The Role of Pentose Phosphate Pathway in Cellular Metabolism
Research Background and Objectives
The Pentose Phosphate Pathway (PPP) is a fundamental metabolic pathway in cellular metabolism, playing a crucial role in maintaining cellular redox balance and providing essential precursors for biosynthesis. This pathway has been the subject of extensive research since its discovery in the 1950s, with its significance in cellular processes becoming increasingly apparent over the decades.
The PPP operates parallel to glycolysis and is divided into two distinct phases: the oxidative phase and the non-oxidative phase. The oxidative phase generates NADPH, a critical reducing agent for biosynthetic reactions and protection against oxidative stress. The non-oxidative phase produces ribose-5-phosphate, a vital component for nucleotide synthesis, and other sugar phosphates that serve as intermediates in various metabolic pathways.
Understanding the PPP's role in cellular metabolism is crucial for several reasons. Firstly, it provides insights into how cells maintain their redox state, which is essential for numerous cellular functions and defense mechanisms against oxidative damage. Secondly, the pathway's involvement in producing ribose-5-phosphate highlights its importance in nucleic acid synthesis and cell proliferation, making it a potential target for cancer research and therapy.
The objectives of studying the PPP extend beyond basic cellular biology. In recent years, there has been growing interest in manipulating this pathway for biotechnological applications. For instance, enhancing PPP flux could lead to improved production of valuable metabolites in industrial microorganisms. Additionally, understanding PPP regulation could provide new strategies for treating metabolic disorders and developing novel therapeutic approaches for diseases such as cancer and diabetes.
From a broader perspective, the PPP serves as a model system for studying metabolic flexibility and cellular adaptation. Its ability to adjust flux based on cellular needs demonstrates the dynamic nature of metabolic networks. This adaptability is particularly relevant in the context of changing environmental conditions and cellular stresses, offering valuable insights into cellular resilience and metabolic reprogramming.
As we delve deeper into the complexities of cellular metabolism, the PPP continues to emerge as a central player in various physiological and pathological processes. Its interconnections with other metabolic pathways, such as glycolysis and the citric acid cycle, underscore the intricate nature of cellular metabolism and the need for a systems-level understanding of these processes.
Metabolic Demand Analysis
The Pentose Phosphate Pathway (PPP) serves as a major source of NADPH, a reducing agent vital for biosynthetic reactions and cellular redox balance. The demand for NADPH is particularly high in rapidly dividing cells, such as cancer cells and stem cells, which require substantial amounts for lipid synthesis and protection against oxidative stress.
In addition to NADPH production, the PPP generates ribose-5-phosphate, a key precursor for nucleotide synthesis. This makes the pathway indispensable for cells undergoing rapid division, as they require a constant supply of nucleotides for DNA replication and RNA synthesis. The demand for ribose-5-phosphate is especially pronounced in tissues with high cell turnover rates, such as the bone marrow and intestinal epithelium.
The PPP also plays a significant role in maintaining cellular redox homeostasis. By producing NADPH, it supports the regeneration of reduced glutathione, a major cellular antioxidant. This function is particularly important in cells exposed to high levels of oxidative stress, such as erythrocytes and liver cells. The demand for PPP activity in these cells is often elevated to counteract the effects of reactive oxygen species and maintain cellular integrity.
Furthermore, the PPP contributes to the production of sugar phosphates used in various metabolic processes. For instance, erythrose-4-phosphate, an intermediate of the pathway, serves as a precursor for aromatic amino acid synthesis. This highlights the pathway's importance in meeting the metabolic demands of cells requiring these specific amino acids for protein synthesis or neurotransmitter production.
The metabolic demand for PPP activity varies significantly across different cell types and physiological states. For example, adipocytes rely heavily on the PPP during lipogenesis, as NADPH is required for fatty acid synthesis. Similarly, immune cells upregulate PPP activity during activation to support rapid proliferation and the production of reactive oxygen species for pathogen elimination.
In pathological conditions, the metabolic demand for PPP activity can be altered. Cancer cells often exhibit increased PPP flux to support their heightened biosynthetic needs and manage oxidative stress. This metabolic reprogramming is considered a hallmark of cancer and has been explored as a potential therapeutic target. Conversely, certain genetic disorders affecting PPP enzymes can lead to decreased pathway activity, resulting in metabolic imbalances and cellular dysfunction.
