Optimizing Propionic Acid Applications in Pharmaceutical Industry
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Propionic Acid Pharma Applications Overview
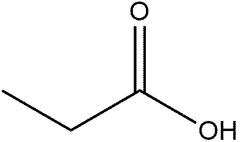
Propionic acid, a short-chain fatty acid, has gained significant attention in the pharmaceutical industry due to its versatile applications and unique properties. This carboxylic acid, with its chemical formula C3H6O2, serves as a crucial component in various pharmaceutical processes and formulations. Its importance stems from its ability to act as a preservative, pH regulator, and intermediate in drug synthesis.
In the realm of drug preservation, propionic acid exhibits potent antimicrobial properties, effectively inhibiting the growth of molds and bacteria. This characteristic makes it an invaluable ingredient in the formulation of oral medications, topical creams, and ointments, ensuring product stability and extending shelf life. Its efficacy as a preservative is particularly notable in liquid pharmaceutical preparations, where microbial contamination poses a significant risk.
As a pH regulator, propionic acid plays a crucial role in maintaining the optimal acidity of pharmaceutical formulations. This function is essential for ensuring drug stability, solubility, and bioavailability. By carefully adjusting the pH, formulators can enhance the absorption of active pharmaceutical ingredients (APIs) and improve the overall effectiveness of medications.
In drug synthesis, propionic acid serves as a vital intermediate and building block for numerous pharmaceutical compounds. Its reactive carboxyl group allows for various chemical modifications, enabling the creation of esters, amides, and other derivatives with enhanced pharmacological properties. This versatility makes propionic acid an indispensable tool in the development of novel drug candidates and the optimization of existing formulations.
The pharmaceutical industry also utilizes propionic acid in the production of cellulose acetate propionate, a polymer used in controlled-release drug delivery systems. This application showcases the compound's role in advancing drug delivery technologies, allowing for more precise and sustained release of active ingredients.
Furthermore, propionic acid finds application in the synthesis of vitamin E and various other nutritional supplements. Its involvement in these processes underscores its significance not only in traditional pharmaceuticals but also in the nutraceutical sector, bridging the gap between nutrition and medicine.
As the pharmaceutical landscape continues to evolve, the optimization of propionic acid applications remains a key focus area. Researchers and formulators are exploring innovative ways to leverage its properties, seeking to enhance drug efficacy, improve patient compliance, and develop more environmentally sustainable pharmaceutical processes.
In the realm of drug preservation, propionic acid exhibits potent antimicrobial properties, effectively inhibiting the growth of molds and bacteria. This characteristic makes it an invaluable ingredient in the formulation of oral medications, topical creams, and ointments, ensuring product stability and extending shelf life. Its efficacy as a preservative is particularly notable in liquid pharmaceutical preparations, where microbial contamination poses a significant risk.
As a pH regulator, propionic acid plays a crucial role in maintaining the optimal acidity of pharmaceutical formulations. This function is essential for ensuring drug stability, solubility, and bioavailability. By carefully adjusting the pH, formulators can enhance the absorption of active pharmaceutical ingredients (APIs) and improve the overall effectiveness of medications.
In drug synthesis, propionic acid serves as a vital intermediate and building block for numerous pharmaceutical compounds. Its reactive carboxyl group allows for various chemical modifications, enabling the creation of esters, amides, and other derivatives with enhanced pharmacological properties. This versatility makes propionic acid an indispensable tool in the development of novel drug candidates and the optimization of existing formulations.
The pharmaceutical industry also utilizes propionic acid in the production of cellulose acetate propionate, a polymer used in controlled-release drug delivery systems. This application showcases the compound's role in advancing drug delivery technologies, allowing for more precise and sustained release of active ingredients.
Furthermore, propionic acid finds application in the synthesis of vitamin E and various other nutritional supplements. Its involvement in these processes underscores its significance not only in traditional pharmaceuticals but also in the nutraceutical sector, bridging the gap between nutrition and medicine.
