Understanding Propionic Acid's Pathway in Environmental Chemistry
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Propionic Acid Background and Research Objectives
Propionic acid, a short-chain fatty acid, has gained significant attention in environmental chemistry due to its widespread occurrence and potential impacts on ecosystems. This compound, with the chemical formula C3H6O2, is naturally produced by various microorganisms and plays a crucial role in numerous biological processes. The study of propionic acid's pathway in environmental chemistry has become increasingly important as researchers seek to understand its fate, transport, and interactions within different environmental compartments.
The evolution of propionic acid research can be traced back to the early 20th century when it was first isolated and characterized. Since then, advancements in analytical techniques and environmental monitoring have led to a deeper understanding of its presence and behavior in natural systems. The growing interest in propionic acid stems from its potential as a renewable resource, its role in atmospheric chemistry, and its implications for water quality and soil health.
Current research objectives in this field encompass a wide range of topics, including the elucidation of propionic acid's formation and degradation pathways in various environmental matrices. Scientists are particularly interested in understanding the microbial processes that govern propionic acid production and consumption in soils, sediments, and aquatic environments. Additionally, there is a focus on investigating the interactions between propionic acid and other environmental constituents, such as minerals, organic matter, and pollutants.
One of the key goals in propionic acid research is to develop comprehensive models that can accurately predict its behavior and distribution in complex environmental systems. This involves integrating knowledge from multiple disciplines, including microbiology, geochemistry, and atmospheric science. Researchers are also exploring the potential use of propionic acid as a biomarker for environmental processes and as an indicator of ecosystem health.
The environmental fate of propionic acid is of particular concern, given its potential to impact water quality and contribute to greenhouse gas emissions. Studies are underway to quantify the fluxes of propionic acid between different environmental compartments and to assess its role in global carbon cycling. Furthermore, there is growing interest in understanding how anthropogenic activities, such as agricultural practices and industrial processes, influence the production and distribution of propionic acid in the environment.
As research in this field progresses, scientists aim to develop innovative strategies for monitoring and managing propionic acid levels in various ecosystems. This includes the development of advanced analytical techniques for accurate detection and quantification, as well as the exploration of potential mitigation measures to address any adverse environmental impacts. The ultimate goal is to gain a holistic understanding of propionic acid's pathway in environmental chemistry, enabling more effective environmental management and policy decisions.
The evolution of propionic acid research can be traced back to the early 20th century when it was first isolated and characterized. Since then, advancements in analytical techniques and environmental monitoring have led to a deeper understanding of its presence and behavior in natural systems. The growing interest in propionic acid stems from its potential as a renewable resource, its role in atmospheric chemistry, and its implications for water quality and soil health.
Current research objectives in this field encompass a wide range of topics, including the elucidation of propionic acid's formation and degradation pathways in various environmental matrices. Scientists are particularly interested in understanding the microbial processes that govern propionic acid production and consumption in soils, sediments, and aquatic environments. Additionally, there is a focus on investigating the interactions between propionic acid and other environmental constituents, such as minerals, organic matter, and pollutants.
One of the key goals in propionic acid research is to develop comprehensive models that can accurately predict its behavior and distribution in complex environmental systems. This involves integrating knowledge from multiple disciplines, including microbiology, geochemistry, and atmospheric science. Researchers are also exploring the potential use of propionic acid as a biomarker for environmental processes and as an indicator of ecosystem health.
The environmental fate of propionic acid is of particular concern, given its potential to impact water quality and contribute to greenhouse gas emissions. Studies are underway to quantify the fluxes of propionic acid between different environmental compartments and to assess its role in global carbon cycling. Furthermore, there is growing interest in understanding how anthropogenic activities, such as agricultural practices and industrial processes, influence the production and distribution of propionic acid in the environment.
As research in this field progresses, scientists aim to develop innovative strategies for monitoring and managing propionic acid levels in various ecosystems. This includes the development of advanced analytical techniques for accurate detection and quantification, as well as the exploration of potential mitigation measures to address any adverse environmental impacts. The ultimate goal is to gain a holistic understanding of propionic acid's pathway in environmental chemistry, enabling more effective environmental management and policy decisions.
