Pentose Phosphate Pathway Contribution to NADPH Production
Pentose Phosphate Pathway and NADPH: Research Background
The Pentose Phosphate Pathway (PPP) is a crucial metabolic pathway that plays a significant role in cellular metabolism, particularly in the production of NADPH (Nicotinamide Adenine Dinucleotide Phosphate). This pathway operates parallel to glycolysis and is essential for maintaining cellular redox balance and providing precursors for biosynthetic processes.
The PPP consists of two distinct phases: the oxidative phase and the non-oxidative phase. The oxidative phase is responsible for generating NADPH, which serves as a reducing agent in various cellular processes, including lipid biosynthesis, nucleotide synthesis, and protection against oxidative stress. This phase involves the conversion of glucose-6-phosphate to ribulose-5-phosphate, generating two molecules of NADPH for each glucose-6-phosphate molecule oxidized.
In the context of NADPH production, the PPP is of paramount importance. NADPH is a critical cofactor in numerous anabolic reactions and serves as the primary source of reducing power for biosynthetic processes. It is essential for maintaining glutathione in its reduced form, which is crucial for cellular defense against oxidative damage. Additionally, NADPH is required for the synthesis of fatty acids, cholesterol, and other lipids, making it indispensable for cell growth and proliferation.
The contribution of the PPP to NADPH production varies among different cell types and physiological conditions. In rapidly dividing cells, such as cancer cells or developing tissues, the PPP is often upregulated to meet the increased demand for NADPH and ribose-5-phosphate, a precursor for nucleotide synthesis. In contrast, in differentiated cells with lower biosynthetic requirements, the PPP may operate at a lower rate.
The regulation of the PPP is tightly controlled to balance the cellular needs for NADPH and ribose-5-phosphate with the requirements of other metabolic pathways. Key regulatory enzymes, such as glucose-6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme of the oxidative phase, are subject to various control mechanisms, including allosteric regulation and transcriptional control.
Understanding the contribution of the PPP to NADPH production is crucial for various fields, including cancer research, drug development, and metabolic engineering. Modulation of the PPP has been explored as a potential therapeutic strategy in diseases characterized by altered cellular metabolism, such as cancer and metabolic disorders. Furthermore, the PPP's role in NADPH production has implications for biotechnology applications, where optimizing NADPH availability can enhance the production of valuable compounds in engineered microorganisms.
Metabolic Demand for NADPH
The metabolic demand for NADPH in cells is substantial and diverse, playing a crucial role in various cellular processes. NADPH serves as a key reducing agent in biosynthetic reactions, antioxidant defense mechanisms, and cellular redox balance maintenance. In the context of the Pentose Phosphate Pathway (PPP) contribution to NADPH production, it is essential to analyze the metabolic demands that drive this pathway's activity.
One of the primary metabolic demands for NADPH is in lipid biosynthesis. The synthesis of fatty acids, cholesterol, and other lipid molecules requires significant amounts of NADPH as a reducing agent. This demand is particularly high in rapidly proliferating cells, such as cancer cells, which have an increased need for membrane lipid synthesis to support their growth and division.
Another major consumer of NADPH is the glutathione system, which is critical for cellular antioxidant defense. Glutathione peroxidase uses NADPH to reduce oxidized glutathione, maintaining the cell's ability to neutralize reactive oxygen species (ROS) and prevent oxidative damage. This demand is especially pronounced in cells exposed to high levels of oxidative stress, such as those in the liver or immune system.
The biosynthesis of amino acids, particularly aromatic amino acids like phenylalanine and tyrosine, also requires NADPH. These processes contribute to the overall metabolic demand for NADPH, although to a lesser extent than lipid synthesis and antioxidant defense.
In specialized cells, such as steroidogenic cells in the adrenal glands and gonads, there is a high demand for NADPH to support steroid hormone synthesis. The cytochrome P450 enzymes involved in steroidogenesis rely heavily on NADPH as an electron donor for their catalytic activities.
The metabolic demand for NADPH also varies significantly across different cell types and physiological states. For instance, adipocytes have a high demand during lipogenesis, while immune cells require substantial NADPH during the respiratory burst to generate ROS for pathogen elimination.
Understanding these metabolic demands is crucial for assessing the importance of the PPP in NADPH production. The pathway's activity is often upregulated in response to increased NADPH demand, such as during rapid cell proliferation or under oxidative stress conditions. This adaptive response highlights the PPP's role as a key regulator of cellular NADPH levels and its importance in maintaining metabolic homeostasis.
Pentose Phosphate Pathway in NADPH Production: Challenges and Limitations
Despite the significant role of the Pentose Phosphate Pathway (PPP) in NADPH production, several challenges and limitations hinder its full potential. One of the primary obstacles is the complex regulation of the pathway, which involves multiple enzymes and metabolites. The intricate network of feedback mechanisms and allosteric regulation makes it difficult to manipulate the pathway for enhanced NADPH production without disrupting other cellular processes.
Another significant challenge is the competition for glucose-6-phosphate between the PPP and glycolysis. As both pathways share this common substrate, increasing flux through the PPP often results in a decrease in glycolytic activity, potentially affecting energy production and other essential cellular functions. This trade-off between NADPH generation and energy metabolism poses a significant hurdle in optimizing the PPP for biotechnological applications.
