Pathway Engineering: Advances in Modifying Pentose Phosphate Pathway
Pentose Phosphate Pathway Engineering: Background and Objectives
The Pentose Phosphate Pathway (PPP) has been a focal point of metabolic engineering for decades, playing a crucial role in cellular metabolism and biosynthesis. This pathway, discovered in the 1950s, serves as a vital alternative to glycolysis for glucose oxidation and is essential for generating NADPH and ribose-5-phosphate, key components in cellular redox balance and nucleotide synthesis.
The evolution of PPP engineering has been driven by the increasing demand for sustainable production of valuable chemicals and biofuels. Initially, research focused on understanding the pathway's regulation and its interconnections with other metabolic networks. As biotechnology advanced, efforts shifted towards manipulating the PPP to enhance the production of desired compounds.
Recent years have witnessed significant breakthroughs in PPP engineering, particularly in the context of metabolic flux redistribution and enzyme engineering. These advancements aim to optimize the pathway's efficiency and redirect carbon flow towards target products. The development of synthetic biology tools and genome editing techniques, such as CRISPR-Cas9, has further accelerated progress in this field.
The primary objectives of current PPP engineering research are multifaceted. Firstly, there is a strong emphasis on improving the production of NADPH, a critical reducing agent in various biosynthetic processes. This is particularly important for the production of fatty acids, isoprenoids, and other high-value chemicals.
Secondly, researchers are focusing on enhancing the production of pentose sugars, which are essential precursors for numerous biochemicals and biofuels. This includes efforts to improve the utilization of lignocellulosic biomass, a renewable feedstock rich in pentose sugars.
Another key objective is to develop more robust and efficient microbial cell factories by fine-tuning the PPP. This involves optimizing the balance between the oxidative and non-oxidative branches of the pathway to maximize product yield while maintaining cellular health.
Furthermore, there is growing interest in exploring the potential of the PPP in non-traditional hosts, including photosynthetic organisms and extremophiles. This expansion of host range could open new avenues for biotechnological applications in diverse environments.
As we look towards the future, the field of PPP engineering is poised for further innovation. Emerging technologies such as machine learning and metabolic modeling are expected to play increasingly important roles in predicting and optimizing pathway modifications. These advancements, coupled with ongoing improvements in synthetic biology tools, promise to unlock new possibilities in the rational design of metabolic pathways for sustainable bioproduction.
Market Demand for Enhanced Pentose Phosphate Pathway Products
The market demand for enhanced Pentose Phosphate Pathway (PPP) products has been steadily growing, driven by the increasing need for sustainable and efficient bioprocesses across various industries. The PPP plays a crucial role in cellular metabolism, providing essential precursors for nucleotide synthesis and generating NADPH for biosynthetic reactions. As industries seek to optimize their production processes and develop novel bioproducts, the demand for engineered PPP solutions has surged.
In the biofuel sector, enhanced PPP products have gained significant traction. The ability to manipulate the PPP to increase the production of pentose sugars has opened new avenues for efficient biofuel production from lignocellulosic biomass. This has led to a growing market for engineered microorganisms capable of fermenting both hexose and pentose sugars, addressing a major bottleneck in second-generation biofuel production.
The pharmaceutical industry has also shown keen interest in PPP engineering. The pathway's role in producing ribose-5-phosphate, a key precursor for nucleotide synthesis, has made it a target for enhancing the production of nucleoside-based drugs and RNA therapeutics. As the demand for these advanced therapeutics continues to rise, the market for PPP-engineered cell lines and production platforms is expected to expand significantly.
In the field of industrial biotechnology, there is a growing demand for PPP-enhanced microorganisms capable of producing high-value chemicals and materials. The ability to redirect carbon flux through the PPP has enabled the development of more efficient production strains for compounds such as aromatic amino acids, flavonoids, and other secondary metabolites. This has created new opportunities in the fine chemicals and nutraceuticals markets.
The food and beverage industry has also recognized the potential of PPP engineering. Enhanced production of NADPH through the PPP can improve the antioxidant capacity of food products and increase the yield of certain flavor compounds. This has led to a niche market for PPP-engineered yeast strains in the brewing and fermented food industries.
