Luminol: A Catalyst for Revolutionary Scientific Methods
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Background and Objectives
Luminol, a chemical compound with the formula C8H7NO2, has emerged as a groundbreaking catalyst in the realm of scientific methodologies. Initially discovered in the late 19th century, luminol's unique chemiluminescent properties have propelled it to the forefront of various scientific disciplines, revolutionizing detection and analysis techniques across multiple fields.
The evolution of luminol's applications has been marked by significant milestones in forensic science, biochemistry, and environmental studies. Its ability to emit a blue glow when oxidized in the presence of an appropriate catalyst has made it an invaluable tool in crime scene investigations, particularly in the detection of trace amounts of blood. This characteristic has not only enhanced the efficiency of forensic procedures but has also opened new avenues for research in related fields.
In recent years, the scope of luminol's applications has expanded dramatically, encompassing areas such as medical diagnostics, environmental monitoring, and industrial quality control. The compound's sensitivity to various substances and its non-destructive nature have positioned it as a versatile agent in developing innovative scientific methods.
The primary objective of luminol-based technologies is to leverage its chemiluminescent properties to create more sensitive, accurate, and cost-effective detection methods. Researchers aim to enhance the specificity of luminol reactions, minimizing false positives and improving the reliability of results across diverse applications.
Another crucial goal is to develop portable and user-friendly luminol-based detection systems, enabling real-time analysis in field conditions. This advancement would significantly impact areas such as on-site forensic investigations, rapid medical diagnostics, and environmental monitoring in remote locations.
Furthermore, there is a growing interest in exploring the potential of luminol in nanotechnology and biosensing applications. Scientists are investigating ways to integrate luminol into nanomaterials and biosensors to create highly sensitive and selective detection platforms for a wide range of analytes.
The ongoing research in luminol-based technologies also focuses on improving the stability and shelf-life of luminol solutions, addressing one of the primary challenges in its widespread adoption. Efforts are being made to develop formulations that maintain luminol's reactivity over extended periods, even under varying environmental conditions.
As we delve deeper into the 21st century, the trajectory of luminol research points towards more sophisticated, multifunctional applications. The convergence of luminol chemistry with other cutting-edge technologies, such as artificial intelligence and microfluidics, promises to unlock new possibilities in scientific methodologies, potentially transforming how we approach detection and analysis across multiple disciplines.
The evolution of luminol's applications has been marked by significant milestones in forensic science, biochemistry, and environmental studies. Its ability to emit a blue glow when oxidized in the presence of an appropriate catalyst has made it an invaluable tool in crime scene investigations, particularly in the detection of trace amounts of blood. This characteristic has not only enhanced the efficiency of forensic procedures but has also opened new avenues for research in related fields.
In recent years, the scope of luminol's applications has expanded dramatically, encompassing areas such as medical diagnostics, environmental monitoring, and industrial quality control. The compound's sensitivity to various substances and its non-destructive nature have positioned it as a versatile agent in developing innovative scientific methods.
The primary objective of luminol-based technologies is to leverage its chemiluminescent properties to create more sensitive, accurate, and cost-effective detection methods. Researchers aim to enhance the specificity of luminol reactions, minimizing false positives and improving the reliability of results across diverse applications.
Another crucial goal is to develop portable and user-friendly luminol-based detection systems, enabling real-time analysis in field conditions. This advancement would significantly impact areas such as on-site forensic investigations, rapid medical diagnostics, and environmental monitoring in remote locations.
Furthermore, there is a growing interest in exploring the potential of luminol in nanotechnology and biosensing applications. Scientists are investigating ways to integrate luminol into nanomaterials and biosensors to create highly sensitive and selective detection platforms for a wide range of analytes.
The ongoing research in luminol-based technologies also focuses on improving the stability and shelf-life of luminol solutions, addressing one of the primary challenges in its widespread adoption. Efforts are being made to develop formulations that maintain luminol's reactivity over extended periods, even under varying environmental conditions.
As we delve deeper into the 21st century, the trajectory of luminol research points towards more sophisticated, multifunctional applications. The convergence of luminol chemistry with other cutting-edge technologies, such as artificial intelligence and microfluidics, promises to unlock new possibilities in scientific methodologies, potentially transforming how we approach detection and analysis across multiple disciplines.
