Targeting Pentose Phosphate Pathway for Metabolic Disorders
Pentose Phosphate Pathway for Metabolic Disorders: Background and Objectives
The Pentose Phosphate Pathway (PPP) has emerged as a critical metabolic pathway in the context of metabolic disorders. This pathway, discovered in the 1950s, plays a crucial role in glucose metabolism and cellular redox balance. The PPP is responsible for generating NADPH, a key molecule in cellular antioxidant defense, and ribose-5-phosphate, essential for nucleotide synthesis. In recent years, research has increasingly focused on the PPP's involvement in various metabolic disorders, including diabetes, obesity, and cancer.
The evolution of PPP research has seen significant milestones, from its initial discovery to the elucidation of its complex regulatory mechanisms. Early studies primarily focused on the pathway's role in glucose metabolism. However, as our understanding of cellular metabolism deepened, the PPP's importance in maintaining redox balance and supporting cell proliferation became apparent. This shift in focus has led to a surge in research exploring the PPP's potential as a therapeutic target for metabolic disorders.
Current technological advancements, particularly in metabolomics and systems biology, have enabled a more comprehensive understanding of the PPP's intricate connections within cellular metabolism. These tools have revealed how alterations in PPP flux can contribute to the development and progression of metabolic disorders. For instance, dysregulation of the PPP has been linked to insulin resistance in diabetes and enhanced tumor growth in cancer.
The primary objective of targeting the PPP for metabolic disorders is to modulate its activity to achieve therapeutic benefits. This approach aims to restore metabolic balance by influencing glucose utilization, redox status, and cellular proliferation. Specific goals include developing inhibitors or activators of key PPP enzymes, such as glucose-6-phosphate dehydrogenase (G6PD), to regulate pathway flux.
Another crucial objective is to elucidate the cross-talk between the PPP and other metabolic pathways, such as glycolysis and lipid metabolism. Understanding these interactions is vital for developing comprehensive therapeutic strategies that address the complex nature of metabolic disorders. Additionally, researchers aim to identify biomarkers related to PPP activity, which could aid in early diagnosis and treatment monitoring of metabolic diseases.
As we look towards future developments, the field anticipates breakthroughs in targeted drug delivery systems that can modulate PPP activity in specific tissues or cell types. This precision approach could potentially minimize side effects while maximizing therapeutic efficacy. Furthermore, there is growing interest in exploring the PPP's role in emerging areas of metabolic research, such as immunometabolism and the gut microbiome's influence on host metabolism.
Market Analysis for Pentose Phosphate Pathway-Targeted Therapies
The market for therapies targeting the Pentose Phosphate Pathway (PPP) in metabolic disorders is experiencing significant growth and attracting considerable attention from pharmaceutical companies and investors. This pathway plays a crucial role in glucose metabolism and redox balance, making it an attractive target for treating various metabolic conditions, including diabetes, obesity, and cancer.
The global market for metabolic disorder treatments is substantial, with diabetes alone representing a market value of over $50 billion annually. As PPP-targeted therapies offer a novel approach to addressing these conditions, they are poised to capture a significant portion of this market. Industry analysts project that PPP-targeted therapies could potentially reach a market size of several billion dollars within the next decade, driven by the increasing prevalence of metabolic disorders worldwide.
Key factors contributing to the market growth of PPP-targeted therapies include the rising incidence of diabetes and obesity, growing awareness of metabolic disorders, and the limitations of current treatment options. Additionally, the potential for PPP-targeted therapies to address multiple metabolic conditions simultaneously makes them particularly attractive to both patients and healthcare providers.
Several pharmaceutical companies are actively developing PPP-targeted therapies, with a number of compounds in various stages of clinical trials. These range from small molecule inhibitors to biologics targeting specific enzymes within the pathway. The competitive landscape is diverse, with both established pharmaceutical giants and innovative biotech startups vying for market share.
Geographically, North America and Europe currently dominate the market for metabolic disorder treatments, and this trend is expected to continue for PPP-targeted therapies. However, rapidly growing economies in Asia-Pacific, particularly China and India, are anticipated to become significant markets due to their large populations and increasing prevalence of metabolic disorders.