Environmental Impact and Market Analysis
Propionic acid, a short-chain fatty acid, plays a significant role in environmental chemistry and has substantial market implications. Its presence in the environment stems from both natural processes and human activities, particularly in agricultural and industrial sectors. The environmental impact of propionic acid is multifaceted, affecting soil, water, and air quality.
In soil environments, propionic acid can influence microbial communities and nutrient cycling. It acts as a carbon source for certain microorganisms, potentially altering soil biodiversity and ecosystem functions. However, excessive concentrations may lead to soil acidification, affecting plant growth and soil structure. The acid's mobility in soil depends on factors such as pH, organic matter content, and soil texture, which determine its potential for leaching into groundwater.
Aquatic ecosystems are also affected by propionic acid. When introduced into water bodies, it can contribute to changes in pH levels and dissolved oxygen content. This may impact aquatic organisms, particularly sensitive species of fish and invertebrates. The acid's biodegradability in water is generally high, which mitigates long-term accumulation but may cause short-term oxygen depletion during rapid breakdown processes.
Atmospheric release of propionic acid, primarily from industrial sources, contributes to the formation of secondary organic aerosols. These aerosols can affect air quality and climate by influencing cloud formation and radiative forcing. However, the atmospheric lifetime of propionic acid is relatively short, limiting its global impact compared to more persistent pollutants.
From a market perspective, propionic acid has diverse applications, driving its demand across various industries. In agriculture, it serves as a preservative for animal feed and grain, reducing spoilage and improving feed efficiency. The food industry utilizes propionic acid as a preservative in baked goods and cheese products, extending shelf life and preventing mold growth. Additionally, it finds applications in pharmaceuticals, plastics, and herbicides.
The global market for propionic acid has been experiencing steady growth, driven by increasing demand in emerging economies and expanding applications in various sectors. The Asia-Pacific region, in particular, has shown significant market expansion due to rapid industrialization and growing agricultural activities. Environmental regulations and sustainability concerns are shaping market trends, with a growing emphasis on bio-based production methods to reduce the environmental footprint of propionic acid manufacturing.
As awareness of environmental impacts grows, there is an increasing focus on developing sustainable production methods and mitigating the negative effects of propionic acid in the environment. This includes research into bio-based production using renewable resources, improved waste management practices in industries utilizing propionic acid, and the development of more environmentally friendly alternatives for certain applications.
In soil environments, propionic acid can influence microbial communities and nutrient cycling. It acts as a carbon source for certain microorganisms, potentially altering soil biodiversity and ecosystem functions. However, excessive concentrations may lead to soil acidification, affecting plant growth and soil structure. The acid's mobility in soil depends on factors such as pH, organic matter content, and soil texture, which determine its potential for leaching into groundwater.
Aquatic ecosystems are also affected by propionic acid. When introduced into water bodies, it can contribute to changes in pH levels and dissolved oxygen content. This may impact aquatic organisms, particularly sensitive species of fish and invertebrates. The acid's biodegradability in water is generally high, which mitigates long-term accumulation but may cause short-term oxygen depletion during rapid breakdown processes.
Atmospheric release of propionic acid, primarily from industrial sources, contributes to the formation of secondary organic aerosols. These aerosols can affect air quality and climate by influencing cloud formation and radiative forcing. However, the atmospheric lifetime of propionic acid is relatively short, limiting its global impact compared to more persistent pollutants.
From a market perspective, propionic acid has diverse applications, driving its demand across various industries. In agriculture, it serves as a preservative for animal feed and grain, reducing spoilage and improving feed efficiency. The food industry utilizes propionic acid as a preservative in baked goods and cheese products, extending shelf life and preventing mold growth. Additionally, it finds applications in pharmaceuticals, plastics, and herbicides.
The global market for propionic acid has been experiencing steady growth, driven by increasing demand in emerging economies and expanding applications in various sectors. The Asia-Pacific region, in particular, has shown significant market expansion due to rapid industrialization and growing agricultural activities. Environmental regulations and sustainability concerns are shaping market trends, with a growing emphasis on bio-based production methods to reduce the environmental footprint of propionic acid manufacturing.
As awareness of environmental impacts grows, there is an increasing focus on developing sustainable production methods and mitigating the negative effects of propionic acid in the environment. This includes research into bio-based production using renewable resources, improved waste management practices in industries utilizing propionic acid, and the development of more environmentally friendly alternatives for certain applications.
