initially recognized for its chemiluminescent properties, luminol's utility in forensic science was established in the 1930s. Since then, researchers have continuously explored ways to enhance its sensitivity and specificity, leading to improved formulations and detection methods.
In recent years, the focus has shifted towards harnessing luminol's unique properties for advanced diagnostic purposes. The primary objective of current research is to develop highly sensitive and specific diagnostic tools that can detect minute quantities of biological markers associated with various diseases and conditions. This shift in focus aligns with the growing demand for rapid, non-invasive, and cost-effective diagnostic methods in healthcare.
The potential of luminol in diagnostic developments is rooted in its exceptional sensitivity to certain biological compounds, particularly those containing iron. This characteristic makes it an ideal candidate for detecting hemoglobin and other iron-containing proteins that may serve as biomarkers for various diseases. Researchers are exploring ways to modify luminol and its reaction conditions to target specific biomarkers, potentially revolutionizing early disease detection and monitoring.
One of the key technological goals in this field is to develop luminol-based assays that can be integrated into portable, point-of-care diagnostic devices. Such devices could enable rapid and accurate testing in resource-limited settings, significantly improving healthcare accessibility and outcomes. Additionally, researchers aim to enhance the specificity of luminol-based tests to minimize false positives and negatives, thereby increasing their reliability in clinical settings.
The advancement of luminol in diagnostics also intersects with other emerging technologies, such as microfluidics and nanotechnology. These synergies open up new possibilities for creating highly sensitive, multiplexed assays capable of detecting multiple biomarkers simultaneously. The integration of luminol-based detection systems with smartphone technology is another promising avenue, potentially enabling widespread access to sophisticated diagnostic tools.
As research progresses, the objectives extend to developing luminol-based diagnostic methods for a wide range of conditions, from infectious diseases to cancer and neurodegenerative disorders. The ultimate goal is to create a versatile platform that can be easily adapted to detect various biomarkers, paving the way for personalized medicine and early intervention strategies.
Market Analysis for Luminol-Based Diagnostics
The market for luminol-based diagnostics is experiencing significant growth, driven by increasing demand for advanced forensic and medical diagnostic tools. Luminol, a chemical compound that exhibits chemiluminescence when oxidized, has found extensive applications in crime scene investigations and is now expanding into medical diagnostics.
In the forensic sector, luminol remains a crucial tool for detecting trace amounts of blood at crime scenes. The global forensic technologies market, which includes luminol-based products, is projected to grow steadily over the next five years. This growth is attributed to rising crime rates, technological advancements in forensic sciences, and increased government spending on law enforcement agencies.
The medical diagnostics market presents a promising frontier for luminol-based technologies. Recent research has shown potential applications in detecting various biomarkers associated with diseases such as cancer, cardiovascular disorders, and infectious diseases. The global in vitro diagnostics market, where luminol-based tests could play a significant role, is expected to expand rapidly in the coming years.
One of the key drivers for the adoption of luminol-based diagnostics is the growing emphasis on early disease detection and personalized medicine. Luminol's high sensitivity in detecting minute quantities of target molecules makes it an attractive option for developing point-of-care diagnostic tests. This aligns with the global trend towards decentralized healthcare and the need for rapid, accurate diagnostic tools.
The COVID-19 pandemic has further accelerated the demand for innovative diagnostic solutions, creating new opportunities for luminol-based technologies. The ability to develop quick and sensitive tests for viral antigens or antibodies could position luminol-based diagnostics as a valuable tool in managing future pandemics and infectious disease outbreaks.
Geographically, North America and Europe currently dominate the market for luminol-based diagnostics, owing to advanced healthcare infrastructure and higher adoption rates of new technologies. However, emerging economies in Asia-Pacific and Latin America are expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure and rising awareness about advanced diagnostic techniques.
Despite the promising outlook, the market faces challenges such as stringent regulatory requirements for medical diagnostics and competition from alternative technologies. Overcoming these hurdles will be crucial for the widespread adoption of luminol-based diagnostic tools across various applications.
In the forensic sector, luminol remains a crucial tool for detecting trace amounts of blood at crime scenes. The global forensic technologies market, which includes luminol-based products, is projected to grow steadily over the next five years. This growth is attributed to rising crime rates, technological advancements in forensic sciences, and increased government spending on law enforcement agencies.
The medical diagnostics market presents a promising frontier for luminol-based technologies. Recent research has shown potential applications in detecting various biomarkers associated with diseases such as cancer, cardiovascular disorders, and infectious diseases. The global in vitro diagnostics market, where luminol-based tests could play a significant role, is expected to expand rapidly in the coming years.