Investors are showing keen interest in companies developing PPP-targeted therapies, with several successful funding rounds reported in recent years. This influx of capital is fueling research and development efforts, accelerating the path to market for promising therapies.
Despite the positive outlook, challenges remain in the market development of PPP-targeted therapies. These include regulatory hurdles, the need for long-term safety data, and potential competition from established treatments. Additionally, the complexity of the PPP and its interactions with other metabolic pathways necessitates careful consideration in drug development and marketing strategies.
In conclusion, the market for PPP-targeted therapies in metabolic disorders shows strong potential for growth, driven by unmet medical needs and advancing scientific understanding. As clinical trials progress and more data becomes available, the market is likely to see increased investment and development activity in this promising therapeutic area.
Current Challenges in Pentose Phosphate Pathway Modulation
Despite significant advancements in understanding the pentose phosphate pathway (PPP), several challenges persist in effectively modulating this metabolic pathway for therapeutic purposes. One of the primary obstacles is the complexity and interconnectedness of the PPP with other metabolic pathways. The PPP plays a crucial role in glucose metabolism, NADPH production, and nucleotide synthesis, making it challenging to target specific aspects without disrupting other essential cellular processes.
Another significant challenge lies in the development of selective and potent inhibitors or activators of key PPP enzymes. While some compounds have shown promise in preclinical studies, their translation to clinical applications has been limited due to off-target effects and potential toxicity. The glucose-6-phosphate dehydrogenase (G6PD) enzyme, for instance, has been a target of interest, but developing drugs that can modulate its activity without affecting other critical cellular functions remains difficult.
The tissue-specific regulation of the PPP presents another hurdle in developing effective therapeutic strategies. Different tissues and cell types exhibit varying levels of PPP activity and regulation, necessitating a more nuanced approach to pathway modulation. This heterogeneity complicates the development of systemic treatments and may require the exploration of targeted delivery methods or tissue-specific interventions.
Furthermore, the dynamic nature of metabolic disorders poses a challenge in PPP modulation. The pathway's activity and importance can fluctuate based on the metabolic state of the cell, making it difficult to achieve consistent therapeutic effects. This variability necessitates a deeper understanding of how PPP regulation changes under different physiological and pathological conditions.
The lack of reliable biomarkers for assessing PPP activity in vivo also hinders progress in this field. Current methods for measuring PPP flux often rely on invasive techniques or complex isotope tracing studies, limiting their applicability in clinical settings. Developing non-invasive imaging or blood-based biomarkers for PPP activity would greatly facilitate the evaluation of therapeutic interventions and patient monitoring.
Lastly, the potential for compensatory metabolic adaptations presents a significant challenge in PPP modulation. Cells may activate alternative pathways or mechanisms to maintain redox balance and nucleotide synthesis when the PPP is inhibited, potentially limiting the efficacy of therapeutic interventions. Understanding and addressing these adaptive responses will be crucial for developing effective long-term treatments targeting the PPP in metabolic disorders.
Existing Approaches to Targeting Pentose Phosphate Pathway for Metabolic Disorders
01 Genetic engineering of the pentose phosphate pathway
Genetic modifications are made to enhance or alter the pentose phosphate pathway in microorganisms. This can involve introducing new genes, modifying existing ones, or altering regulatory elements to improve flux through the pathway. These modifications aim to increase the production of desired compounds or improve the efficiency of cellular processes.- Genetic engineering of the pentose phosphate pathway: Genetic modification techniques are used to enhance or alter the pentose phosphate pathway in organisms. This can involve introducing new genes, modifying existing ones, or regulating gene expression to improve metabolic efficiency or produce desired compounds. Such modifications can lead to increased production of valuable metabolites or improved cellular processes.
- Utilization of the pentose phosphate pathway in biofuel production: The pentose phosphate pathway is exploited for the production of biofuels. This involves engineering microorganisms to efficiently convert pentose sugars into biofuel precursors or directly into biofuels. Optimizing this pathway can lead to improved yields and more cost-effective biofuel production processes.