Current Challenges in Propionic Acid Pathway Elucidation
Despite significant advancements in environmental chemistry, elucidating the complete pathway of propionic acid in various ecosystems remains a complex challenge. One of the primary obstacles is the diverse and dynamic nature of environmental matrices, which can significantly influence the behavior and transformation of propionic acid.
The heterogeneity of environmental samples, ranging from soil and sediments to water bodies, presents difficulties in developing standardized analytical methods. Each matrix requires specific extraction and detection techniques, making it challenging to compare results across different studies and environmental conditions.
Another major hurdle is the complex microbial interactions involved in propionic acid metabolism. The pathway can vary depending on the microbial community present, which is often influenced by factors such as pH, temperature, and nutrient availability. Identifying and characterizing all the microorganisms involved in each step of the pathway requires advanced molecular techniques and bioinformatics tools.
The low concentrations of propionic acid and its intermediates in environmental samples pose significant analytical challenges. Current detection methods often lack the sensitivity required to accurately quantify these compounds at environmentally relevant concentrations. This limitation hinders our ability to track the complete pathway and understand the kinetics of each transformation step.
Furthermore, the rapid turnover of propionic acid in many environmental systems makes it difficult to capture and analyze all intermediates. Some metabolites may be short-lived or present in trace amounts, requiring sophisticated time-resolved sampling and analysis techniques that are not yet widely available or standardized.
The potential for abiotic transformations of propionic acid in the environment adds another layer of complexity. Distinguishing between biotic and abiotic processes and understanding their relative contributions to the overall pathway is crucial but often challenging in real-world scenarios.
Lastly, the lack of comprehensive databases and standardized reporting formats for propionic acid pathway studies hinders the integration and comparison of results from different research groups. This fragmentation of knowledge makes it difficult to build a coherent understanding of the pathway across various environmental conditions and ecosystems.
Addressing these challenges requires a multidisciplinary approach, combining advanced analytical chemistry, microbiology, and environmental science. Developing more sensitive and selective detection methods, improving in situ sampling techniques, and enhancing our understanding of microbial ecology in various environments are critical steps towards fully elucidating the propionic acid pathway in environmental chemistry.
The heterogeneity of environmental samples, ranging from soil and sediments to water bodies, presents difficulties in developing standardized analytical methods. Each matrix requires specific extraction and detection techniques, making it challenging to compare results across different studies and environmental conditions.
Another major hurdle is the complex microbial interactions involved in propionic acid metabolism. The pathway can vary depending on the microbial community present, which is often influenced by factors such as pH, temperature, and nutrient availability. Identifying and characterizing all the microorganisms involved in each step of the pathway requires advanced molecular techniques and bioinformatics tools.
The low concentrations of propionic acid and its intermediates in environmental samples pose significant analytical challenges. Current detection methods often lack the sensitivity required to accurately quantify these compounds at environmentally relevant concentrations. This limitation hinders our ability to track the complete pathway and understand the kinetics of each transformation step.
Furthermore, the rapid turnover of propionic acid in many environmental systems makes it difficult to capture and analyze all intermediates. Some metabolites may be short-lived or present in trace amounts, requiring sophisticated time-resolved sampling and analysis techniques that are not yet widely available or standardized.
The potential for abiotic transformations of propionic acid in the environment adds another layer of complexity. Distinguishing between biotic and abiotic processes and understanding their relative contributions to the overall pathway is crucial but often challenging in real-world scenarios.
Lastly, the lack of comprehensive databases and standardized reporting formats for propionic acid pathway studies hinders the integration and comparison of results from different research groups. This fragmentation of knowledge makes it difficult to build a coherent understanding of the pathway across various environmental conditions and ecosystems.
Addressing these challenges requires a multidisciplinary approach, combining advanced analytical chemistry, microbiology, and environmental science. Developing more sensitive and selective detection methods, improving in situ sampling techniques, and enhancing our understanding of microbial ecology in various environments are critical steps towards fully elucidating the propionic acid pathway in environmental chemistry.
Existing Methodologies for Pathway Analysis
01 Production methods of propionic acid
Various methods are employed for the production of propionic acid, including fermentation processes, chemical synthesis, and catalytic reactions. These methods often involve the use of specific microorganisms, catalysts, or chemical precursors to efficiently produce propionic acid on an industrial scale.- Production methods of propionic acid: Various methods are employed for the production of propionic acid, including fermentation processes, chemical synthesis, and catalytic reactions. These methods often involve the use of specific microorganisms, catalysts, or chemical precursors to efficiently produce propionic acid on an industrial scale.