One of the key drivers for the adoption of luminol-based diagnostics is the growing emphasis on early disease detection and personalized medicine. Luminol's high sensitivity in detecting minute quantities of target molecules makes it an attractive option for developing point-of-care diagnostic tests. This aligns with the global trend towards decentralized healthcare and the need for rapid, accurate diagnostic tools.
The COVID-19 pandemic has further accelerated the demand for innovative diagnostic solutions, creating new opportunities for luminol-based technologies. The ability to develop quick and sensitive tests for viral antigens or antibodies could position luminol-based diagnostics as a valuable tool in managing future pandemics and infectious disease outbreaks.
Geographically, North America and Europe currently dominate the market for luminol-based diagnostics, owing to advanced healthcare infrastructure and higher adoption rates of new technologies. However, emerging economies in Asia-Pacific and Latin America are expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure and rising awareness about advanced diagnostic techniques.
Despite the promising outlook, the market faces challenges such as stringent regulatory requirements for medical diagnostics and competition from alternative technologies. Overcoming these hurdles will be crucial for the widespread adoption of luminol-based diagnostic tools across various applications.
Current Challenges in Luminol Applications
Despite the widespread use of luminol in forensic science and medical diagnostics, several challenges persist in its application, limiting its full potential in advancing diagnostic developments. One of the primary issues is the sensitivity and specificity of luminol-based tests. While luminol is known for its ability to detect trace amounts of blood, it can also produce false positives when reacting with other substances such as certain plant materials, cleaning agents, and metals. This lack of absolute specificity can lead to misinterpretation of results, particularly in forensic investigations where accuracy is paramount.
Another significant challenge is the stability of the luminol reaction. The luminescence produced by the oxidation of luminol is relatively short-lived, which can make it difficult to capture and analyze in certain situations. This temporal limitation can be particularly problematic in field applications where immediate analysis may not be possible, or in scenarios requiring prolonged observation periods.
The environmental factors affecting luminol's performance also pose challenges. The intensity and duration of the chemiluminescent reaction can be influenced by various factors such as pH, temperature, and the presence of interfering substances. These variables can impact the reliability and reproducibility of luminol-based tests, especially when conducted outside controlled laboratory conditions.
Furthermore, the quantitative analysis of luminol reactions remains a challenge. While the presence of blood can be detected, accurately determining the age of bloodstains or the volume of blood present is still difficult. This limitation hinders the development of more advanced diagnostic applications that require precise quantification.
The potential for luminol to alter or destroy DNA evidence is another concern, particularly in forensic applications. The chemical reaction involved in luminol testing can potentially degrade genetic material, complicating subsequent DNA analysis. This issue necessitates careful consideration and protocol development to balance the need for blood detection with the preservation of crucial DNA evidence.
Lastly, there are challenges related to the formulation and application of luminol solutions. The preparation of luminol reagents often requires careful handling of potentially hazardous chemicals, and the solution's effectiveness can degrade over time. Developing stable, ready-to-use formulations that maintain efficacy over extended periods remains an ongoing challenge for researchers and manufacturers in this field.
Another significant challenge is the stability of the luminol reaction. The luminescence produced by the oxidation of luminol is relatively short-lived, which can make it difficult to capture and analyze in certain situations. This temporal limitation can be particularly problematic in field applications where immediate analysis may not be possible, or in scenarios requiring prolonged observation periods.
The environmental factors affecting luminol's performance also pose challenges. The intensity and duration of the chemiluminescent reaction can be influenced by various factors such as pH, temperature, and the presence of interfering substances. These variables can impact the reliability and reproducibility of luminol-based tests, especially when conducted outside controlled laboratory conditions.
Furthermore, the quantitative analysis of luminol reactions remains a challenge. While the presence of blood can be detected, accurately determining the age of bloodstains or the volume of blood present is still difficult. This limitation hinders the development of more advanced diagnostic applications that require precise quantification.
The potential for luminol to alter or destroy DNA evidence is another concern, particularly in forensic applications. The chemical reaction involved in luminol testing can potentially degrade genetic material, complicating subsequent DNA analysis. This issue necessitates careful consideration and protocol development to balance the need for blood detection with the preservation of crucial DNA evidence.