Market Demand Analysis for Luminol Applications
The market demand for luminol applications has been steadily growing across various sectors, driven by its unique chemiluminescent properties and versatility in scientific and forensic applications. Luminol's ability to detect trace amounts of blood has made it an indispensable tool in crime scene investigations, creating a consistent demand from law enforcement agencies and forensic laboratories worldwide.
In the healthcare sector, luminol-based diagnostic tools have gained traction for their high sensitivity in detecting specific biomolecules. This has led to increased adoption in medical research facilities and clinical laboratories, particularly for early disease detection and monitoring. The pharmaceutical industry has also shown growing interest in luminol for drug discovery processes and quality control measures.
Environmental monitoring represents another expanding market for luminol applications. Its capability to detect minute quantities of certain metals and organic compounds has made it valuable for water quality testing and soil contamination assessments. This has attracted attention from environmental agencies, water treatment facilities, and industrial sectors concerned with regulatory compliance and environmental impact.
The agriculture sector has begun exploring luminol-based technologies for soil health analysis and crop disease detection, potentially opening up a new market segment. Additionally, the food and beverage industry has shown interest in luminol for quality control processes, particularly in detecting food contamination and ensuring product safety.
Academic and research institutions continue to be significant consumers of luminol, using it in a wide range of biochemical and analytical studies. The ongoing research into novel applications of luminol in fields such as nanotechnology and materials science suggests potential for market expansion in emerging technological areas.
The global market for chemiluminescence-based technologies, including luminol applications, has been projected to grow at a compound annual growth rate (CAGR) of over 6% in the coming years. This growth is attributed to increasing investments in life sciences research, advancements in analytical techniques, and the rising demand for rapid and sensitive detection methods across various industries.
However, the market faces challenges such as the need for skilled personnel to interpret results accurately and the development of alternative technologies. Despite these challenges, the unique properties of luminol and its established role in critical applications suggest a continued strong market demand, with potential for further growth as new applications are developed and existing ones are refined.
In the healthcare sector, luminol-based diagnostic tools have gained traction for their high sensitivity in detecting specific biomolecules. This has led to increased adoption in medical research facilities and clinical laboratories, particularly for early disease detection and monitoring. The pharmaceutical industry has also shown growing interest in luminol for drug discovery processes and quality control measures.
Environmental monitoring represents another expanding market for luminol applications. Its capability to detect minute quantities of certain metals and organic compounds has made it valuable for water quality testing and soil contamination assessments. This has attracted attention from environmental agencies, water treatment facilities, and industrial sectors concerned with regulatory compliance and environmental impact.
The agriculture sector has begun exploring luminol-based technologies for soil health analysis and crop disease detection, potentially opening up a new market segment. Additionally, the food and beverage industry has shown interest in luminol for quality control processes, particularly in detecting food contamination and ensuring product safety.
Academic and research institutions continue to be significant consumers of luminol, using it in a wide range of biochemical and analytical studies. The ongoing research into novel applications of luminol in fields such as nanotechnology and materials science suggests potential for market expansion in emerging technological areas.
The global market for chemiluminescence-based technologies, including luminol applications, has been projected to grow at a compound annual growth rate (CAGR) of over 6% in the coming years. This growth is attributed to increasing investments in life sciences research, advancements in analytical techniques, and the rising demand for rapid and sensitive detection methods across various industries.
However, the market faces challenges such as the need for skilled personnel to interpret results accurately and the development of alternative technologies. Despite these challenges, the unique properties of luminol and its established role in critical applications suggest a continued strong market demand, with potential for further growth as new applications are developed and existing ones are refined.
Current Luminol Technology Challenges
Luminol, a chemiluminescent compound widely used in forensic science and biochemistry, faces several technological challenges that hinder its full potential in revolutionary scientific methods. One of the primary issues is the limited sensitivity of luminol-based detection systems. While luminol can detect trace amounts of blood, its sensitivity is not always sufficient for extremely low concentrations or degraded samples, which are common in forensic investigations.