Understanding the metabolic demands met by the PPP is crucial for developing targeted therapies and interventions. By modulating PPP activity, it may be possible to influence cellular metabolism in various contexts, from cancer treatment to managing metabolic disorders. As research in this field progresses, a more nuanced understanding of the PPP's role in meeting diverse metabolic demands will likely emerge, opening new avenues for therapeutic interventions and metabolic engineering strategies.
Current Status and Challenges
Recent advancements have significantly improved our understanding of PPP's functions, particularly in redox homeostasis and biosynthesis. However, several challenges persist in fully elucidating its mechanisms and potential applications.
One of the primary challenges is the intricate regulation of PPP flux. While the pathway's importance in generating NADPH and ribose-5-phosphate is well-established, the precise mechanisms controlling its activity remain partially understood. Researchers are grappling with the task of unraveling the complex interplay between enzymatic activities, metabolite concentrations, and cellular signaling pathways that modulate PPP flux.
Another significant challenge lies in the heterogeneity of PPP activity across different cell types and physiological conditions. The pathway's contribution to cellular metabolism varies considerably between normal and pathological states, making it difficult to develop universally applicable models or therapeutic strategies. This variability necessitates a more nuanced approach to studying PPP in diverse cellular contexts.
The integration of PPP with other metabolic pathways presents yet another hurdle. While connections between PPP and glycolysis are well-documented, the extent of its interactions with other pathways, such as the TCA cycle and lipid metabolism, requires further investigation. Elucidating these cross-pathway interactions is crucial for a comprehensive understanding of cellular metabolism.
Technological limitations also pose challenges in studying PPP dynamics in real-time within living cells. Current methods often rely on endpoint measurements or disruptive techniques, which may not capture the pathway's true behavior under physiological conditions. Developing non-invasive, high-resolution techniques for monitoring PPP flux in vivo remains an active area of research.
From a therapeutic perspective, targeting PPP for disease treatment presents both opportunities and challenges. While the pathway's involvement in cancer metabolism and neurodegenerative disorders has been established, developing selective and effective interventions without disrupting essential cellular functions remains a significant hurdle.
Lastly, the emerging role of PPP in cellular processes beyond metabolism, such as cell signaling and epigenetic regulation, opens new avenues for research while simultaneously complicating our understanding of its functions. Unraveling these non-canonical roles and their implications for cellular homeostasis represents a frontier in PPP research.
Current Mechanisms of Pentose Phosphate Pathway
01 Genetic engineering of the pentose phosphate pathway
Genetic modifications are made to enhance or alter the pentose phosphate pathway in microorganisms. This can involve introducing new genes, modifying existing ones, or altering regulatory elements to improve flux through the pathway. Such modifications can lead to increased production of desired metabolites or improved utilization of pentose sugars.- Genetic engineering of the pentose phosphate pathway: Genetic modifications are made to enhance or alter the pentose phosphate pathway in microorganisms. This can involve introducing new genes, modifying existing genes, or altering regulatory elements to improve flux through the pathway. Such modifications can lead to increased production of desired compounds or improved utilization of pentose sugars.
- Metabolic engineering for improved pentose utilization: Strategies are developed to enhance the ability of microorganisms to utilize pentose sugars, particularly in the context of biofuel or biochemical production. This can involve optimizing enzymes in the pentose phosphate pathway, introducing heterologous pathways, or modifying sugar transport systems to improve pentose uptake and metabolism.
- Regulation and control of the pentose phosphate pathway: Research focuses on understanding and manipulating the regulatory mechanisms of the pentose phosphate pathway. This includes studying transcriptional and post-translational regulation, metabolic flux analysis, and developing methods to control pathway activity for desired outcomes in biotechnology applications.
- Applications of the pentose phosphate pathway in biotechnology: The pentose phosphate pathway is exploited for various biotechnological applications, including the production of biofuels, pharmaceuticals, and fine chemicals. Strategies are developed to harness the pathway's ability to generate reducing power and precursor molecules for biosynthesis of valuable compounds.
- Analytical methods for studying the pentose phosphate pathway: Development of analytical techniques to study and quantify the activity and flux through the pentose phosphate pathway. This includes metabolomics approaches, isotope labeling experiments, and the development of biosensors or reporter systems to monitor pathway activity in real-time.