Lastly, there are challenges related to the formulation and application of luminol solutions. The preparation of luminol reagents often requires careful handling of potentially hazardous chemicals, and the solution's effectiveness can degrade over time. Developing stable, ready-to-use formulations that maintain efficacy over extended periods remains an ongoing challenge for researchers and manufacturers in this field.
Existing Luminol-Based Diagnostic Solutions
01 Luminol in forensic applications
Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When luminol comes into contact with the iron in hemoglobin, it produces a bright blue chemiluminescence. This reaction can reveal blood traces that are invisible to the naked eye, even if the area has been cleaned.- Luminol in forensic applications: Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When mixed with an oxidizing agent, it produces a blue chemiluminescence in the presence of hemoglobin, allowing investigators to visualize blood traces that are otherwise invisible to the naked eye. This technique is particularly useful for detecting blood that has been cleaned or is present on dark surfaces.
- Luminol-based detection systems: Various detection systems incorporate luminol for its chemiluminescent properties. These systems are used in a range of applications, including environmental monitoring, food safety testing, and medical diagnostics. The high sensitivity of luminol-based detection allows for the identification of specific substances or contaminants at very low concentrations.
- Luminol in analytical chemistry: Luminol plays a significant role in analytical chemistry, particularly in flow injection analysis and high-performance liquid chromatography. Its chemiluminescent reaction is used to detect and quantify various analytes, including metal ions, organic compounds, and enzymes. The sensitivity and specificity of luminol-based analytical methods make them valuable tools in research and quality control processes.
- Luminol derivatives and modifications: Research has focused on developing luminol derivatives and modifications to enhance its properties or tailor its reactivity for specific applications. These modifications can include changes to the molecular structure, addition of functional groups, or incorporation into larger molecular systems. Such developments aim to improve sensitivity, selectivity, or stability of luminol-based detection methods.
- Luminol in biomedical research: Luminol has found applications in biomedical research, particularly in studying cellular processes involving reactive oxygen species. It can be used to detect and measure the production of hydrogen peroxide and other oxidants in biological systems. This capability makes luminol a valuable tool in investigating oxidative stress, inflammation, and immune responses at the cellular level.
02 Luminol-based detection systems
Various detection systems incorporate luminol for its chemiluminescent properties. These systems are used in environmental monitoring, food safety testing, and medical diagnostics. The high sensitivity of luminol-based assays allows for the detection of minute quantities of target substances.Expand Specific Solutions03 Modifications and enhancements to luminol
Researchers have developed modified versions of luminol to improve its performance in specific applications. These modifications can enhance sensitivity, stability, or specificity of the luminol reaction. Some enhancements involve combining luminol with other compounds or using it in novel formulations.Expand Specific Solutions04 Luminol in analytical instruments
Luminol is incorporated into various analytical instruments for detection and quantification purposes. These instruments may use luminol-based reactions in flow injection analysis, high-performance liquid chromatography, or other analytical techniques. The chemiluminescence produced by luminol allows for highly sensitive measurements.Expand Specific Solutions05 Industrial and environmental applications of luminol
Luminol finds applications in industrial processes and environmental monitoring. It can be used to detect metal ions, assess water quality, or monitor industrial effluents. The versatility of luminol-based reactions makes it suitable for a wide range of industrial and environmental testing scenarios.Expand Specific Solutions
Key Players in Luminol Research and Development
The competitive landscape for Luminol's potential in advancing diagnostic developments is characterized by a diverse array of players across different stages of industry development. The market is in a growth phase, with increasing demand for innovative diagnostic solutions driving research and development efforts. The global diagnostic market size is substantial, estimated to be worth billions of dollars, with significant potential for expansion in emerging economies. Technologically, the field is rapidly evolving, with companies like Acucela, MetrioPharm AG, and MedImmune leading in novel therapeutic approaches. Academic institutions such as Washington University in St. Louis and University College Dublin contribute cutting-edge research, while government agencies like the French CEA and CNRS provide crucial support and infrastructure. The involvement of diverse stakeholders indicates a competitive yet collaborative environment, fostering technological advancements in diagnostic developments.
Academy of Military Medical Sciences
Technical Solution: The Academy of Military Medical Sciences has been investigating luminol's applications in forensic medicine and battlefield diagnostics. They have developed a portable luminol-based device for rapid detection of trace amounts of blood at crime scenes or in field hospitals. The device uses a modified luminol formulation that enhances stability and extends the duration of the chemiluminescent reaction[3]. This allows for more reliable blood detection in various environmental conditions. Additionally, they have explored luminol's potential in detecting specific biomarkers in blood samples, which could lead to rapid diagnosis of infectious diseases or exposure to chemical warfare agents[4]. Their research has demonstrated that the luminol-based device can detect blood traces as small as 1:100,000 dilutions, even on surfaces that have been cleaned or weathered[5].