- Regulation and manipulation of pentose phosphate pathway enzymes: Research focuses on understanding and manipulating the enzymes involved in the pentose phosphate pathway. This includes studying enzyme kinetics, developing inhibitors or activators, and engineering enzymes for improved activity or specificity. Such work can lead to better control of metabolic flux and enhanced production of desired compounds.
- Application of the pentose phosphate pathway in metabolic engineering: The pentose phosphate pathway is a key target in metabolic engineering efforts. By redirecting metabolic flux through this pathway, researchers aim to improve the production of various compounds, including amino acids, nucleotides, and other high-value chemicals. This involves systems biology approaches and metabolic modeling to optimize pathway performance.
- Role of the pentose phosphate pathway in cellular stress response: Studies investigate the importance of the pentose phosphate pathway in cellular stress responses, particularly in oxidative stress. The pathway's role in generating NADPH, a key molecule in maintaining cellular redox balance, is explored. Understanding this function can lead to new strategies for improving cell survival under stress conditions or developing treatments for oxidative stress-related disorders.
02 Metabolic engineering for improved pentose utilization
Strategies are developed to enhance the ability of microorganisms to utilize pentose sugars, such as xylose and arabinose, through the pentose phosphate pathway. This can involve introducing or optimizing genes for pentose transport and metabolism, as well as modifying regulatory networks to improve pentose fermentation efficiency.Expand Specific Solutions03 Regulation and control of the pentose phosphate pathway
Methods are developed to regulate and control the flux through the pentose phosphate pathway. This can involve manipulating key enzymes, altering cofactor availability, or modifying regulatory proteins to direct metabolic flux towards desired products or cellular processes. The goal is to optimize the pathway's performance for specific applications.Expand Specific Solutions04 Applications of the pentose phosphate pathway in biotechnology
The pentose phosphate pathway is utilized in various biotechnological applications, such as the production of biofuels, chemicals, and pharmaceuticals. Engineered microorganisms with enhanced pentose phosphate pathway capabilities are developed for efficient conversion of biomass-derived sugars into valuable products.Expand Specific Solutions05 Analysis and modeling of the pentose phosphate pathway
Advanced analytical techniques and computational models are developed to study and predict the behavior of the pentose phosphate pathway. These tools help in understanding the pathway's dynamics, identifying bottlenecks, and guiding metabolic engineering efforts to improve its performance in various applications.Expand Specific Solutions
Key Players in Pentose Phosphate Pathway-Related Drug Development
The targeting of the Pentose Phosphate Pathway for metabolic disorders is an emerging field in the early stages of development. The market size is growing as research intensifies, driven by the increasing prevalence of metabolic diseases. While the technology is still in its infancy, several key players are advancing research and development. Companies like BioMarin Pharmaceutical, Amgen, and Pfizer are leveraging their expertise in drug development to explore this pathway. Academic institutions such as Columbia University and Zhejiang University are contributing fundamental research. The involvement of diverse entities, from pharmaceutical giants to specialized biotech firms like Incyte Corp. and VeroScience LLC, indicates a competitive landscape with potential for significant breakthroughs in metabolic disorder treatments.
BioMarin Pharmaceutical, Inc.
Amgen, Inc.