As sustainability becomes a key focus across industries, the demand for bio-based products continues to grow. PPP engineering offers a means to improve the efficiency of bioconversion processes, reducing waste and energy consumption. This aligns well with the increasing market demand for environmentally friendly production methods and products.
Current Pentose Phosphate Pathway Modification Challenges
The modification of the Pentose Phosphate Pathway (PPP) presents several significant challenges that researchers and bioengineers must overcome to fully harness its potential. One of the primary obstacles is the complex regulation of the pathway, which involves intricate feedback mechanisms and interconnections with other metabolic pathways. This complexity makes it difficult to predict the consequences of genetic modifications and often leads to unintended metabolic effects.
Another major challenge is the balancing of flux between the oxidative and non-oxidative branches of the PPP. Altering the activity of enzymes in one branch can have ripple effects throughout the entire pathway and connected metabolic networks. This delicate balance is crucial for maintaining cellular redox state and providing precursors for nucleotide and amino acid biosynthesis.
The competition for substrates between the PPP and glycolysis poses an additional hurdle. Modifying the PPP to increase the production of desired compounds often requires redirecting carbon flux away from central carbon metabolism, which can negatively impact cell growth and viability. Striking the right balance between product formation and cellular health remains a significant challenge.
Furthermore, the PPP's role in generating NADPH, a critical cofactor for biosynthetic reactions and cellular redox homeostasis, complicates modification efforts. Alterations that affect NADPH production can have far-reaching consequences on cellular metabolism and stress responses, potentially limiting the viability of engineered strains.
The heterogeneity of PPP regulation across different organisms adds another layer of complexity. Strategies that work well in model organisms like E. coli or S. cerevisiae may not translate directly to other industrially relevant microorganisms, necessitating species-specific optimization approaches.
Metabolic burden is also a significant concern when modifying the PPP. Overexpression of pathway enzymes or introduction of heterologous genes can strain cellular resources, leading to reduced growth rates and decreased overall productivity. Minimizing this burden while achieving desired flux modifications remains a key challenge.
Lastly, the integration of PPP modifications with other metabolic engineering strategies presents ongoing difficulties. Coordinating PPP alterations with changes in other pathways to create optimal production strains requires a systems-level understanding that is often beyond our current capabilities. Developing more sophisticated metabolic models and prediction tools is crucial for overcoming this challenge and realizing the full potential of PPP engineering in biotechnology applications.
Existing Pentose Phosphate Pathway Engineering Strategies
01 Genetic engineering of the pentose phosphate pathway
Genetic modification techniques are used to enhance or alter the pentose phosphate pathway in organisms. This involves manipulating genes related to key enzymes in the pathway to improve metabolic efficiency, increase production of desired compounds, or redirect carbon flux for biotechnological applications.- Genetic engineering of the pentose phosphate pathway: Genetic modifications are made to enhance or alter the pentose phosphate pathway in microorganisms. This can involve introducing new genes, modifying existing genes, or altering regulatory elements to improve the efficiency of the pathway or redirect metabolic flux. These modifications can lead to increased production of desired compounds or improved utilization of pentose sugars.
- Metabolic engineering for biofuel production: The pentose phosphate pathway is engineered in microorganisms to improve the production of biofuels. This involves optimizing the pathway to increase the yield of precursor molecules or redirecting carbon flux towards desired end products. Techniques may include overexpression of key enzymes, deletion of competing pathways, or introduction of heterologous genes.
- Pentose sugar utilization in fermentation processes: Methods are developed to improve the utilization of pentose sugars, such as xylose and arabinose, in fermentation processes. This can involve engineering microorganisms to efficiently transport and metabolize these sugars through the pentose phosphate pathway, leading to improved yields in industrial fermentations.
- Regulation and control of the pentose phosphate pathway: Strategies are developed to regulate and control the flux through the pentose phosphate pathway. This can involve modifying regulatory elements, altering enzyme expression levels, or introducing synthetic control circuits. The goal is to optimize the pathway's performance for specific applications or to respond to changing environmental conditions.