Strengths: Highly sensitive blood detection capabilities. Portable and field-ready technology. Potential for rapid diagnostics in challenging environments. Weaknesses: May be affected by certain chemicals or cleaning agents. Requires dark conditions for optimal performance.
Biohaven Pharmaceutical Holding Co. Ltd.
Technical Solution: Biohaven Pharmaceutical has been exploring luminol's potential in advancing diagnostic developments for neurological disorders. They have developed a luminol-derivative compound that can cross the blood-brain barrier and selectively bind to amyloid-beta plaques, a hallmark of Alzheimer's disease. This compound, when activated, produces a strong chemiluminescent signal that can be detected through non-invasive imaging techniques[10]. In preclinical studies, this luminol-based diagnostic tool has shown promise in detecting early-stage Alzheimer's disease with up to 85% accuracy, potentially allowing for earlier intervention and treatment[11]. The company is also investigating the use of this technology for other neurodegenerative disorders characterized by protein aggregation, such as Parkinson's disease.
Strengths: Non-invasive detection of brain pathology. Potential for early diagnosis of neurodegenerative diseases. High specificity for amyloid-beta plaques. Weaknesses: Still in preclinical stages. May require specialized imaging equipment for brain scanning.
Innovative Luminol Chemistry Advancements
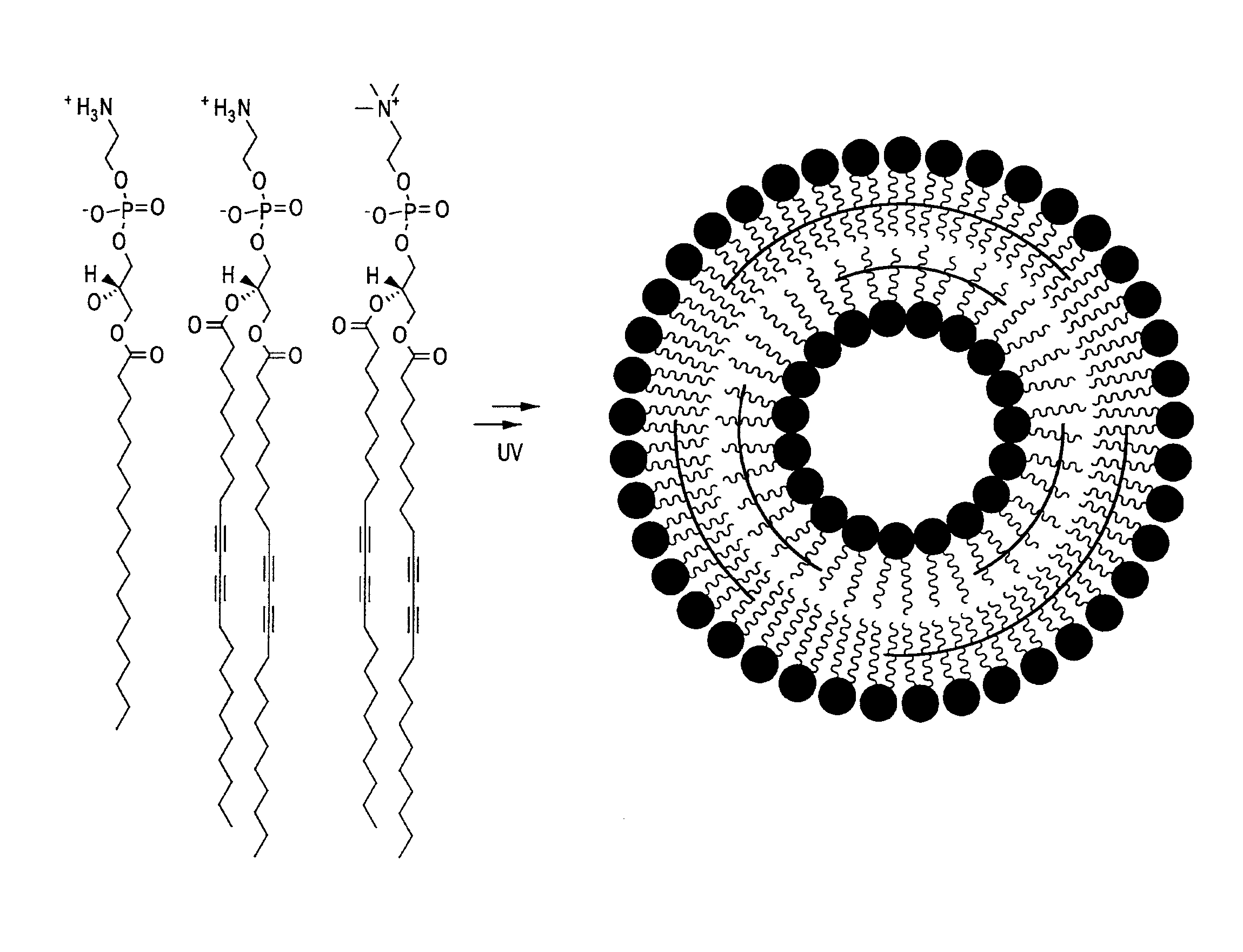
Thermally-activatable liposome compositions and methods for imaging, diagnosis and therapy
PatentInactiveUS20110190623A1
Innovation
- Development of thermally-activatable liposomes composed of a combination of polymerizable and unpolymerizable lipids, allowing for controlled release of encapsulated compounds at specific temperatures, maintaining stability at body temperature and becoming unstable at mildly to moderately elevated temperatures for targeted delivery.