The compartmentalization of the PPP also presents limitations. While the oxidative branch of the pathway is primarily localized in the cytosol, some enzymes of the non-oxidative branch are found in both the cytosol and plastids in plant cells. This compartmentalization can lead to bottlenecks in metabolite transport and may limit the overall efficiency of NADPH production.
Furthermore, the PPP's sensitivity to cellular redox state can be both an advantage and a limitation. While this sensitivity allows for dynamic regulation of NADPH production in response to cellular needs, it can also make the pathway challenging to control in engineered systems where consistent NADPH output is desired.
The genetic manipulation of PPP enzymes to enhance NADPH production is often complicated by pleiotropic effects. Overexpression or knockout of key enzymes can lead to unexpected metabolic imbalances, growth defects, or reduced cellular fitness. This complexity makes it challenging to fine-tune the pathway for optimal NADPH production without compromising other cellular functions.
Lastly, the PPP's contribution to NADPH production can vary significantly between different cell types and physiological conditions. This variability makes it difficult to develop universal strategies for enhancing NADPH production via the PPP across diverse biological systems and applications. Researchers must often tailor their approaches to specific organisms or cell types, limiting the broad applicability of PPP-based NADPH production strategies.
Current Pentose Phosphate Pathway Flux Measurement
01 Genetic engineering for enhanced NADPH production
Modifying genes involved in the pentose phosphate pathway to increase NADPH production. This can involve overexpressing key enzymes or altering regulatory elements to enhance flux through the pathway, resulting in higher NADPH yields.- Genetic engineering for enhanced NADPH production: Modifying genes involved in the pentose phosphate pathway to increase NADPH production. This can involve overexpressing key enzymes or altering regulatory elements to enhance flux through the pathway, resulting in higher NADPH yields.
- Optimization of culture conditions: Adjusting fermentation parameters such as pH, temperature, and nutrient composition to favor the pentose phosphate pathway and increase NADPH production. This approach can enhance the activity of key enzymes and redirect metabolic flux towards NADPH generation.
- Use of alternative carbon sources: Utilizing different carbon sources, such as xylose or other pentose sugars, to promote flux through the pentose phosphate pathway and increase NADPH production. This strategy can be particularly effective in microorganisms engineered to metabolize these alternative substrates.
- Metabolic engineering for cofactor balance: Modifying metabolic pathways to alter the balance between NADPH and NADH, favoring NADPH production. This can involve redirecting flux from glycolysis to the pentose phosphate pathway or engineering enzymes to prefer NADP+ over NAD+ as a cofactor.
- Integration of synthetic pathways: Introducing synthetic metabolic pathways or enzymes that can generate NADPH independently of the native pentose phosphate pathway. This approach can complement the existing NADPH production mechanisms and increase overall yields.
02 Optimization of culture conditions
Adjusting fermentation parameters such as pH, temperature, and nutrient composition to maximize NADPH production through the pentose phosphate pathway. This approach focuses on creating an optimal environment for the pathway's enzymes to function efficiently.Expand Specific Solutions03 Use of alternative carbon sources
Utilizing different carbon sources, such as xylose or other pentose sugars, to directly feed into the pentose phosphate pathway and increase NADPH production. This strategy can be particularly effective in microorganisms engineered to metabolize these alternative substrates.Expand Specific Solutions04 Metabolic flux redirection
Redirecting metabolic flux towards the pentose phosphate pathway by modifying competing pathways or regulatory mechanisms. This can involve downregulating glycolysis or other NADPH-producing pathways to favor NADPH production through the pentose phosphate pathway.Expand Specific Solutions05 Integration with other NADPH-producing pathways
Combining the pentose phosphate pathway with other NADPH-producing pathways, such as the malic enzyme pathway or isocitrate dehydrogenase, to synergistically increase overall NADPH production. This approach can involve coordinated genetic modifications or pathway engineering.Expand Specific Solutions
Key Players in Research
The Pentose Phosphate Pathway's contribution to NADPH production is a mature field of study, with ongoing research and development across various industries. The market is in a growth phase, driven by increasing applications in biotechnology, pharmaceuticals, and industrial processes. The global market size for related products and technologies is estimated to be in the billions of dollars. Companies like BASF SE, Ajinomoto Co., Inc., and DuPont de Nemours, Inc. are leading players, leveraging their extensive R&D capabilities to develop innovative solutions. Academic institutions such as Jiangnan University and Zhejiang University of Technology are also contributing significantly to advancing the understanding and application of this pathway, indicating a high level of technological maturity and potential for further breakthroughs.
BASF SE
Ajinomoto Co., Inc.
