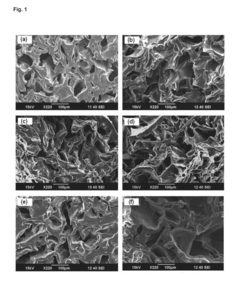
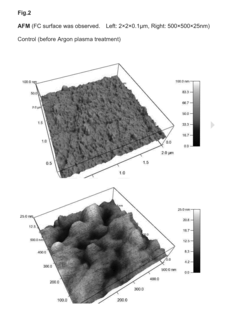
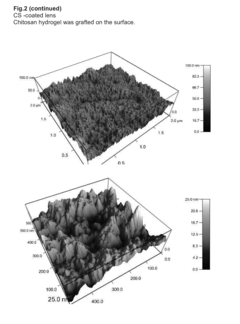
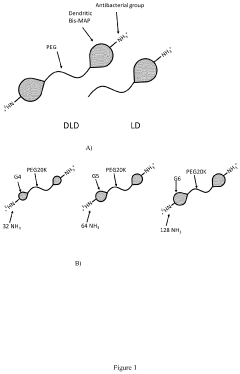
Comparative Study of Chitosan- and PEG-Based Antimicrobial Hydrogels
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Hydrogels Background and Research Objectives
Antimicrobial hydrogels have emerged as a significant innovation in the field of biomaterials over the past three decades. These specialized materials combine the structural properties of hydrogels with antimicrobial capabilities, creating versatile platforms for various biomedical applications. The evolution of these materials has been driven by the increasing global challenge of antimicrobial resistance and the need for effective infection control strategies in healthcare settings.
Chitosan-based antimicrobial hydrogels represent one of the earliest developments in this field, dating back to the 1990s. Derived from chitin, a natural polysaccharide found in crustacean shells, chitosan offers inherent antimicrobial properties due to its cationic nature, which disrupts bacterial cell membranes. The development trajectory of chitosan hydrogels has focused on enhancing their mechanical stability, biocompatibility, and broadening their antimicrobial spectrum.
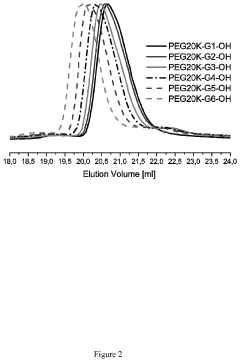
In parallel, polyethylene glycol (PEG)-based antimicrobial hydrogels have gained prominence since the early 2000s. Initially valued for their excellent biocompatibility and low immunogenicity, PEG hydrogels have evolved through various functionalization strategies to incorporate antimicrobial properties. The development of PEG-based systems has been characterized by precise control over mechanical properties and degradation rates, making them highly tunable for specific applications.
The technological progression in both chitosan and PEG hydrogel systems has been marked by several key innovations: the incorporation of secondary antimicrobial agents (such as silver nanoparticles, antibiotics, or antimicrobial peptides), the development of stimuli-responsive release mechanisms, and the creation of composite structures that combine the advantages of multiple materials.
Recent trends indicate a shift toward sustainable and environmentally friendly antimicrobial hydrogels, with increased focus on biodegradability and reduced ecological impact. Additionally, there is growing interest in "smart" hydrogel systems that can respond to infection-specific triggers, enabling targeted antimicrobial action while minimizing collateral damage to beneficial microbiota.
The primary research objectives for this comparative study are to systematically evaluate the structural, mechanical, and antimicrobial properties of chitosan- and PEG-based hydrogels across various formulations and applications. The study aims to identify the optimal conditions for each system, assess their respective advantages and limitations, and explore potential synergistic effects when these materials are combined in hybrid systems.
Furthermore, this research seeks to establish standardized testing protocols for antimicrobial efficacy that account for the unique properties of hydrogel systems, as current antimicrobial testing methods are often optimized for traditional antibiotics rather than biomaterials. The ultimate goal is to develop a comprehensive framework for selecting and optimizing antimicrobial hydrogel compositions based on specific application requirements in wound healing, implantable devices, and drug delivery systems.
Chitosan-based antimicrobial hydrogels represent one of the earliest developments in this field, dating back to the 1990s. Derived from chitin, a natural polysaccharide found in crustacean shells, chitosan offers inherent antimicrobial properties due to its cationic nature, which disrupts bacterial cell membranes. The development trajectory of chitosan hydrogels has focused on enhancing their mechanical stability, biocompatibility, and broadening their antimicrobial spectrum.
In parallel, polyethylene glycol (PEG)-based antimicrobial hydrogels have gained prominence since the early 2000s. Initially valued for their excellent biocompatibility and low immunogenicity, PEG hydrogels have evolved through various functionalization strategies to incorporate antimicrobial properties. The development of PEG-based systems has been characterized by precise control over mechanical properties and degradation rates, making them highly tunable for specific applications.