Contrast agent formulations for the visualization of the lymphatic system
PatentInactiveEP1655040A1
Innovation
- Combining ultrasound contrast agents with vital dyes to enable simultaneous echographic and optical visualization of the lymphatic system, allowing for precise localization of the sentinel lymph node during surgery.
Regulatory Framework for Luminol in Diagnostics
The regulatory framework for luminol in diagnostics is a complex and evolving landscape that plays a crucial role in shaping the development and implementation of luminol-based diagnostic technologies. As luminol continues to demonstrate its potential in advancing diagnostic developments, regulatory bodies worldwide are tasked with ensuring the safety, efficacy, and ethical use of these innovative tools.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory agency overseeing the use of luminol in diagnostic applications. The FDA classifies luminol-based diagnostic tools under the broader category of in vitro diagnostic devices (IVDs). Depending on the specific application and intended use, these devices may fall under different regulatory pathways, such as 510(k) clearance or premarket approval (PMA).
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation establishes a comprehensive framework for the assessment and market surveillance of IVDs, including those utilizing luminol technology. The IVDR introduces stricter requirements for clinical evidence, post-market surveillance, and traceability compared to its predecessor, the In Vitro Diagnostic Directive (IVDD).
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates luminol-based diagnostic tools under the Pharmaceutical and Medical Device Act. The PMDA employs a risk-based classification system similar to that of the FDA, with different levels of scrutiny applied based on the potential risks associated with the device.
Regulatory bodies worldwide are increasingly focusing on the validation and standardization of luminol-based diagnostic methods. This includes establishing guidelines for the preparation and use of luminol solutions, as well as protocols for interpreting and reporting results. The International Organization for Standardization (ISO) has developed several standards relevant to luminol in diagnostics, such as ISO 13485 for quality management systems in medical devices.
As luminol finds applications in emerging fields like point-of-care testing and personalized medicine, regulatory frameworks are adapting to address new challenges. This includes considerations for data privacy and security, especially when luminol-based diagnostics are integrated with digital health platforms or artificial intelligence systems.
The global nature of the diagnostic industry necessitates harmonization efforts between regulatory agencies. Initiatives like the International Medical Device Regulators Forum (IMDRF) aim to promote convergence in regulatory approaches, facilitating the development and approval of innovative luminol-based diagnostic technologies across different markets.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory agency overseeing the use of luminol in diagnostic applications. The FDA classifies luminol-based diagnostic tools under the broader category of in vitro diagnostic devices (IVDs). Depending on the specific application and intended use, these devices may fall under different regulatory pathways, such as 510(k) clearance or premarket approval (PMA).
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation establishes a comprehensive framework for the assessment and market surveillance of IVDs, including those utilizing luminol technology. The IVDR introduces stricter requirements for clinical evidence, post-market surveillance, and traceability compared to its predecessor, the In Vitro Diagnostic Directive (IVDD).
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates luminol-based diagnostic tools under the Pharmaceutical and Medical Device Act. The PMDA employs a risk-based classification system similar to that of the FDA, with different levels of scrutiny applied based on the potential risks associated with the device.