- Applications of propionic acid in food preservation: Propionic acid is widely used as a food preservative due to its antimicrobial properties. It is effective in inhibiting the growth of mold and certain bacteria, making it valuable in extending the shelf life of various food products, particularly baked goods and dairy products.
- Use of propionic acid in pharmaceutical formulations: Propionic acid and its derivatives find applications in the pharmaceutical industry. They are used in the formulation of various medications, including topical treatments for skin conditions and as intermediates in the synthesis of certain drugs.
- Industrial applications of propionic acid: Propionic acid has diverse industrial applications beyond food and pharmaceuticals. It is used in the production of plastics, herbicides, and as a chemical intermediate in various manufacturing processes. Its versatility makes it a valuable compound in multiple industries.
- Environmental and safety considerations in propionic acid handling: The handling and storage of propionic acid require specific safety measures due to its corrosive nature and potential environmental impact. Proper containment, neutralization techniques, and waste management practices are essential to ensure safe use and minimize environmental risks associated with propionic acid.
02 Applications of propionic acid in food preservation
Propionic acid is widely used as a food preservative due to its antimicrobial properties. It is effective in preventing mold growth and extending the shelf life of various food products, particularly in bakery items and animal feed. The acid and its salts are recognized as safe food additives in many countries.Expand Specific Solutions03 Propionic acid derivatives and their uses
Derivatives of propionic acid, such as esters and salts, have diverse applications in industries including pharmaceuticals, cosmetics, and agriculture. These compounds often exhibit enhanced properties or specific functionalities compared to the parent acid, making them valuable in various formulations and processes.Expand Specific Solutions04 Environmental and safety considerations in propionic acid handling
The production, storage, and use of propionic acid require careful consideration of environmental and safety factors. This includes proper containment methods, waste management, and worker protection measures. Innovations in these areas focus on minimizing environmental impact and ensuring safe handling of the acid in industrial settings.Expand Specific Solutions05 Analytical methods for propionic acid detection and quantification
Various analytical techniques are employed for the detection and quantification of propionic acid in different matrices. These methods are crucial for quality control in production processes, monitoring food preservation efficacy, and environmental testing. Advancements in analytical technologies aim to improve the accuracy, sensitivity, and efficiency of propionic acid analysis.Expand Specific Solutions
Key Players in Propionic Acid Research
The environmental chemistry pathway of propionic acid is currently in a developing stage, with growing market potential due to increasing environmental concerns and sustainable practices. The market size is expanding, driven by applications in various industries such as agriculture, food preservation, and pharmaceuticals. Technologically, the field is advancing, with companies like Nippon Shokubai, Dow Global Technologies, and Genomatica leading research efforts. Universities such as Campinas, Queensland, and Ohio State are contributing significantly to the scientific understanding. While established chemical companies like BASF and Evonik are involved, emerging players and research institutions are also making notable progress, indicating a competitive and evolving landscape in this area.
Dow Global Technologies LLC
Technical Solution: Dow has developed advanced catalytic processes for propionic acid production, focusing on environmentally friendly routes. Their approach involves the carbonylation of ethylene using carbon monoxide and water in the presence of a homogeneous catalyst system[1]. This method reduces the environmental impact by avoiding the use of petrochemical feedstocks. Dow has also explored bio-based pathways, utilizing renewable resources to produce propionic acid through fermentation processes[2]. Their research extends to the development of novel catalysts that enhance selectivity and yield, while minimizing byproduct formation[3].
Strengths: Innovative catalytic processes, focus on renewable resources, and expertise in large-scale chemical production. Weaknesses: Potential high costs associated with new technology implementation and competition from established petrochemical routes.
Genomatica, Inc.
Technical Solution: Genomatica has pioneered a bio-based approach to propionic acid production, leveraging their expertise in industrial biotechnology. Their process utilizes engineered microorganisms to convert renewable feedstocks, such as sugar or glycerin, into propionic acid[4]. The company has developed proprietary strains of bacteria that can efficiently produce propionic acid under anaerobic conditions, reducing energy requirements and greenhouse gas emissions[5]. Genomatica's technology also incorporates advanced fermentation and separation techniques to achieve high purity and yield[6]. This bio-based route aligns with principles of green chemistry and contributes to the circular economy.