As the pharmaceutical landscape continues to evolve, the optimization of propionic acid applications remains a key focus area. Researchers and formulators are exploring innovative ways to leverage its properties, seeking to enhance drug efficacy, improve patient compliance, and develop more environmentally sustainable pharmaceutical processes.
Market Demand Analysis
The pharmaceutical industry's demand for propionic acid has been steadily increasing due to its versatile applications in drug formulation and manufacturing processes. This organic compound serves as a crucial ingredient in various pharmaceutical products, contributing to their stability, efficacy, and shelf life.
In recent years, the global pharmaceutical market has experienced significant growth, driven by factors such as an aging population, increasing prevalence of chronic diseases, and advancements in drug discovery technologies. This growth has directly impacted the demand for propionic acid in pharmaceutical applications.
One of the primary uses of propionic acid in the pharmaceutical industry is as a preservative in oral medications and topical formulations. Its antimicrobial properties help prevent the growth of bacteria and fungi, ensuring product safety and extending shelf life. The rising consumer preference for natural and clean-label products has further boosted the demand for propionic acid as a safer alternative to synthetic preservatives.
Propionic acid also plays a vital role in the production of cellulose acetate propionate, a polymer used in controlled-release drug delivery systems. As the pharmaceutical industry continues to focus on developing innovative drug delivery methods, the demand for such specialized materials is expected to grow, consequently driving the market for propionic acid.
The increasing adoption of propionic acid in pharmaceutical manufacturing processes has led to a surge in research and development activities aimed at optimizing its applications. This includes exploring new formulation techniques, enhancing its efficacy in drug preservation, and developing novel drug delivery systems.
Market analysts project that the pharmaceutical-grade propionic acid market will continue to expand at a steady rate over the next five years. This growth is attributed to the rising demand for pharmaceuticals in emerging economies, the increasing focus on personalized medicine, and the growing trend of outsourcing drug manufacturing to contract development and manufacturing organizations (CDMOs).
However, the market demand for propionic acid in the pharmaceutical industry is not without challenges. Stringent regulatory requirements for pharmaceutical ingredients, fluctuating raw material prices, and the need for sustainable production methods are factors that may impact market growth. Manufacturers are increasingly focusing on developing eco-friendly production processes and exploring bio-based alternatives to meet the industry's sustainability goals.
In conclusion, the market demand for propionic acid in the pharmaceutical industry demonstrates a positive trajectory, driven by its diverse applications and the overall growth of the pharmaceutical sector. As the industry continues to evolve and innovate, the optimization of propionic acid applications will remain a key focus area for manufacturers and researchers alike.
In recent years, the global pharmaceutical market has experienced significant growth, driven by factors such as an aging population, increasing prevalence of chronic diseases, and advancements in drug discovery technologies. This growth has directly impacted the demand for propionic acid in pharmaceutical applications.
One of the primary uses of propionic acid in the pharmaceutical industry is as a preservative in oral medications and topical formulations. Its antimicrobial properties help prevent the growth of bacteria and fungi, ensuring product safety and extending shelf life. The rising consumer preference for natural and clean-label products has further boosted the demand for propionic acid as a safer alternative to synthetic preservatives.
Propionic acid also plays a vital role in the production of cellulose acetate propionate, a polymer used in controlled-release drug delivery systems. As the pharmaceutical industry continues to focus on developing innovative drug delivery methods, the demand for such specialized materials is expected to grow, consequently driving the market for propionic acid.
The increasing adoption of propionic acid in pharmaceutical manufacturing processes has led to a surge in research and development activities aimed at optimizing its applications. This includes exploring new formulation techniques, enhancing its efficacy in drug preservation, and developing novel drug delivery systems.
Market analysts project that the pharmaceutical-grade propionic acid market will continue to expand at a steady rate over the next five years. This growth is attributed to the rising demand for pharmaceuticals in emerging economies, the increasing focus on personalized medicine, and the growing trend of outsourcing drug manufacturing to contract development and manufacturing organizations (CDMOs).