Breakthrough Modulation Strategies for Pentose Phosphate Pathway
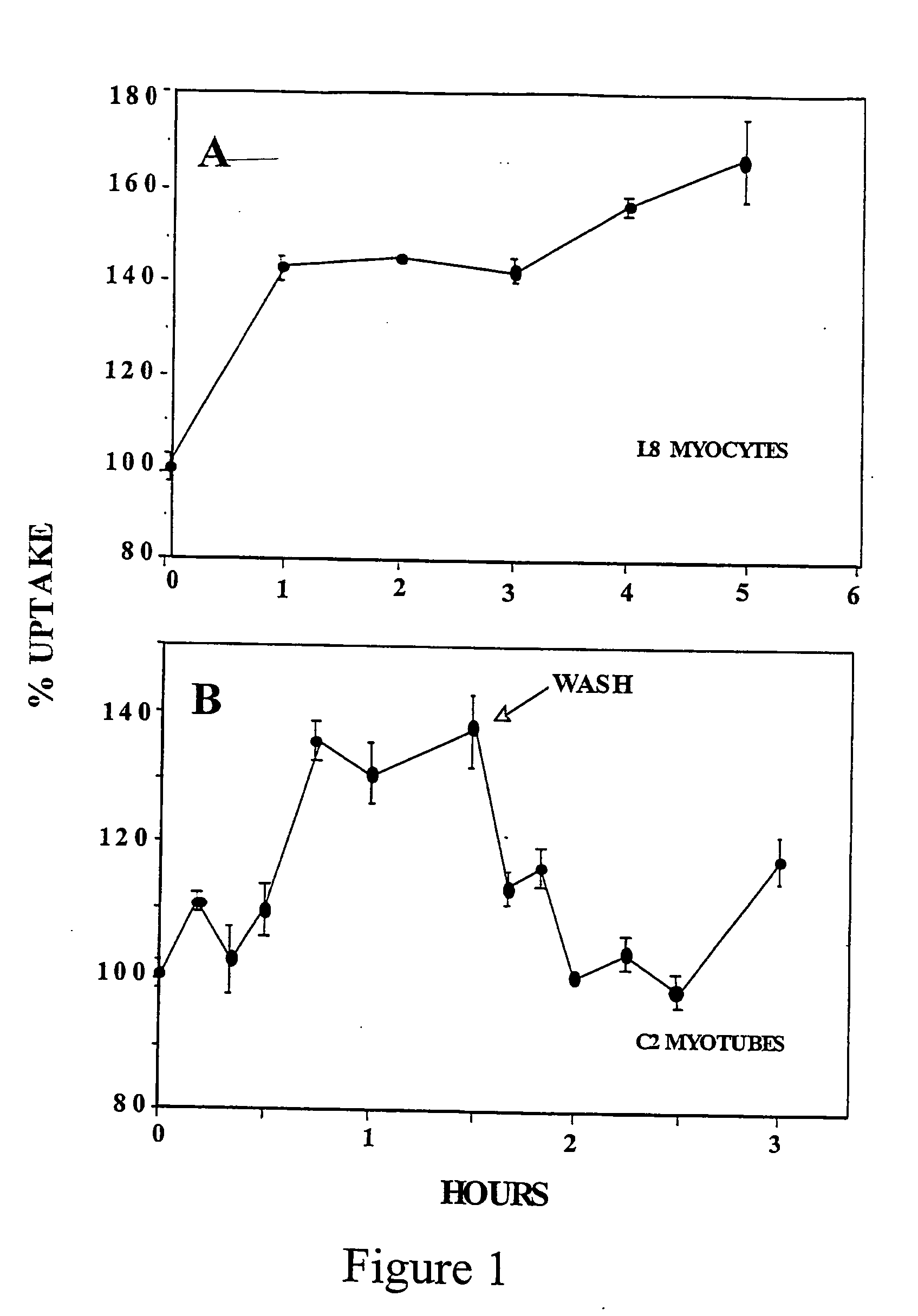
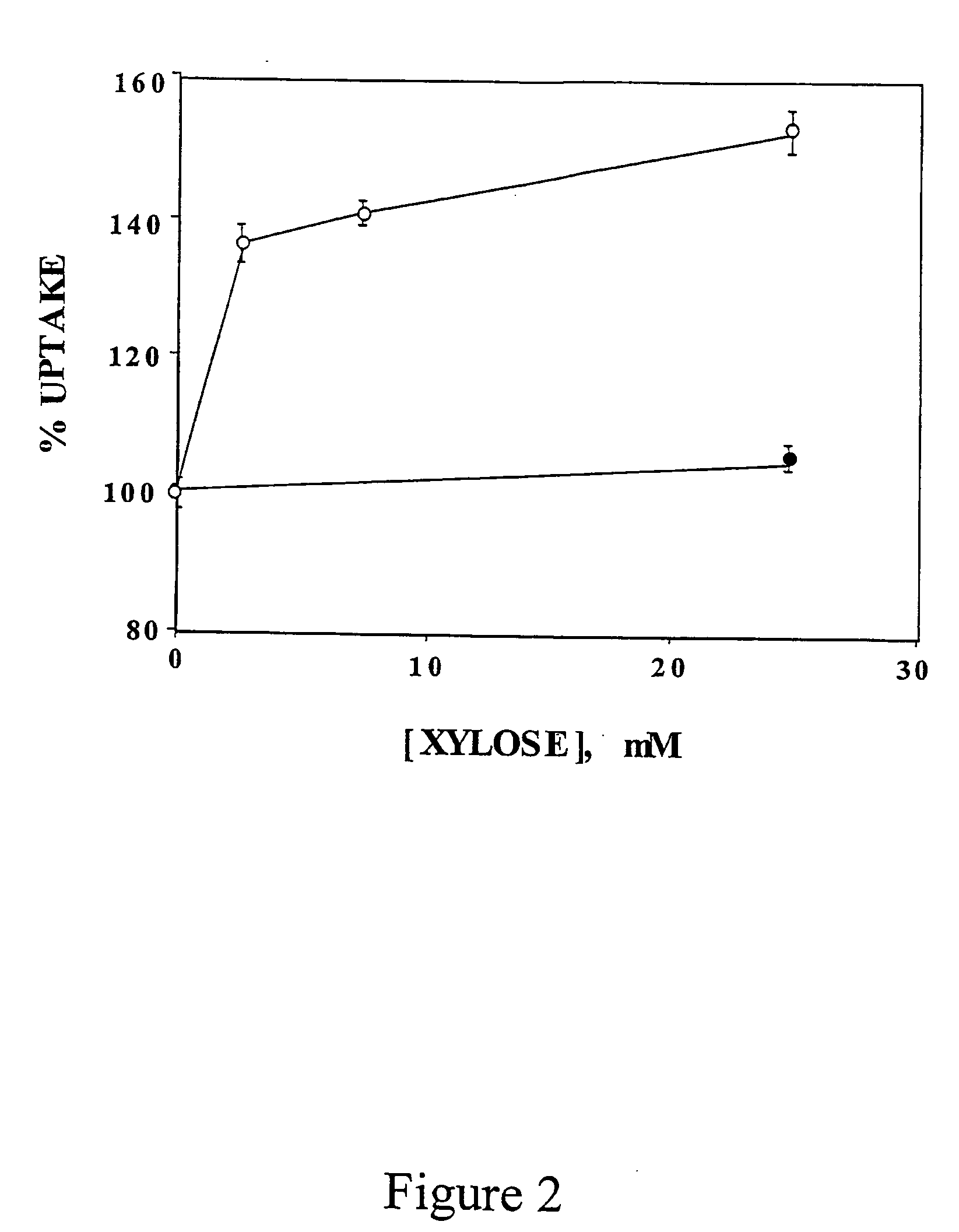
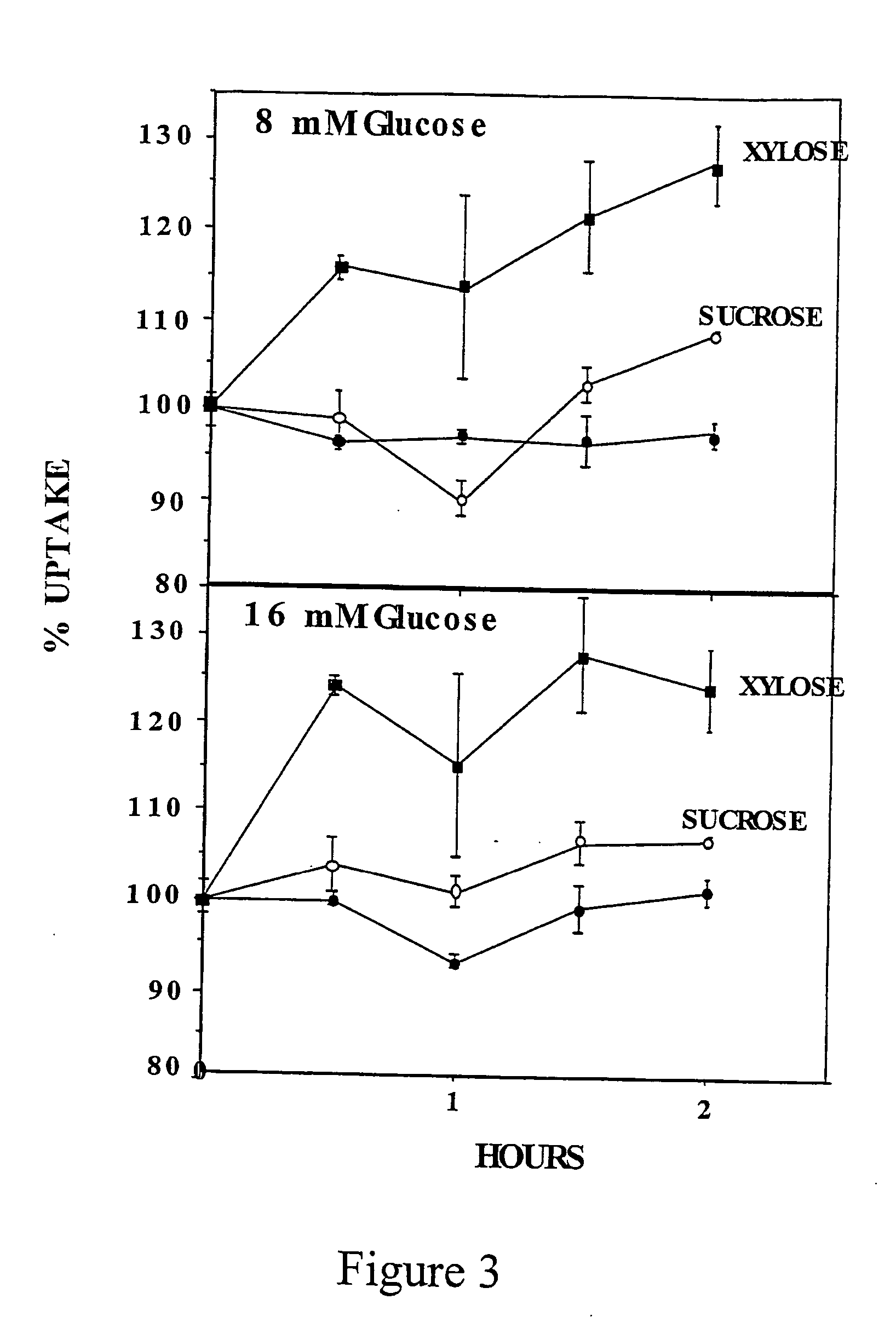
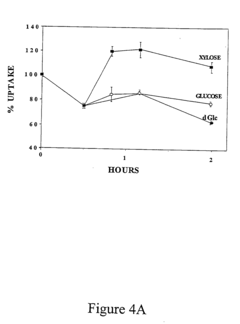
- Development of novel pentose derivatives, such as D-xylose derivatives, that increase glucose transport in a non-insulin dependent manner, enhancing cellular glucose uptake and metabolism, thereby addressing hyperglycemia and its complications.
- Development of pentose derivatives, such as xylose, ribose, and lyxose derivatives, that increase glucose transport in a non-insulin dependent manner, enhancing cellular glucose uptake and metabolism, thereby reducing blood sugar levels and addressing insulin resistance.
Regulatory Landscape for Metabolic Disorder Treatments
The regulatory landscape for metabolic disorder treatments is complex and constantly evolving, reflecting the growing understanding of these conditions and the development of innovative therapies. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the approval and regulation of treatments for metabolic disorders, including those targeting the pentose phosphate pathway.