Strengths: Sustainable bio-based production, reduced carbon footprint, and potential for cost-effective scaling. Weaknesses: Dependence on feedstock availability and potential challenges in achieving petrochemical-grade purity.
Breakthrough Studies in Propionic Acid Biochemistry
Improved propionibacterium strains for the production of propionic acid
PatentWO2017055932A2
Innovation
- Genome shuffling between selected Propionibacterium strains, such as P. acidipropionici ATCC 4875 and P. acidipropionici ATCC 55737, to generate novel strains with enhanced growth rates and propionic acid production, utilizing genetic material exchange to create strains with improved metabolic pathways and regulatory mechanisms.
Method for production of propionic acid from inulins
PatentPendingIN202141042851A
Innovation
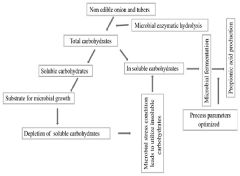
- A single-step process optimizing enzymatic hydrolysis of inulin from onion waste and non-edible tubers to convert insoluble carbohydrates into fermentable sugars, followed by microbial fermentation to produce propionic acid, addressing issues like bi-product formation and downstream processing through optimized microbial growth conditions and stress parameters.
Regulatory Framework for Environmental Chemicals
The regulatory framework for environmental chemicals plays a crucial role in understanding and managing the environmental impact of substances like propionic acid. This framework encompasses a complex network of laws, regulations, and guidelines established by various governmental and international bodies to protect human health and the environment.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) provide guidelines and recommendations for the management of environmental chemicals. These frameworks often serve as a basis for national and regional regulations.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body responsible for implementing and enforcing environmental chemical regulations. The Toxic Substances Control Act (TSCA) and the Clean Water Act (CWA) are two key pieces of legislation that govern the use, disposal, and environmental release of chemicals like propionic acid.
The European Union has implemented the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to assess and manage the risks associated with chemicals they produce or import. This comprehensive approach aims to improve the protection of human health and the environment through better and earlier identification of the intrinsic properties of chemical substances.
Many countries have established their own regulatory frameworks, often modeled after or in compliance with international standards. These frameworks typically include requirements for chemical registration, risk assessment, labeling, and reporting of environmental releases.
Specific to propionic acid, regulations often focus on its potential environmental impacts, particularly in aquatic ecosystems. Guidelines may include maximum permissible concentrations in water bodies, soil, and air, as well as requirements for proper handling, storage, and disposal to prevent environmental contamination.
Regulatory frameworks also often include provisions for monitoring and reporting environmental levels of chemicals like propionic acid. This data collection helps inform policy decisions and assess the effectiveness of existing regulations.
As scientific understanding of environmental chemistry evolves, regulatory frameworks are periodically updated to incorporate new knowledge and address emerging concerns. This dynamic nature of environmental regulations underscores the importance of ongoing research into the environmental pathways and impacts of chemicals like propionic acid.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) provide guidelines and recommendations for the management of environmental chemicals. These frameworks often serve as a basis for national and regional regulations.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body responsible for implementing and enforcing environmental chemical regulations. The Toxic Substances Control Act (TSCA) and the Clean Water Act (CWA) are two key pieces of legislation that govern the use, disposal, and environmental release of chemicals like propionic acid.
The European Union has implemented the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to assess and manage the risks associated with chemicals they produce or import. This comprehensive approach aims to improve the protection of human health and the environment through better and earlier identification of the intrinsic properties of chemical substances.
Many countries have established their own regulatory frameworks, often modeled after or in compliance with international standards. These frameworks typically include requirements for chemical registration, risk assessment, labeling, and reporting of environmental releases.
Specific to propionic acid, regulations often focus on its potential environmental impacts, particularly in aquatic ecosystems. Guidelines may include maximum permissible concentrations in water bodies, soil, and air, as well as requirements for proper handling, storage, and disposal to prevent environmental contamination.
Regulatory frameworks also often include provisions for monitoring and reporting environmental levels of chemicals like propionic acid. This data collection helps inform policy decisions and assess the effectiveness of existing regulations.