However, the market demand for propionic acid in the pharmaceutical industry is not without challenges. Stringent regulatory requirements for pharmaceutical ingredients, fluctuating raw material prices, and the need for sustainable production methods are factors that may impact market growth. Manufacturers are increasingly focusing on developing eco-friendly production processes and exploring bio-based alternatives to meet the industry's sustainability goals.
In conclusion, the market demand for propionic acid in the pharmaceutical industry demonstrates a positive trajectory, driven by its diverse applications and the overall growth of the pharmaceutical sector. As the industry continues to evolve and innovate, the optimization of propionic acid applications will remain a key focus area for manufacturers and researchers alike.
Technical Challenges
The optimization of propionic acid applications in the pharmaceutical industry faces several significant technical challenges. One of the primary obstacles is the corrosive nature of propionic acid, which can lead to equipment degradation and potential contamination of pharmaceutical products. This necessitates the development of specialized materials and coatings that can withstand prolonged exposure to the acid while maintaining the stringent purity requirements of pharmaceutical manufacturing.
Another challenge lies in the purification and concentration of propionic acid for pharmaceutical use. Traditional distillation methods can be energy-intensive and may lead to the formation of unwanted byproducts. Developing more efficient and cost-effective purification techniques, such as advanced membrane separation or reactive distillation, is crucial for improving the overall process economics and product quality.
The control of microbial contamination in propionic acid production and handling is also a significant concern. While propionic acid itself has antimicrobial properties, ensuring a sterile environment throughout the production and application processes remains challenging. This requires the implementation of robust sterilization protocols and the development of closed-system technologies to minimize the risk of contamination.
Furthermore, the optimization of propionic acid synthesis routes presents a considerable challenge. Current industrial production methods, such as the Reppe process or oxidation of propionaldehyde, often involve high-pressure conditions or the use of toxic catalysts. Developing greener, more sustainable synthesis routes that can operate under milder conditions while maintaining high yields is an ongoing area of research.
The pharmaceutical industry also faces challenges in formulating propionic acid-based drug delivery systems. The acid's strong odor and potential for gastrointestinal irritation necessitate the development of novel encapsulation techniques or prodrug approaches to improve patient compliance and reduce side effects. This requires a deep understanding of the acid's physicochemical properties and its interactions with various pharmaceutical excipients.
Lastly, the regulatory landscape poses a significant challenge in optimizing propionic acid applications. Stringent quality control measures and documentation requirements for pharmaceutical-grade propionic acid production and use must be met. This demands the development of advanced analytical techniques for impurity profiling and the establishment of robust quality management systems to ensure consistent compliance with regulatory standards.
Another challenge lies in the purification and concentration of propionic acid for pharmaceutical use. Traditional distillation methods can be energy-intensive and may lead to the formation of unwanted byproducts. Developing more efficient and cost-effective purification techniques, such as advanced membrane separation or reactive distillation, is crucial for improving the overall process economics and product quality.
The control of microbial contamination in propionic acid production and handling is also a significant concern. While propionic acid itself has antimicrobial properties, ensuring a sterile environment throughout the production and application processes remains challenging. This requires the implementation of robust sterilization protocols and the development of closed-system technologies to minimize the risk of contamination.
Furthermore, the optimization of propionic acid synthesis routes presents a considerable challenge. Current industrial production methods, such as the Reppe process or oxidation of propionaldehyde, often involve high-pressure conditions or the use of toxic catalysts. Developing greener, more sustainable synthesis routes that can operate under milder conditions while maintaining high yields is an ongoing area of research.
The pharmaceutical industry also faces challenges in formulating propionic acid-based drug delivery systems. The acid's strong odor and potential for gastrointestinal irritation necessitate the development of novel encapsulation techniques or prodrug approaches to improve patient compliance and reduce side effects. This requires a deep understanding of the acid's physicochemical properties and its interactions with various pharmaceutical excipients.