Another significant challenge is the interference from other substances that can produce false-positive results. Luminol reacts with various oxidizing agents and metal ions, not just blood, which can lead to misinterpretation of evidence. This lack of specificity necessitates additional confirmatory tests, increasing the time and resources required for analysis.
The stability of luminol solutions is also a concern. Once prepared, luminol solutions have a limited shelf life and can degrade rapidly, affecting the reliability of test results. This instability requires frequent preparation of fresh solutions, which can be time-consuming and costly in laboratory settings.
Furthermore, the application of luminol in crime scene investigations faces practical challenges. The need for darkness during luminol application and photography can be problematic in certain environments. Additionally, the potential for luminol to interfere with subsequent DNA analysis raises concerns about evidence preservation and the order of forensic procedures.
In biochemical applications, luminol-based assays face challenges in quantification accuracy. The intensity of luminescence can be affected by various factors, including pH, temperature, and the presence of inhibitors, making precise quantification difficult in complex biological samples.
The development of more selective and sensitive luminol derivatives is an ongoing challenge. While modifications to the luminol molecule have shown promise in enhancing its properties, achieving a balance between improved performance and cost-effectiveness remains elusive.
Lastly, the integration of luminol-based detection with advanced imaging technologies presents both opportunities and challenges. While combining luminol with high-sensitivity cameras and image processing software can enhance detection capabilities, optimizing these systems for field use and ensuring their reliability under various conditions is an area that requires further development.
Another significant challenge is the interference from other substances that can produce false-positive results. Luminol reacts with various oxidizing agents and metal ions, not just blood, which can lead to misinterpretation of evidence. This lack of specificity necessitates additional confirmatory tests, increasing the time and resources required for analysis.
The stability of luminol solutions is also a concern. Once prepared, luminol solutions have a limited shelf life and can degrade rapidly, affecting the reliability of test results. This instability requires frequent preparation of fresh solutions, which can be time-consuming and costly in laboratory settings.
Furthermore, the application of luminol in crime scene investigations faces practical challenges. The need for darkness during luminol application and photography can be problematic in certain environments. Additionally, the potential for luminol to interfere with subsequent DNA analysis raises concerns about evidence preservation and the order of forensic procedures.
In biochemical applications, luminol-based assays face challenges in quantification accuracy. The intensity of luminescence can be affected by various factors, including pH, temperature, and the presence of inhibitors, making precise quantification difficult in complex biological samples.
The development of more selective and sensitive luminol derivatives is an ongoing challenge. While modifications to the luminol molecule have shown promise in enhancing its properties, achieving a balance between improved performance and cost-effectiveness remains elusive.
Lastly, the integration of luminol-based detection with advanced imaging technologies presents both opportunities and challenges. While combining luminol with high-sensitivity cameras and image processing software can enhance detection capabilities, optimizing these systems for field use and ensuring their reliability under various conditions is an area that requires further development.
Existing Luminol-based Detection Methods
01 Luminol in forensic applications
Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When luminol comes into contact with the iron in hemoglobin, it produces a bright blue chemiluminescence. This reaction can reveal blood traces that are invisible to the naked eye, even if the area has been cleaned.- Luminol-based chemiluminescence detection methods: Luminol is widely used in chemiluminescence detection methods for various applications. These methods utilize the light-emitting properties of luminol when it reacts with specific substances, allowing for sensitive and selective detection of target analytes. The techniques are employed in forensic science, environmental monitoring, and biomedical research.
- Luminol derivatives and modifications: Research focuses on developing luminol derivatives and modifications to enhance its properties for specific applications. These modifications aim to improve sensitivity, selectivity, or stability of luminol-based detection systems. Some approaches involve chemical alterations to the luminol molecule or incorporation into larger structures.
- Luminol in forensic applications: Luminol plays a crucial role in forensic science, particularly in the detection of blood traces at crime scenes. The compound reacts with the iron in hemoglobin, producing a blue luminescence that can reveal otherwise invisible blood stains. Forensic investigators use luminol-based solutions to identify and document potential evidence.
- Luminol in biomedical research and diagnostics: Luminol-based assays are employed in biomedical research and diagnostics for detecting various biomolecules and cellular processes. These applications include the measurement of enzyme activities, detection of reactive oxygen species, and development of immunoassays. The high sensitivity of luminol chemiluminescence makes it valuable for detecting low concentrations of analytes.