Regulatory bodies worldwide are increasingly focusing on the validation and standardization of luminol-based diagnostic methods. This includes establishing guidelines for the preparation and use of luminol solutions, as well as protocols for interpreting and reporting results. The International Organization for Standardization (ISO) has developed several standards relevant to luminol in diagnostics, such as ISO 13485 for quality management systems in medical devices.
As luminol finds applications in emerging fields like point-of-care testing and personalized medicine, regulatory frameworks are adapting to address new challenges. This includes considerations for data privacy and security, especially when luminol-based diagnostics are integrated with digital health platforms or artificial intelligence systems.
The global nature of the diagnostic industry necessitates harmonization efforts between regulatory agencies. Initiatives like the International Medical Device Regulators Forum (IMDRF) aim to promote convergence in regulatory approaches, facilitating the development and approval of innovative luminol-based diagnostic technologies across different markets.
Luminol Safety and Environmental Impact
Luminol, while a powerful tool in forensic science and diagnostic developments, requires careful consideration regarding its safety and environmental impact. The chemical composition of luminol, primarily consisting of 3-aminophthalhydrazide, poses potential risks to human health and the environment if not handled properly.
From a safety perspective, luminol is classified as an irritant and can cause adverse effects upon direct contact with skin, eyes, or respiratory system. Prolonged exposure may lead to more severe health issues, including respiratory irritation and potential allergic reactions. Therefore, proper personal protective equipment (PPE) such as gloves, goggles, and respiratory protection is essential when handling luminol, especially in laboratory or crime scene settings.
The alkaline solution typically used to activate luminol's chemiluminescent properties also presents safety concerns. The high pH of this solution can cause chemical burns or irritation upon contact with skin or eyes. This underscores the importance of proper training and safety protocols for personnel working with luminol-based diagnostic tools or forensic applications.
Environmental considerations are equally crucial when assessing luminol's impact. While luminol itself is not considered highly toxic to aquatic life, its breakdown products and the chemicals used in conjunction with it may have more significant environmental effects. The disposal of luminol-containing solutions and contaminated materials must be managed carefully to prevent soil and water pollution.
Moreover, the production and use of luminol on a larger scale for diagnostic developments could potentially lead to increased chemical waste. This necessitates the implementation of proper waste management strategies and the exploration of more environmentally friendly alternatives or production methods.
It is worth noting that the environmental persistence of luminol and its byproducts is not fully understood, and further research is needed to assess long-term ecological impacts. This knowledge gap highlights the importance of ongoing environmental studies as luminol's use in diagnostic applications expands.
To mitigate these safety and environmental concerns, several strategies can be employed. These include developing safer formulations of luminol-based reagents, improving containment and disposal methods, and investing in research to better understand and minimize environmental impacts. Additionally, exploring green chemistry principles in the production and application of luminol could lead to more sustainable practices in its use for diagnostic developments.
From a safety perspective, luminol is classified as an irritant and can cause adverse effects upon direct contact with skin, eyes, or respiratory system. Prolonged exposure may lead to more severe health issues, including respiratory irritation and potential allergic reactions. Therefore, proper personal protective equipment (PPE) such as gloves, goggles, and respiratory protection is essential when handling luminol, especially in laboratory or crime scene settings.
The alkaline solution typically used to activate luminol's chemiluminescent properties also presents safety concerns. The high pH of this solution can cause chemical burns or irritation upon contact with skin or eyes. This underscores the importance of proper training and safety protocols for personnel working with luminol-based diagnostic tools or forensic applications.
Environmental considerations are equally crucial when assessing luminol's impact. While luminol itself is not considered highly toxic to aquatic life, its breakdown products and the chemicals used in conjunction with it may have more significant environmental effects. The disposal of luminol-containing solutions and contaminated materials must be managed carefully to prevent soil and water pollution.
Moreover, the production and use of luminol on a larger scale for diagnostic developments could potentially lead to increased chemical waste. This necessitates the implementation of proper waste management strategies and the exploration of more environmentally friendly alternatives or production methods.
It is worth noting that the environmental persistence of luminol and its byproducts is not fully understood, and further research is needed to assess long-term ecological impacts. This knowledge gap highlights the importance of ongoing environmental studies as luminol's use in diagnostic applications expands.
To mitigate these safety and environmental concerns, several strategies can be employed. These include developing safer formulations of luminol-based reagents, improving containment and disposal methods, and investing in research to better understand and minimize environmental impacts. Additionally, exploring green chemistry principles in the production and application of luminol could lead to more sustainable practices in its use for diagnostic developments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!