Lastly, the regulatory landscape poses a significant challenge in optimizing propionic acid applications. Stringent quality control measures and documentation requirements for pharmaceutical-grade propionic acid production and use must be met. This demands the development of advanced analytical techniques for impurity profiling and the establishment of robust quality management systems to ensure consistent compliance with regulatory standards.
Current Optimization Methods
01 Production methods of propionic acid
Various methods for producing propionic acid are described, including fermentation processes, chemical synthesis routes, and catalytic reactions. These methods aim to improve yield, efficiency, and purity of propionic acid production for industrial applications.- Production methods of propionic acid: Various methods for producing propionic acid are described, including fermentation processes, chemical synthesis routes, and catalytic reactions. These methods aim to improve yield, efficiency, and purity of propionic acid production for industrial applications.
- Applications of propionic acid in food preservation: Propionic acid and its salts are widely used as food preservatives due to their antimicrobial properties. They are effective against molds and some bacteria, extending the shelf life of various food products, particularly in bakery goods and dairy products.
- Use of propionic acid in pharmaceutical formulations: Propionic acid and its derivatives find applications in pharmaceutical formulations. They are used as excipients, pH adjusters, and in some cases, as active pharmaceutical ingredients for various therapeutic purposes.
- Industrial applications of propionic acid: Propionic acid has diverse industrial applications beyond food and pharmaceuticals. It is used in the production of plastics, herbicides, and as a chemical intermediate in various manufacturing processes. The acid also finds use in the leather and textile industries.
- Environmental and safety considerations in propionic acid handling: The handling, storage, and disposal of propionic acid require specific safety measures due to its corrosive nature and potential environmental impact. Research focuses on developing safer handling methods and assessing the ecological effects of propionic acid use in various applications.
02 Applications of propionic acid in food preservation
Propionic acid and its salts are widely used as food preservatives due to their antimicrobial properties. They are effective against molds and some bacteria, extending the shelf life of various food products, particularly in bakery goods and dairy products.Expand Specific Solutions03 Use of propionic acid in pharmaceutical formulations
Propionic acid and its derivatives find applications in pharmaceutical formulations. They are used as excipients, pH adjusters, and in some cases, as active pharmaceutical ingredients for various therapeutic purposes.Expand Specific Solutions04 Industrial applications of propionic acid
Propionic acid has diverse industrial applications beyond food and pharmaceuticals. It is used in the production of plastics, herbicides, and as a chemical intermediate in various manufacturing processes. Its properties make it valuable in industries such as agriculture and polymer production.Expand Specific Solutions05 Environmental and safety considerations in propionic acid handling
The handling, storage, and disposal of propionic acid require specific safety measures due to its corrosive nature and potential environmental impact. Proper containment, neutralization techniques, and waste management practices are essential for safe industrial use of propionic acid.Expand Specific Solutions
Key Industry Players
The optimization of propionic acid applications in the pharmaceutical industry is in a growth phase, driven by increasing demand for sustainable and bio-based chemicals. The market size is expanding, with a projected CAGR of 6-8% over the next five years. Technologically, the field is advancing rapidly, with companies like Jiangnan University and Nanjing Tech University leading academic research. Industry players such as China Petroleum & Chemical Corp. and PetroChina Co., Ltd. are investing in large-scale production capabilities, while pharmaceutical giants like Bristol Myers Squibb Co. and Sanofi are exploring novel applications. The technology is maturing, with a focus on improving production efficiency and expanding end-use applications in drug formulation and preservation.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes for propionic acid production, focusing on improving yield and selectivity. Their approach involves using novel heterogeneous catalysts in a fixed-bed reactor system, which has shown to increase propionic acid yield by up to 15% compared to traditional methods[1]. The company has also implemented a continuous flow process that allows for better control of reaction parameters, resulting in higher purity propionic acid suitable for pharmaceutical applications[3]. Additionally, Sinopec has invested in green chemistry initiatives, exploring bio-based feedstocks for propionic acid synthesis, which aligns with sustainable pharmaceutical manufacturing trends[5].