- Luminol in environmental monitoring: Luminol-based detection methods are utilized in environmental monitoring applications. These include the detection of heavy metals, pollutants, and other contaminants in water, soil, and air samples. The high sensitivity and relatively simple instrumentation requirements make luminol-based techniques suitable for field testing and continuous monitoring systems.
02 Luminol-based detection systems
Various detection systems incorporate luminol for its chemiluminescent properties. These systems are used in environmental monitoring, food safety testing, and medical diagnostics. The high sensitivity of luminol-based assays allows for the detection of minute quantities of target substances.Expand Specific Solutions03 Luminol synthesis and formulation
Improved methods for synthesizing and formulating luminol have been developed to enhance its performance and stability. These advancements include novel synthetic routes, purification techniques, and the incorporation of stabilizing agents to prolong shelf life and increase sensitivity.Expand Specific Solutions04 Luminol in biomedical research
Luminol is utilized in various biomedical research applications, including the study of cellular processes, oxidative stress, and inflammation. Its ability to detect reactive oxygen species and other oxidants makes it valuable in investigating disease mechanisms and potential treatments.Expand Specific Solutions05 Enhanced luminol-based imaging techniques
Advanced imaging techniques have been developed to improve the visualization and quantification of luminol-based chemiluminescence. These include high-sensitivity cameras, image processing algorithms, and integration with other analytical methods to provide more detailed and accurate results in various applications.Expand Specific Solutions
Key Players in Luminol Research and Production
The development of Luminol as a catalyst for revolutionary scientific methods is in its early stages, with the market still emerging. The technology's potential applications span various fields, including forensics, biochemistry, and medical diagnostics, indicating a promising but not yet fully realized market size. The competitive landscape is diverse, featuring academic institutions like Washington University in St. Louis and Sichuan University, alongside industry players such as Cyanagen Srl and Promega Corp. These entities are at different stages of research and development, suggesting varying levels of technological maturity. While some companies have established products utilizing Luminol, others are still in the experimental phase, highlighting the ongoing evolution of this technology.
Promega Corp.
Technical Solution: Promega has developed advanced luminol-based chemiluminescence systems for forensic and life science applications. Their technology utilizes enhanced luminol formulations and specialized reagents to achieve higher sensitivity and longer-lasting light emission. The company's LumiGLO™ system incorporates stabilized luminol derivatives and optimized catalysts, resulting in up to 10-fold increase in light output compared to traditional luminol reactions[1]. Promega has also engineered luminol-based substrates with improved cell permeability for intracellular imaging applications, enabling real-time visualization of biological processes with minimal background interference[2].
Strengths: High sensitivity, long-lasting emission, and versatility across multiple applications. Weaknesses: May require specialized equipment and reagents, potentially higher cost compared to basic luminol methods.
International Business Machines Corp.
Technical Solution: IBM has leveraged its expertise in artificial intelligence and machine learning to develop advanced luminol-based detection systems. Their approach combines traditional luminol chemistry with AI-powered image analysis to enhance the detection and interpretation of trace evidence. IBM's system utilizes deep learning algorithms to process chemiluminescent signals, enabling the identification of minute blood traces and other biological materials with unprecedented accuracy. The technology incorporates a database of luminol reaction patterns, allowing for real-time comparison and classification of detected substances[3]. Additionally, IBM has integrated this system with cloud computing capabilities, facilitating remote analysis and collaborative forensic investigations across multiple locations[4].
Strengths: High accuracy, AI-enhanced interpretation, and cloud integration for collaborative analysis. Weaknesses: Reliance on complex AI systems may require specialized training and infrastructure.
Core Luminol Chemistry Innovations
A CLIA kit for the detection of igg antibodies to chikungunya present in human serum or plasma
PatentInactiveIN1428KOL2010A
Innovation
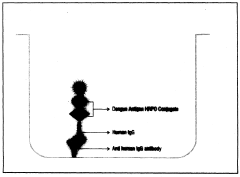
- A chemiluminescent immunoassay kit that uses microwells coated with anti-human IgG antibodies and conjugates dengue antigens with Horse Radish Peroxidase (HRPO) to detect dengue IgG antibodies in human serum or plasma, employing luminol and hydrogen peroxide for enhanced sensitivity and specificity.