As scientific understanding of environmental chemistry evolves, regulatory frameworks are periodically updated to incorporate new knowledge and address emerging concerns. This dynamic nature of environmental regulations underscores the importance of ongoing research into the environmental pathways and impacts of chemicals like propionic acid.
Ecological Risk Assessment of Propionic Acid
Ecological risk assessment of propionic acid is a critical process in understanding its potential impacts on the environment. This assessment involves evaluating the likelihood and severity of adverse effects that may occur due to exposure to propionic acid in various ecosystems.
The first step in this assessment is hazard identification, which involves determining the inherent toxicity of propionic acid to different organisms. Studies have shown that propionic acid exhibits low to moderate toxicity to aquatic organisms, with varying effects on different species. For example, fish species such as rainbow trout and bluegill sunfish have shown different levels of sensitivity to propionic acid exposure.
Exposure assessment is the next crucial component, focusing on the pathways through which propionic acid enters the environment and the concentrations at which organisms may be exposed. This includes considering its release from industrial processes, agricultural runoff, and natural sources. The environmental fate of propionic acid, including its biodegradation and potential for bioaccumulation, plays a significant role in determining exposure levels.
Dose-response assessment involves quantifying the relationship between the level of exposure to propionic acid and the severity of adverse effects on organisms. This step typically involves analyzing data from laboratory toxicity tests and field studies to establish threshold concentrations for different ecological endpoints.
Risk characterization, the final step, integrates the information from the previous steps to estimate the likelihood and magnitude of ecological effects. This involves comparing estimated environmental concentrations with toxicity thresholds and considering factors such as the spatial and temporal distribution of propionic acid in the environment.
Uncertainty analysis is an essential aspect of the ecological risk assessment process for propionic acid. This involves identifying and quantifying sources of variability and uncertainty in the data and models used throughout the assessment. Factors such as limited toxicity data for certain species, variability in environmental conditions, and uncertainties in exposure estimates must be carefully considered.
Mitigation strategies based on the risk assessment findings may include recommendations for reducing propionic acid emissions, implementing treatment technologies, or establishing environmental quality standards. These strategies aim to minimize the potential ecological impacts of propionic acid while balancing its beneficial uses in various industries.
Ongoing monitoring and research are crucial for refining the ecological risk assessment of propionic acid. As new data becomes available and analytical techniques improve, the assessment can be updated to provide more accurate and comprehensive evaluations of its environmental impacts.
The first step in this assessment is hazard identification, which involves determining the inherent toxicity of propionic acid to different organisms. Studies have shown that propionic acid exhibits low to moderate toxicity to aquatic organisms, with varying effects on different species. For example, fish species such as rainbow trout and bluegill sunfish have shown different levels of sensitivity to propionic acid exposure.
Exposure assessment is the next crucial component, focusing on the pathways through which propionic acid enters the environment and the concentrations at which organisms may be exposed. This includes considering its release from industrial processes, agricultural runoff, and natural sources. The environmental fate of propionic acid, including its biodegradation and potential for bioaccumulation, plays a significant role in determining exposure levels.
Dose-response assessment involves quantifying the relationship between the level of exposure to propionic acid and the severity of adverse effects on organisms. This step typically involves analyzing data from laboratory toxicity tests and field studies to establish threshold concentrations for different ecological endpoints.
Risk characterization, the final step, integrates the information from the previous steps to estimate the likelihood and magnitude of ecological effects. This involves comparing estimated environmental concentrations with toxicity thresholds and considering factors such as the spatial and temporal distribution of propionic acid in the environment.
Uncertainty analysis is an essential aspect of the ecological risk assessment process for propionic acid. This involves identifying and quantifying sources of variability and uncertainty in the data and models used throughout the assessment. Factors such as limited toxicity data for certain species, variability in environmental conditions, and uncertainties in exposure estimates must be carefully considered.
Mitigation strategies based on the risk assessment findings may include recommendations for reducing propionic acid emissions, implementing treatment technologies, or establishing environmental quality standards. These strategies aim to minimize the potential ecological impacts of propionic acid while balancing its beneficial uses in various industries.
Ongoing monitoring and research are crucial for refining the ecological risk assessment of propionic acid. As new data becomes available and analytical techniques improve, the assessment can be updated to provide more accurate and comprehensive evaluations of its environmental impacts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!