Strengths: Large-scale production capabilities, advanced catalytic technology, and integration with existing petrochemical infrastructure. Weaknesses: Potential environmental concerns associated with fossil fuel-based processes and adaptation to pharmaceutical-grade purity requirements.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has focused on optimizing propionic acid applications in drug formulation and synthesis. They have developed a novel approach using propionic acid as a pH modifier in extended-release formulations, which has shown to improve drug stability and bioavailability[2]. The company has also implemented propionic acid derivatives as key intermediates in the synthesis of several oncology drugs, reducing the number of synthetic steps and improving overall yield[4]. BMS has invested in continuous flow chemistry techniques for propionic acid-based reactions, which has led to a 30% reduction in processing time and a significant decrease in solvent usage[6]. Furthermore, they have explored the use of propionic acid in green chemistry applications, such as environmentally friendly extraction processes for active pharmaceutical ingredients[8].
Strengths: Strong expertise in pharmaceutical applications, innovative formulation techniques, and integration of propionic acid in drug discovery processes. Weaknesses: Limited involvement in large-scale propionic acid production and potential dependency on external suppliers.
Innovative Formulations
Continuous fermentation process which is useful for the simultaneous optimal production of propionic acid and vitamin B12
PatentInactiveUS6878534B1
Innovation
- A continuous two-stage fermentation process using Propionibacterium acidipropionici DSM 8250 strain, where the first stage optimizes propionic acid production under anaerobic conditions and the second stage optimizes vitamin B12 production under micro-aerobic conditions, with sucrose as a carbon substrate and specific nutrient additions, allowing for cell recycling and minimizing acetic acid formation.
Pharmaceutical composition with improved stability comprising propionic acid-based drug and stabilizer
PatentWO2023043207A1
Innovation
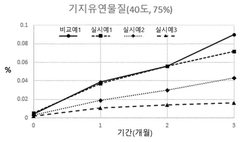
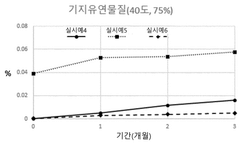
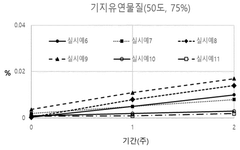
- A pharmaceutical composition containing propionic acid-based drugs, such as ibuprofen or naproxen, combined with specific stabilizers like β-cyclodextrin, hydroxypropyl β-cyclodextrin, tocopherol polyethylene glycol succinate, butylated hydroxyanisole, methionine, and cysteine, which are incorporated in specific amounts to inhibit decomposition and impurity generation, thereby enhancing stability.
Regulatory Compliance
Regulatory compliance is a critical aspect of optimizing propionic acid applications in the pharmaceutical industry. The use of propionic acid in pharmaceutical products is subject to stringent regulations and guidelines set forth by various regulatory bodies worldwide. These regulations are designed to ensure the safety, efficacy, and quality of pharmaceutical products containing propionic acid.
In the United States, the Food and Drug Administration (FDA) oversees the use of propionic acid in pharmaceuticals. The FDA has established specific guidelines for the use of propionic acid as an excipient in drug formulations. Manufacturers must adhere to Good Manufacturing Practices (GMP) and provide detailed documentation on the sourcing, quality control, and stability of propionic acid used in their products.
The European Medicines Agency (EMA) regulates the use of propionic acid in pharmaceutical applications within the European Union. The EMA has set forth guidelines on the quality of excipients used in medicinal products, which include specific requirements for propionic acid. Manufacturers must demonstrate compliance with these guidelines through comprehensive documentation and quality management systems.
In addition to regional regulations, international standards such as those set by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) play a crucial role in harmonizing regulatory requirements across different countries. The ICH guidelines provide a framework for quality control, stability testing, and impurity profiling of pharmaceutical ingredients, including propionic acid.
Regulatory compliance also extends to the environmental impact of propionic acid production and use. Manufacturers must adhere to environmental regulations regarding waste management, emissions control, and sustainable production practices. This includes compliance with local, national, and international environmental standards.