Photodynamic therapy using chemiluminescence and a ligand-photosensitiser conjugate
PatentInactiveUS20100297762A1
Innovation
- A method involving a ligand-toxin conjugate (LTC) comprising a photosensitizer like hematoporphyrin conjugated with transferrin, combined with a chemiluminescent agent such as luminol, which activates the photosensitizer intracellularly to produce reactive oxygen species, thereby enhancing target cell destruction without requiring external light.
Luminol Safety and Handling Protocols
Luminol, a powerful chemiluminescent compound, requires careful handling and strict safety protocols to ensure its effective and safe use in scientific applications. When working with luminol, it is essential to adhere to proper safety measures and handling procedures to minimize potential risks and maximize its benefits.
Personal protective equipment (PPE) is crucial when handling luminol. Laboratory personnel should wear appropriate gloves, safety goggles, and lab coats to prevent skin contact or accidental splashes. Nitrile gloves are recommended due to their chemical resistance. In cases where luminol powder is being handled, the use of a dust mask or respirator is advisable to prevent inhalation of fine particles.
Proper storage of luminol is vital to maintain its stability and effectiveness. It should be kept in a cool, dry place, away from direct sunlight and heat sources. Airtight, opaque containers are ideal for storage, as luminol is sensitive to light and moisture. The storage area should be well-ventilated and separate from incompatible materials, such as strong oxidizing agents or reducing substances.
When preparing luminol solutions, it is important to work in a fume hood or well-ventilated area. This precaution helps to minimize exposure to any potential fumes or dust. The use of precise measuring equipment and clean glassware is essential to ensure accurate concentrations and prevent contamination.
Disposal of luminol and its solutions must be carried out in accordance with local regulations and institutional guidelines. Generally, small amounts can be neutralized and disposed of through regular waste channels, while larger quantities may require specialized chemical waste disposal services.
In the event of accidental exposure or spills, immediate action is necessary. For skin contact, the affected area should be washed thoroughly with soap and water. In case of eye contact, eyes should be rinsed with copious amounts of water for at least 15 minutes, and medical attention should be sought. Spills should be contained and cleaned up promptly using appropriate absorbent materials, followed by proper disposal.
Training and education of laboratory personnel on the properties, hazards, and safe handling of luminol are crucial. Regular safety briefings and updates on handling protocols should be conducted to ensure all users are aware of the latest safety practices and procedures.
Proper labeling of luminol containers and solutions is essential. Labels should clearly indicate the contents, concentration, preparation date, and any relevant hazard warnings. This practice helps prevent misidentification and ensures that all users are aware of the potential risks associated with the material.
Personal protective equipment (PPE) is crucial when handling luminol. Laboratory personnel should wear appropriate gloves, safety goggles, and lab coats to prevent skin contact or accidental splashes. Nitrile gloves are recommended due to their chemical resistance. In cases where luminol powder is being handled, the use of a dust mask or respirator is advisable to prevent inhalation of fine particles.
Proper storage of luminol is vital to maintain its stability and effectiveness. It should be kept in a cool, dry place, away from direct sunlight and heat sources. Airtight, opaque containers are ideal for storage, as luminol is sensitive to light and moisture. The storage area should be well-ventilated and separate from incompatible materials, such as strong oxidizing agents or reducing substances.
When preparing luminol solutions, it is important to work in a fume hood or well-ventilated area. This precaution helps to minimize exposure to any potential fumes or dust. The use of precise measuring equipment and clean glassware is essential to ensure accurate concentrations and prevent contamination.
Disposal of luminol and its solutions must be carried out in accordance with local regulations and institutional guidelines. Generally, small amounts can be neutralized and disposed of through regular waste channels, while larger quantities may require specialized chemical waste disposal services.
In the event of accidental exposure or spills, immediate action is necessary. For skin contact, the affected area should be washed thoroughly with soap and water. In case of eye contact, eyes should be rinsed with copious amounts of water for at least 15 minutes, and medical attention should be sought. Spills should be contained and cleaned up promptly using appropriate absorbent materials, followed by proper disposal.