The FDA has established specific guidelines for the development and approval of drugs targeting metabolic disorders. These guidelines emphasize the importance of demonstrating safety and efficacy through rigorous clinical trials. For treatments targeting the pentose phosphate pathway, the FDA requires comprehensive preclinical studies to establish the mechanism of action and potential side effects before progressing to human trials.
In recent years, the FDA has shown increased flexibility in its approach to metabolic disorder treatments, recognizing the urgent need for new therapies. This has led to the implementation of accelerated approval pathways for promising treatments that address unmet medical needs. However, post-marketing surveillance requirements remain stringent to ensure long-term safety and efficacy.
The European Medicines Agency (EMA) has similar regulatory frameworks in place for metabolic disorder treatments. The EMA's approach emphasizes the importance of patient-reported outcomes and quality of life measures in addition to traditional clinical endpoints. This holistic approach aligns with the complex nature of metabolic disorders and their impact on patients' daily lives.
Globally, regulatory bodies are increasingly collaborating to harmonize their approaches to metabolic disorder treatments. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been instrumental in developing guidelines that streamline the drug development and approval process across different regions.
Regulatory challenges specific to pentose phosphate pathway-targeted therapies include the need for validated biomarkers to assess treatment efficacy and the potential for long-term metabolic effects. Regulatory agencies are working closely with researchers and pharmaceutical companies to establish appropriate endpoints and trial designs for these novel therapies.
As the field of metabolic disorders continues to advance, regulatory frameworks are adapting to accommodate emerging technologies and treatment modalities. This includes the development of guidelines for gene therapies and personalized medicine approaches that may target specific metabolic pathways, including the pentose phosphate pathway.
Biomarkers for Pentose Phosphate Pathway-Targeted Therapy Efficacy
Biomarkers play a crucial role in assessing the efficacy of therapies targeting the Pentose Phosphate Pathway (PPP) for metabolic disorders. These molecular indicators provide valuable insights into the pathway's activity and the effectiveness of interventions aimed at modulating PPP function.
One key biomarker for PPP-targeted therapy efficacy is the level of NADPH (nicotinamide adenine dinucleotide phosphate) in cells. As the PPP is a major source of NADPH production, changes in NADPH levels can directly reflect the pathway's activity. Increased NADPH levels following treatment may indicate successful upregulation of the PPP, while decreased levels could suggest pathway inhibition.
Glucose-6-phosphate dehydrogenase (G6PD) activity serves as another important biomarker. G6PD is the rate-limiting enzyme of the PPP, and its activity correlates strongly with overall pathway flux. Monitoring G6PD activity through enzymatic assays or expression levels can provide valuable information on the effectiveness of PPP-targeted therapies.
Metabolomic profiling of PPP intermediates, such as ribose-5-phosphate and sedoheptulose-7-phosphate, offers a comprehensive view of pathway activity. Changes in the concentrations of these metabolites can indicate alterations in PPP flux and help assess therapy efficacy.
Oxidative stress markers, including glutathione levels and lipid peroxidation products, can indirectly reflect PPP activity. As the PPP plays a crucial role in maintaining cellular redox balance, changes in oxidative stress markers can indicate the pathway's functional status and the effectiveness of targeted interventions.
Gene expression analysis of key PPP enzymes, such as transketolase and transaldolase, can provide insights into the pathway's regulation and response to therapy. Alterations in the expression levels of these enzymes may indicate successful modulation of the PPP.
Advanced imaging techniques, such as hyperpolarized 13C magnetic resonance spectroscopy, allow for real-time monitoring of PPP flux in vivo. This non-invasive approach enables the assessment of therapy efficacy in living organisms, providing valuable translational data.
Integrating multiple biomarkers through systems biology approaches can offer a more comprehensive understanding of PPP-targeted therapy efficacy. Machine learning algorithms can be employed to analyze complex datasets and identify patterns that correlate with treatment outcomes.
By leveraging these biomarkers, researchers and clinicians can more effectively evaluate the impact of PPP-targeted therapies on metabolic disorders, optimize treatment strategies, and develop personalized approaches for individual patients.