To ensure regulatory compliance, pharmaceutical companies must implement robust quality management systems that cover all aspects of propionic acid use, from sourcing and manufacturing to storage and incorporation into final drug products. This includes maintaining detailed records of batch production, conducting regular quality control tests, and implementing traceability systems throughout the supply chain.
Furthermore, regulatory bodies require ongoing monitoring and reporting of any adverse events or quality issues related to pharmaceutical products containing propionic acid. Companies must have systems in place to promptly investigate and address any such issues, as well as to implement corrective and preventive actions as necessary.
As regulations evolve, pharmaceutical companies must stay abreast of changes and adapt their processes accordingly. This may involve regular training of personnel, updating standard operating procedures, and investing in new technologies to meet evolving regulatory requirements. Compliance with these regulations is essential not only for legal and ethical reasons but also for maintaining consumer trust and ensuring the continued success of propionic acid applications in the pharmaceutical industry.
In the United States, the Food and Drug Administration (FDA) oversees the use of propionic acid in pharmaceuticals. The FDA has established specific guidelines for the use of propionic acid as an excipient in drug formulations. Manufacturers must adhere to Good Manufacturing Practices (GMP) and provide detailed documentation on the sourcing, quality control, and stability of propionic acid used in their products.
The European Medicines Agency (EMA) regulates the use of propionic acid in pharmaceutical applications within the European Union. The EMA has set forth guidelines on the quality of excipients used in medicinal products, which include specific requirements for propionic acid. Manufacturers must demonstrate compliance with these guidelines through comprehensive documentation and quality management systems.
In addition to regional regulations, international standards such as those set by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) play a crucial role in harmonizing regulatory requirements across different countries. The ICH guidelines provide a framework for quality control, stability testing, and impurity profiling of pharmaceutical ingredients, including propionic acid.
Regulatory compliance also extends to the environmental impact of propionic acid production and use. Manufacturers must adhere to environmental regulations regarding waste management, emissions control, and sustainable production practices. This includes compliance with local, national, and international environmental standards.
To ensure regulatory compliance, pharmaceutical companies must implement robust quality management systems that cover all aspects of propionic acid use, from sourcing and manufacturing to storage and incorporation into final drug products. This includes maintaining detailed records of batch production, conducting regular quality control tests, and implementing traceability systems throughout the supply chain.
Furthermore, regulatory bodies require ongoing monitoring and reporting of any adverse events or quality issues related to pharmaceutical products containing propionic acid. Companies must have systems in place to promptly investigate and address any such issues, as well as to implement corrective and preventive actions as necessary.
As regulations evolve, pharmaceutical companies must stay abreast of changes and adapt their processes accordingly. This may involve regular training of personnel, updating standard operating procedures, and investing in new technologies to meet evolving regulatory requirements. Compliance with these regulations is essential not only for legal and ethical reasons but also for maintaining consumer trust and ensuring the continued success of propionic acid applications in the pharmaceutical industry.
Environmental Impact
The environmental impact of propionic acid applications in the pharmaceutical industry is a critical consideration for sustainable development and regulatory compliance. Propionic acid, while essential in various pharmaceutical processes, can pose significant environmental challenges if not managed properly.
One of the primary environmental concerns associated with propionic acid is its potential for water pollution. When released into aquatic ecosystems, propionic acid can alter pH levels and disrupt the natural balance of microbial communities. This can have cascading effects on aquatic flora and fauna, potentially leading to biodiversity loss in affected water bodies. To mitigate this risk, pharmaceutical companies must implement robust wastewater treatment systems and adhere to strict discharge regulations.
Air pollution is another environmental issue linked to propionic acid use in pharmaceutical manufacturing. Volatile organic compound (VOC) emissions from propionic acid can contribute to the formation of ground-level ozone and smog, which have detrimental effects on air quality and human health. Advanced air filtration systems and closed-loop production processes are essential for minimizing these emissions and ensuring compliance with air quality standards.