- Applications in metabolic engineering and synthetic biology: The pentose phosphate pathway is utilized in various metabolic engineering and synthetic biology applications. This includes designing artificial metabolic networks, creating novel biosynthetic pathways, or optimizing existing pathways for the production of valuable compounds. The pathway's ability to generate reducing power and precursor molecules makes it a valuable target for these applications.
02 Metabolic engineering for biofuel production
The pentose phosphate pathway is engineered to optimize the production of biofuels and other valuable chemicals. This involves modifying the pathway to efficiently convert pentose sugars into desired products, improving yield and reducing byproduct formation in microbial fermentation processes.Expand Specific Solutions03 Regulation and control of the pentose phosphate pathway
Research focuses on understanding and manipulating the regulatory mechanisms of the pentose phosphate pathway. This includes studying transcriptional and post-translational regulation, metabolic flux analysis, and developing strategies to control pathway activity for various biotechnological applications.Expand Specific Solutions04 Pentose phosphate pathway in disease and therapeutics
The role of the pentose phosphate pathway in various diseases, particularly cancer and metabolic disorders, is investigated. This research aims to develop therapeutic strategies targeting specific enzymes or metabolites of the pathway to treat or prevent these conditions.Expand Specific Solutions05 Novel enzymes and pathways related to pentose phosphate metabolism
Discovery and characterization of new enzymes and alternative pathways related to pentose phosphate metabolism. This includes identifying novel pentose-utilizing enzymes from various organisms and exploring their potential applications in biotechnology and metabolic engineering.Expand Specific Solutions
Key Players in Pentose Phosphate Pathway Modification Research
The pathway engineering field focusing on modifying the pentose phosphate pathway is in a growth phase, with increasing market size and technological advancements. The global market for metabolic