02 Metabolic engineering for improved pentose utilization
Strategies are developed to enhance the ability of microorganisms to utilize pentose sugars, particularly xylose and arabinose. This can involve introducing or optimizing pentose transport systems, enhancing pentose isomerase activity, or improving the connection between the pentose phosphate pathway and central carbon metabolism.Expand Specific Solutions03 Application of the pentose phosphate pathway in biofuel production
The pentose phosphate pathway is leveraged for the production of biofuels and other valuable chemicals. This can involve redirecting carbon flux through the pathway to increase NADPH production, which is crucial for many biosynthetic processes, or using pentose sugars as feedstocks for fermentation.Expand Specific Solutions04 Regulation and control of the pentose phosphate pathway
Research focuses on understanding and manipulating the regulatory mechanisms of the pentose phosphate pathway. This includes studying transcriptional and post-translational regulation, metabolic flux analysis, and developing strategies to overcome feedback inhibition or other regulatory constraints.Expand Specific Solutions05 Novel enzymes and pathways related to pentose phosphate metabolism
Discovery and characterization of new enzymes or alternative pathways related to pentose phosphate metabolism. This can include identifying novel isomerases, oxidoreductases, or other enzymes that can improve pentose utilization or provide new routes for valuable product synthesis.Expand Specific Solutions
Key Players in Pentose Phosphate Pathway Research
The pentose phosphate pathway (PPP) in cellular metabolism is a rapidly evolving field with increasing market potential. The industry is in a growth phase, driven by advancements in metabolic engineering and biotechnology. The global market for PPP-related research and applications is expanding, with projections indicating significant growth in the coming years. Technologically, the field is progressing from basic research to practical applications, with companies like BASF SE, Ajinomoto Co., Inc., and Danisco US, Inc. leading the way in industrial biotechnology. Academic institutions such as Fudan University and the University of California are contributing to fundamental research, while pharmaceutical companies like AVEO Pharmaceuticals, Inc. are exploring PPP's role in drug development. This diverse landscape indicates a maturing field with potential for breakthrough innovations in metabolic manipulation and cellular engineering.
Beth Israel Deaconess Medical Center, Inc.
The Regents of the University of California
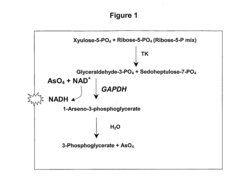
Key Pentose Phosphate Pathway Enzymes and Reactions
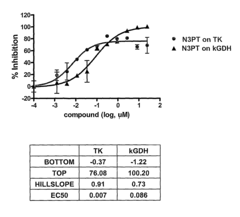
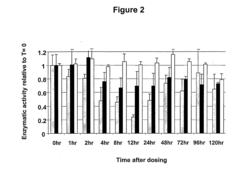
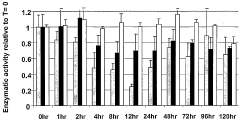
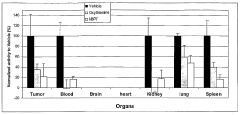
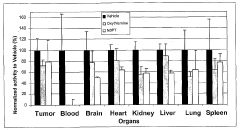
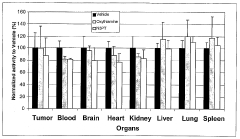
- A fluorescence-based assay that measures the enzymatic activities of these enzymes in cell lysates, tissues, and blood, providing increased sensitivity and convenience by eliminating the need for enzyme isolation and reducing sample manipulation, allowing for high-throughput analysis.
- A fluorescence-based assay method that measures the activity of transketolase, alpha-ketoglutarate dehydrogenase, pyruvate dehydrogenase, and glucose-6-phosphate dehydrogenase enzymes by monitoring the production of NADH or NADPH, allowing for more sensitive and rapid detection without the need for extensive sample preparation, enabling monitoring of enzymatic levels over time and optimizing drug dosing and scheduling.
Pentose Phosphate Pathway in Disease and Therapeutics
The Pentose Phosphate Pathway (PPP) plays a crucial role in cellular metabolism and has significant implications in various diseases and therapeutic approaches. Dysregulation of the PPP has been associated with numerous pathological conditions, including cancer, diabetes, and neurodegenerative disorders.