Training and education of laboratory personnel on the properties, hazards, and safe handling of luminol are crucial. Regular safety briefings and updates on handling protocols should be conducted to ensure all users are aware of the latest safety practices and procedures.
Proper labeling of luminol containers and solutions is essential. Labels should clearly indicate the contents, concentration, preparation date, and any relevant hazard warnings. This practice helps prevent misidentification and ensures that all users are aware of the potential risks associated with the material.
Luminol in Forensic Science Advancements
Luminol has emerged as a groundbreaking tool in forensic science, revolutionizing crime scene investigations and enhancing the capabilities of forensic experts. This chemiluminescent compound has become an indispensable asset in the detection and analysis of blood traces, even in cases where traditional methods fall short.
The application of luminol in forensic science has significantly advanced the field by providing a non-destructive and highly sensitive method for visualizing latent bloodstains. Its ability to react with the iron in hemoglobin produces a distinctive blue glow, allowing investigators to identify and document blood evidence that may be invisible to the naked eye or that has been subjected to cleaning attempts.
One of the most significant advancements brought about by luminol is its effectiveness in cold cases. Forensic teams can now revisit crime scenes that are months or even years old, uncovering crucial evidence that was previously undetectable. This has led to the resolution of numerous cold cases and has provided closure for victims' families.
The development of more sophisticated luminol formulations has further enhanced its utility in forensic investigations. Modern versions offer improved sensitivity, longer-lasting luminescence, and reduced interference from other substances. These advancements have made luminol an even more reliable tool for crime scene analysts.
Luminol's impact extends beyond blood detection. It has also found applications in the identification of other bodily fluids and certain chemical residues, broadening the scope of forensic examinations. This versatility has made luminol an essential component of forensic toolkits worldwide.
The integration of luminol with digital imaging technologies has revolutionized the documentation of crime scenes. High-sensitivity cameras can now capture the faint luminescence produced by the luminol reaction, creating detailed maps of blood spatter patterns that can be analyzed long after the initial investigation.
Furthermore, the use of luminol has spurred advancements in forensic training and education. Simulated crime scenes using luminol allow forensic science students and law enforcement personnel to gain hands-on experience in blood pattern analysis and evidence collection under realistic conditions.
As forensic science continues to evolve, luminol remains at the forefront of innovation. Ongoing research aims to develop even more specific and sensitive luminol-based techniques, potentially allowing for the determination of blood age or the differentiation between human and animal blood at crime scenes.
The application of luminol in forensic science has significantly advanced the field by providing a non-destructive and highly sensitive method for visualizing latent bloodstains. Its ability to react with the iron in hemoglobin produces a distinctive blue glow, allowing investigators to identify and document blood evidence that may be invisible to the naked eye or that has been subjected to cleaning attempts.
One of the most significant advancements brought about by luminol is its effectiveness in cold cases. Forensic teams can now revisit crime scenes that are months or even years old, uncovering crucial evidence that was previously undetectable. This has led to the resolution of numerous cold cases and has provided closure for victims' families.
The development of more sophisticated luminol formulations has further enhanced its utility in forensic investigations. Modern versions offer improved sensitivity, longer-lasting luminescence, and reduced interference from other substances. These advancements have made luminol an even more reliable tool for crime scene analysts.
Luminol's impact extends beyond blood detection. It has also found applications in the identification of other bodily fluids and certain chemical residues, broadening the scope of forensic examinations. This versatility has made luminol an essential component of forensic toolkits worldwide.
The integration of luminol with digital imaging technologies has revolutionized the documentation of crime scenes. High-sensitivity cameras can now capture the faint luminescence produced by the luminol reaction, creating detailed maps of blood spatter patterns that can be analyzed long after the initial investigation.
Furthermore, the use of luminol has spurred advancements in forensic training and education. Simulated crime scenes using luminol allow forensic science students and law enforcement personnel to gain hands-on experience in blood pattern analysis and evidence collection under realistic conditions.
As forensic science continues to evolve, luminol remains at the forefront of innovation. Ongoing research aims to develop even more specific and sensitive luminol-based techniques, potentially allowing for the determination of blood age or the differentiation between human and animal blood at crime scenes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!