Pentose Phosphate Pathway Enzyme Innovations
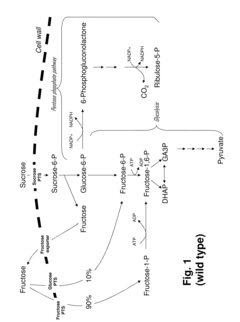
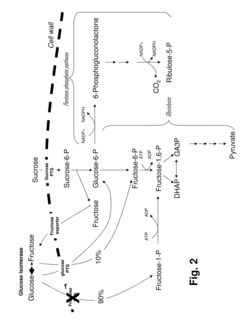
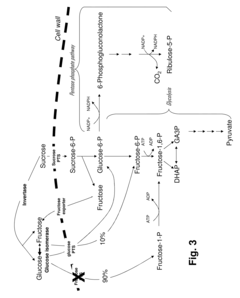
- Development of novel strains with attenuated or blocked fructose transport mechanisms and expression of enzymes like glucose isomerase and glucokinase to drive fructose toward the pentose phosphate pathway, enhancing NADPH production and lysine synthesis by converting sucrose into glucose-6-phosphate for efficient utilization.
- Modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) enzymes with specific amino acid exchanges, particularly at positions 35 and 36, are developed to enhance their specificity for NADP, allowing them to efficiently catalyze reactions with both NAD and NADP, thereby improving the yield of NADP-dependent syntheses like L-lysine production.
Pentose Phosphate Pathway in Disease Models
The Pentose Phosphate Pathway (PPP) plays a crucial role in various disease models, highlighting its significance in understanding pathological processes and developing potential therapeutic strategies. In cancer models, the PPP is often upregulated, contributing to increased NADPH production and enhanced antioxidant capacity. This metabolic shift supports rapid cell proliferation and survival under oxidative stress conditions commonly observed in tumors.
In neurodegenerative disease models, such as Alzheimer's and Parkinson's, alterations in PPP activity have been linked to impaired cellular redox balance and increased susceptibility to oxidative damage. Studies have shown that modulating PPP flux can potentially mitigate neuronal loss and improve cognitive function in these models.
Diabetes models have also revealed important insights into PPP involvement. Hyperglycemia-induced oxidative stress can lead to complications, and the PPP serves as a critical defense mechanism by generating NADPH for antioxidant systems. However, chronic activation of the PPP in diabetes may contribute to the progression of diabetic complications through increased production of reactive oxygen species.
In cardiovascular disease models, the PPP has been implicated in both protective and detrimental roles. While it provides essential NADPH for maintaining vascular health, excessive PPP activation can lead to increased fatty acid synthesis and lipid accumulation, contributing to atherosclerosis development.
Inflammatory disease models have demonstrated the importance of the PPP in immune cell function. NADPH produced by the PPP is crucial for the respiratory burst in phagocytes, a key mechanism in host defense. However, dysregulation of this process can lead to chronic inflammation and tissue damage.
Recent studies in aging models have highlighted the potential of PPP modulation as an anti-aging strategy. Age-related decline in PPP activity has been associated with decreased NADPH production and impaired cellular redox homeostasis. Interventions aimed at enhancing PPP flux have shown promise in extending lifespan and improving healthspan in various model organisms.
Understanding the role of the PPP in these diverse disease models provides valuable insights into the complex interplay between metabolism, redox balance, and pathological processes. This knowledge can guide the development of targeted therapies and interventions aimed at modulating PPP activity for therapeutic benefit across a wide range of diseases.
Metabolic Flux Analysis
Metabolic flux analysis (MFA) is a powerful tool for quantifying the contribution of the pentose phosphate pathway (PPP) to NADPH production in cellular metabolism. This technique provides a comprehensive view of metabolic fluxes, allowing researchers to track the flow of carbon through various pathways, including the PPP. By employing isotope labeling and mathematical modeling, MFA can accurately determine the relative contributions of different pathways to NADPH generation.
In the context of PPP and NADPH production, MFA typically involves the use of 13C-labeled glucose as a substrate. As cells metabolize this labeled glucose, the distribution of isotopes in downstream metabolites reflects the activity of various pathways. The oxidative branch of the PPP, which is a major source of NADPH, can be distinguished from other NADPH-producing reactions by analyzing the labeling patterns in specific metabolites.
One key advantage of MFA in studying PPP contribution to NADPH production is its ability to provide quantitative flux estimates under different physiological conditions. This allows researchers to assess how factors such as nutrient availability, oxidative stress, or genetic modifications affect the relative importance of the PPP in cellular redox balance. For instance, MFA studies have revealed that cancer cells often exhibit increased flux through the PPP, likely to support their elevated demand for NADPH in biosynthesis and antioxidant defense.
Recent advancements in MFA techniques have further enhanced our understanding of PPP dynamics. High-resolution mass spectrometry and nuclear magnetic resonance spectroscopy now enable more precise measurement of isotope distributions, leading to improved flux estimates. Additionally, the integration of MFA with other omics data, such as transcriptomics and proteomics, provides a more holistic view of cellular metabolism and regulatory mechanisms governing PPP activity.
Challenges in applying MFA to study PPP contribution include the complexity of metabolic networks and the presence of multiple NADPH-producing pathways. Researchers must carefully design experiments and develop sophisticated computational models to accurately deconvolute the contributions of different pathways. Despite these challenges, MFA remains an invaluable tool for elucidating the role of the PPP in cellular NADPH homeostasis and its implications for various physiological and pathological processes.