In cancer, the PPP is often upregulated to support rapid cell proliferation and survival. The increased flux through the pathway provides cancer cells with ribose-5-phosphate for nucleotide synthesis and NADPH for redox homeostasis and lipid biosynthesis. This metabolic reprogramming contributes to tumor growth and resistance to oxidative stress-induced cell death. Targeting key enzymes of the PPP, such as glucose-6-phosphate dehydrogenase (G6PD), has shown promise in inhibiting cancer cell growth and enhancing the efficacy of chemotherapeutic agents.
Diabetes mellitus, particularly type 2 diabetes, is characterized by impaired glucose metabolism and insulin resistance. The PPP has been implicated in the pathogenesis of diabetes through its role in regulating cellular redox status and lipid metabolism. Alterations in PPP activity can contribute to oxidative stress and inflammation, exacerbating insulin resistance and β-cell dysfunction. Therapeutic strategies aimed at modulating PPP flux may offer potential benefits in managing diabetes and its complications.
Neurodegenerative disorders, such as Alzheimer's and Parkinson's diseases, are associated with oxidative stress and mitochondrial dysfunction. The PPP serves as a critical source of NADPH, which is essential for maintaining cellular antioxidant defenses. Impairment of the PPP in neuronal cells can lead to increased susceptibility to oxidative damage and neurodegeneration. Enhancing PPP activity through pharmacological interventions or genetic approaches has shown neuroprotective effects in preclinical models of neurodegenerative diseases.
The therapeutic potential of targeting the PPP extends beyond these major disease categories. In cardiovascular diseases, modulation of PPP activity may help protect against ischemia-reperfusion injury and atherosclerosis. In autoimmune disorders, the PPP plays a role in T cell activation and differentiation, offering potential targets for immunomodulatory therapies.
Recent advances in understanding the regulation of the PPP have led to the development of novel therapeutic strategies. These include small molecule inhibitors of key PPP enzymes, metabolic modulators that redirect flux through the pathway, and genetic approaches to manipulate PPP activity. Additionally, the use of metabolomics and systems biology approaches has enabled a more comprehensive understanding of how the PPP interacts with other metabolic pathways in disease states, paving the way for more targeted and effective therapeutic interventions.
Metabolomics and Pentose Phosphate Pathway Analysis
Metabolomics, the comprehensive study of small molecule metabolites in biological systems, plays a crucial role in understanding the intricate workings of cellular metabolism. When applied to the analysis of the Pentose Phosphate Pathway (PPP), metabolomics provides valuable insights into the pathway's function, regulation, and impact on overall cellular metabolism.
The pentose phosphate pathway is a fundamental metabolic pathway that operates parallel to glycolysis, serving two primary functions: generating reducing equivalents in the form of NADPH and producing ribose-5-phosphate for nucleotide synthesis. Metabolomic analysis of the PPP involves the identification and quantification of key metabolites involved in this pathway, including glucose-6-phosphate, 6-phosphogluconate, and ribulose-5-phosphate.
Advanced metabolomic techniques, such as liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) spectroscopy, enable researchers to measure the levels of these metabolites with high precision. By comparing metabolite profiles under different conditions or in various cell types, scientists can gain insights into the flux through the PPP and its regulation in response to cellular demands.
One of the primary applications of metabolomics in PPP analysis is the investigation of the pathway's role in oxidative stress response. The NADPH generated by the PPP is crucial for maintaining cellular redox balance and protecting against oxidative damage. Metabolomic studies have revealed how cells modulate PPP activity to increase NADPH production under oxidative stress conditions, demonstrating the pathway's importance in cellular defense mechanisms.
Furthermore, metabolomic approaches have been instrumental in elucidating the interplay between the PPP and other metabolic pathways. For instance, studies have shown how the PPP interfaces with glycolysis, the tricarboxylic acid (TCA) cycle, and lipid metabolism. This systems-level understanding is critical for comprehending the broader impact of PPP activity on cellular metabolism and energy homeostasis.
In cancer research, metabolomics has provided valuable insights into the altered metabolism of tumor cells, particularly regarding the PPP. Many cancer cells exhibit increased PPP activity to support rapid proliferation and manage oxidative stress. Metabolomic profiling of cancer cells has revealed specific alterations in PPP metabolites, offering potential targets for therapeutic interventions and biomarker development.
The integration of metabolomics with other omics technologies, such as transcriptomics and proteomics, has further enhanced our understanding of PPP regulation. These multi-omics approaches allow researchers to correlate changes in metabolite levels with alterations in gene expression and enzyme activities, providing a more comprehensive view of PPP dynamics in various physiological and pathological states.