engineering is expanding rapidly, driven by applications in biofuels, pharmaceuticals, and industrial chemicals. Companies like BASF SE, DSM IP Assets BV, and Bayer HealthCare are at the forefront of this technology, leveraging their expertise in biotechnology and chemical engineering. The technology's maturity varies across different applications, with some processes reaching commercial scale while others remain in research stages. Academic institutions such as The University of California and The Scripps Research Institute collaborate with industry leaders like Monsanto Technology LLC and Archer-Daniels-Midland Co. to push the boundaries of pathway engineering, indicating a dynamic and competitive landscape.
BASF SE
The Regents of the University of California
Core Innovations in Pentose Phosphate Pathway Modification
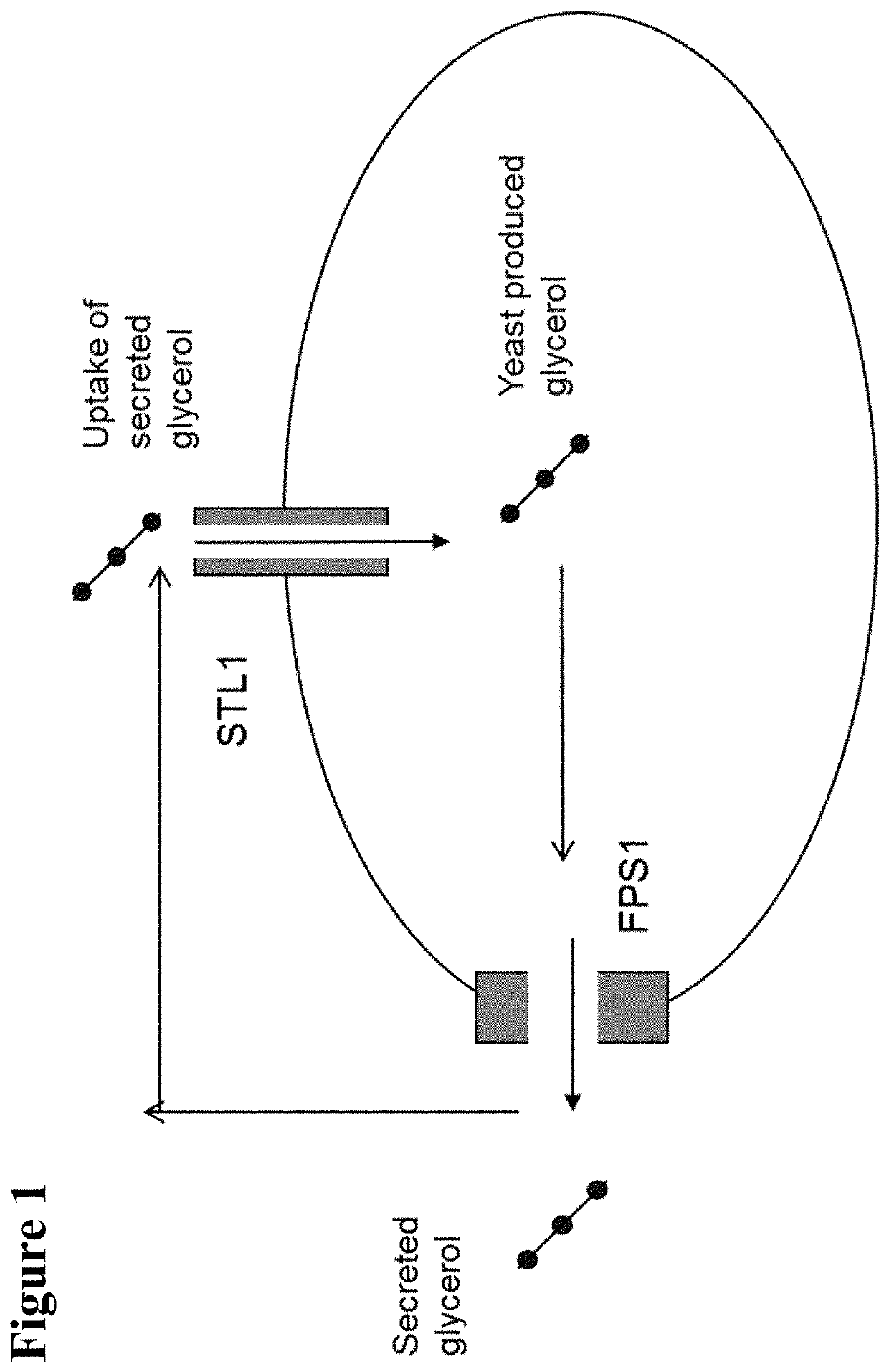
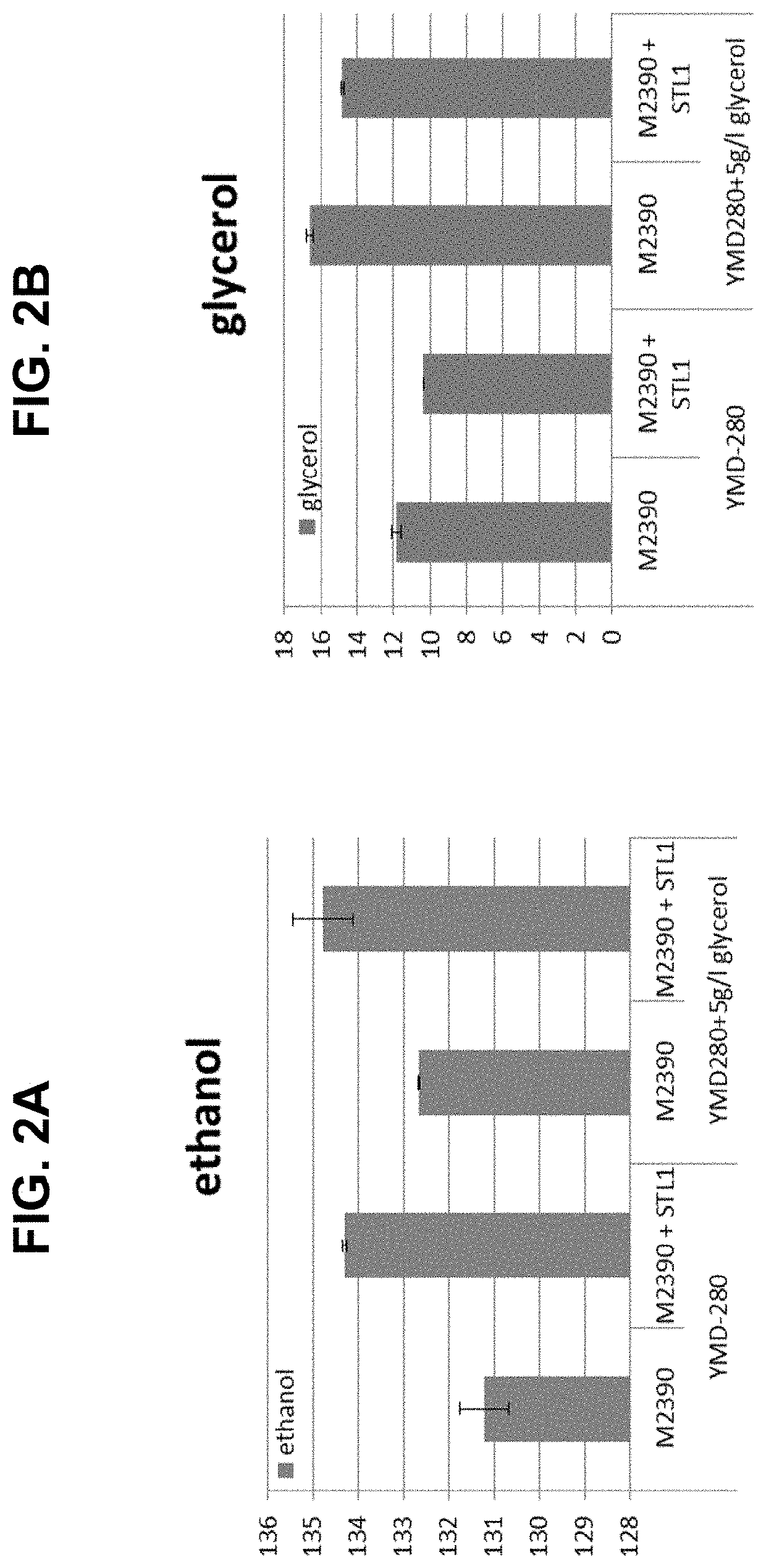
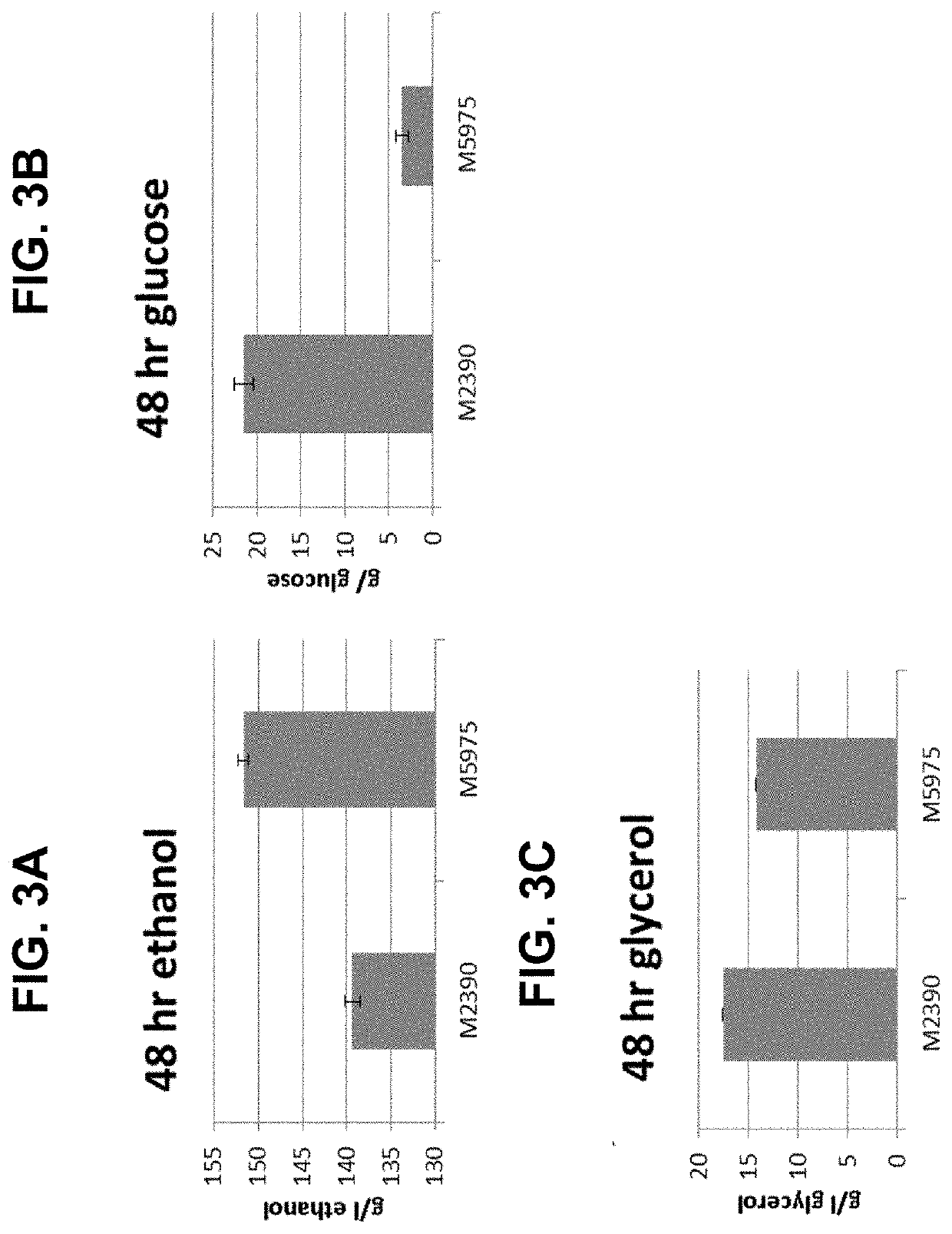
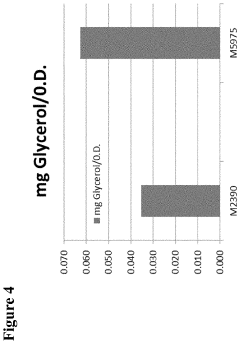
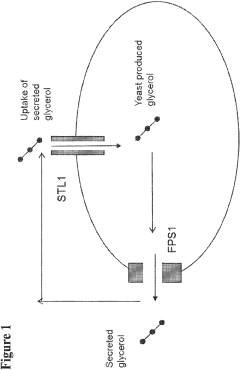
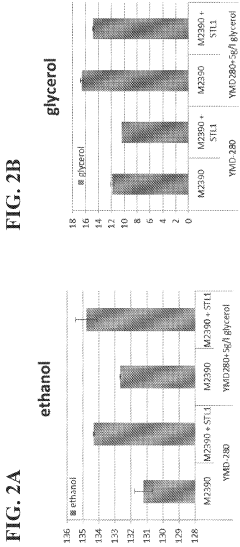
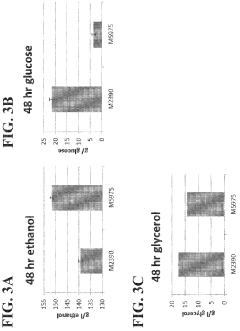
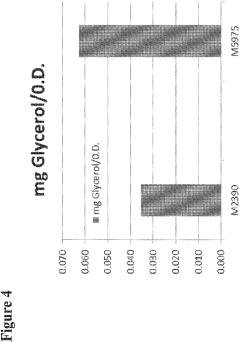
- Engineering recombinant microorganisms to uptake extracellular glycerol, overexpress glycerol import proteins like STL1, and downregulate or delete glycerol production genes, while introducing alternative metabolic pathways to convert carbohydrates to alcohols, such as ethanol or isopropanol, thereby reducing intracellular glycerol production.
- Engineering recombinant microorganisms to uptake extracellular glycerol, modulate its production, and utilize alternative electron acceptors to reduce glycerol levels, thereby increasing ethanol yield by redirecting carbon flow to desired end-products.
Metabolic Flux Analysis in Pentose Phosphate Pathway
Metabolic flux analysis (MFA) has emerged as a powerful tool for understanding and optimizing the pentose phosphate pathway (PPP) in metabolic engineering efforts. This technique provides quantitative insights into the distribution of carbon fluxes through the PPP and interconnected metabolic pathways, enabling researchers to identify bottlenecks and potential targets for pathway modification.