The production of propionic acid itself also carries environmental implications. Traditional petrochemical-based production methods rely on fossil fuels, contributing to greenhouse gas emissions and climate change. However, the industry is increasingly exploring bio-based production methods using renewable resources, which could significantly reduce the carbon footprint of propionic acid manufacturing.
Waste management is a crucial aspect of environmental stewardship in propionic acid applications. Proper handling, storage, and disposal of propionic acid and its byproducts are necessary to prevent soil contamination and protect terrestrial ecosystems. Implementing circular economy principles, such as recycling and reusing propionic acid where possible, can help minimize waste generation and resource consumption.
Energy consumption in propionic acid-related processes is another environmental factor to consider. Optimizing energy efficiency in production, purification, and application processes can lead to reduced greenhouse gas emissions and overall environmental impact. This may involve investing in energy-efficient equipment, heat recovery systems, and renewable energy sources for pharmaceutical operations.
As the pharmaceutical industry strives for greater sustainability, life cycle assessments (LCAs) of propionic acid applications are becoming increasingly important. These assessments help identify environmental hotspots throughout the product lifecycle, from raw material extraction to end-of-life disposal, enabling targeted improvements and more informed decision-making regarding propionic acid use.
Regulatory bodies worldwide are tightening environmental standards for pharmaceutical manufacturing, including the use of chemicals like propionic acid. Compliance with these regulations not only mitigates environmental risks but also drives innovation in cleaner production technologies and green chemistry principles. This regulatory pressure is encouraging the development of more environmentally friendly alternatives and process optimizations in propionic acid applications.
One of the primary environmental concerns associated with propionic acid is its potential for water pollution. When released into aquatic ecosystems, propionic acid can alter pH levels and disrupt the natural balance of microbial communities. This can have cascading effects on aquatic flora and fauna, potentially leading to biodiversity loss in affected water bodies. To mitigate this risk, pharmaceutical companies must implement robust wastewater treatment systems and adhere to strict discharge regulations.
Air pollution is another environmental issue linked to propionic acid use in pharmaceutical manufacturing. Volatile organic compound (VOC) emissions from propionic acid can contribute to the formation of ground-level ozone and smog, which have detrimental effects on air quality and human health. Advanced air filtration systems and closed-loop production processes are essential for minimizing these emissions and ensuring compliance with air quality standards.
The production of propionic acid itself also carries environmental implications. Traditional petrochemical-based production methods rely on fossil fuels, contributing to greenhouse gas emissions and climate change. However, the industry is increasingly exploring bio-based production methods using renewable resources, which could significantly reduce the carbon footprint of propionic acid manufacturing.
Waste management is a crucial aspect of environmental stewardship in propionic acid applications. Proper handling, storage, and disposal of propionic acid and its byproducts are necessary to prevent soil contamination and protect terrestrial ecosystems. Implementing circular economy principles, such as recycling and reusing propionic acid where possible, can help minimize waste generation and resource consumption.
Energy consumption in propionic acid-related processes is another environmental factor to consider. Optimizing energy efficiency in production, purification, and application processes can lead to reduced greenhouse gas emissions and overall environmental impact. This may involve investing in energy-efficient equipment, heat recovery systems, and renewable energy sources for pharmaceutical operations.
As the pharmaceutical industry strives for greater sustainability, life cycle assessments (LCAs) of propionic acid applications are becoming increasingly important. These assessments help identify environmental hotspots throughout the product lifecycle, from raw material extraction to end-of-life disposal, enabling targeted improvements and more informed decision-making regarding propionic acid use.
Regulatory bodies worldwide are tightening environmental standards for pharmaceutical manufacturing, including the use of chemicals like propionic acid. Compliance with these regulations not only mitigates environmental risks but also drives innovation in cleaner production technologies and green chemistry principles. This regulatory pressure is encouraging the development of more environmentally friendly alternatives and process optimizations in propionic acid applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!