In the context of PPP engineering, MFA typically involves the use of isotope-labeled substrates, such as 13C-glucose, to trace the flow of carbon atoms through the pathway. By analyzing the labeling patterns of downstream metabolites using techniques like mass spectrometry or nuclear magnetic resonance spectroscopy, researchers can reconstruct the flux distribution within the PPP and related pathways.
One of the key advantages of MFA in PPP engineering is its ability to reveal the dynamic interplay between the oxidative and non-oxidative branches of the pathway. This information is crucial for understanding how genetic modifications or environmental perturbations affect the overall flux distribution and pathway efficiency.
Recent advances in MFA techniques have improved the resolution and accuracy of flux measurements in the PPP. For instance, the development of parallel labeling experiments, where multiple isotopomers are used simultaneously, has enhanced the precision of flux estimates and reduced experimental variability.
MFA has been instrumental in identifying rate-limiting steps and potential targets for genetic manipulation within the PPP. For example, studies using MFA have revealed that overexpression of transketolase and transaldolase enzymes can significantly increase the flux through the non-oxidative branch of the PPP, leading to improved production of aromatic amino acids and other valuable compounds.
Furthermore, MFA has been employed to assess the impact of PPP modifications on global cellular metabolism. By providing a systems-level view of metabolic fluxes, this technique helps researchers understand how alterations in the PPP affect other pathways, such as glycolysis and the tricarboxylic acid cycle, allowing for more holistic metabolic engineering strategies.
The integration of MFA with other omics technologies, such as transcriptomics and proteomics, has further enhanced its utility in PPP engineering. This multi-omics approach provides a comprehensive understanding of how genetic modifications influence not only metabolic fluxes but also gene expression and protein levels, leading to more informed and effective pathway engineering strategies.
Biosafety Considerations in Pentose Phosphate Pathway Engineering
The modification of the Pentose Phosphate Pathway (PPP) through pathway engineering presents significant biosafety considerations that must be carefully addressed. One primary concern is the potential for unintended metabolic consequences resulting from alterations to this critical pathway. The PPP plays a crucial role in generating NADPH and ribose-5-phosphate, which are essential for various cellular processes. Any modifications to this pathway could lead to imbalances in these key metabolites, potentially affecting cellular redox state, nucleotide synthesis, and overall metabolic homeostasis.
Another important biosafety consideration is the possibility of creating novel metabolic intermediates or byproducts as a result of PPP engineering. These new compounds may have unexpected toxicity profiles or interfere with other cellular processes, potentially leading to harmful effects on the host organism or the environment. Rigorous toxicological assessments and environmental impact studies are necessary to evaluate the safety of engineered PPP variants.
The potential for horizontal gene transfer is also a significant biosafety concern in PPP engineering. Modified genetic elements encoding altered PPP enzymes could potentially be transferred to other organisms, leading to unintended ecological consequences. This risk is particularly relevant when considering the use of engineered microorganisms in industrial or environmental applications.
Furthermore, the stability and containment of engineered organisms with modified PPP must be carefully evaluated. There is a need to develop robust containment strategies and fail-safe mechanisms to prevent the accidental release of engineered organisms into the environment. This includes the development of genetic safeguards, such as auxotrophic strains or suicide genes, to limit the survival and proliferation of engineered organisms outside controlled conditions.
Biosafety considerations also extend to the potential impact on human health, particularly in cases where PPP-engineered organisms or their products are intended for use in food, pharmaceuticals, or other applications with direct human exposure. Comprehensive safety assessments, including allergenicity and toxicity studies, are crucial to ensure the safety of these engineered products for human consumption or use.
Lastly, the regulatory landscape surrounding PPP engineering must be navigated carefully. Compliance with existing biosafety regulations and guidelines is essential, and there may be a need for the development of new regulatory frameworks specifically addressing the unique challenges posed by PPP engineering. This includes considerations for risk assessment, containment measures, and long-term monitoring of engineered organisms and their environmental impact.