The technological progression in both chitosan and PEG hydrogel systems has been marked by several key innovations: the incorporation of secondary antimicrobial agents (such as silver nanoparticles, antibiotics, or antimicrobial peptides), the development of stimuli-responsive release mechanisms, and the creation of composite structures that combine the advantages of multiple materials.
Recent trends indicate a shift toward sustainable and environmentally friendly antimicrobial hydrogels, with increased focus on biodegradability and reduced ecological impact. Additionally, there is growing interest in "smart" hydrogel systems that can respond to infection-specific triggers, enabling targeted antimicrobial action while minimizing collateral damage to beneficial microbiota.
The primary research objectives for this comparative study are to systematically evaluate the structural, mechanical, and antimicrobial properties of chitosan- and PEG-based hydrogels across various formulations and applications. The study aims to identify the optimal conditions for each system, assess their respective advantages and limitations, and explore potential synergistic effects when these materials are combined in hybrid systems.
Furthermore, this research seeks to establish standardized testing protocols for antimicrobial efficacy that account for the unique properties of hydrogel systems, as current antimicrobial testing methods are often optimized for traditional antibiotics rather than biomaterials. The ultimate goal is to develop a comprehensive framework for selecting and optimizing antimicrobial hydrogel compositions based on specific application requirements in wound healing, implantable devices, and drug delivery systems.
Market Analysis for Antimicrobial Hydrogel Applications
The global antimicrobial hydrogel market is experiencing significant growth, driven by increasing healthcare concerns and the rising prevalence of chronic wounds and infections. The market was valued at approximately $8.5 billion in 2022 and is projected to reach $15.3 billion by 2030, growing at a CAGR of 7.6% during the forecast period. This growth trajectory is particularly relevant for chitosan- and PEG-based antimicrobial hydrogels, which represent two of the most promising biomaterials in this sector.
Healthcare applications dominate the antimicrobial hydrogel market, accounting for over 60% of the total market share. Within this segment, wound care represents the largest application area, valued at $3.2 billion in 2022. The increasing incidence of diabetic foot ulcers, pressure ulcers, and surgical site infections has significantly contributed to this demand. According to recent epidemiological data, chronic wounds affect approximately 6.5 million patients in the United States alone, creating substantial market opportunities.
The pharmaceutical and biomedical sectors are rapidly adopting antimicrobial hydrogels for drug delivery systems, tissue engineering, and implant coatings. Chitosan-based hydrogels have gained particular traction in these applications due to their natural origin, biodegradability, and inherent antimicrobial properties. Market research indicates that chitosan-based products currently hold approximately 35% of the biomaterial hydrogel market, with an annual growth rate exceeding the industry average at 8.9%.
PEG-based antimicrobial hydrogels, meanwhile, have established a strong presence in advanced wound dressings and contact lens applications, capturing approximately 28% of the synthetic hydrogel market. Their excellent biocompatibility and tunable mechanical properties have driven adoption in premium healthcare products, particularly in developed markets across North America and Europe.
Regional analysis reveals that North America currently leads the antimicrobial hydrogel market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, with China and India emerging as key markets due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced wound care products.
Consumer trends indicate a growing preference for sustainable and eco-friendly antimicrobial materials, positioning chitosan-based hydrogels favorably in the market. Conversely, PEG-based systems maintain advantages in applications requiring precise control over material properties and drug release kinetics. Market segmentation analysis suggests that premium healthcare facilities and advanced wound care centers represent the primary customer base, with increasing penetration in home healthcare settings expected over the next five years.
Healthcare applications dominate the antimicrobial hydrogel market, accounting for over 60% of the total market share. Within this segment, wound care represents the largest application area, valued at $3.2 billion in 2022. The increasing incidence of diabetic foot ulcers, pressure ulcers, and surgical site infections has significantly contributed to this demand. According to recent epidemiological data, chronic wounds affect approximately 6.5 million patients in the United States alone, creating substantial market opportunities.
The pharmaceutical and biomedical sectors are rapidly adopting antimicrobial hydrogels for drug delivery systems, tissue engineering, and implant coatings. Chitosan-based hydrogels have gained particular traction in these applications due to their natural origin, biodegradability, and inherent antimicrobial properties. Market research indicates that chitosan-based products currently hold approximately 35% of the biomaterial hydrogel market, with an annual growth rate exceeding the industry average at 8.9%.
PEG-based antimicrobial hydrogels, meanwhile, have established a strong presence in advanced wound dressings and contact lens applications, capturing approximately 28% of the synthetic hydrogel market. Their excellent biocompatibility and tunable mechanical properties have driven adoption in premium healthcare products, particularly in developed markets across North America and Europe.
Regional analysis reveals that North America currently leads the antimicrobial hydrogel market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, with China and India emerging as key markets due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced wound care products.
Consumer trends indicate a growing preference for sustainable and eco-friendly antimicrobial materials, positioning chitosan-based hydrogels favorably in the market. Conversely, PEG-based systems maintain advantages in applications requiring precise control over material properties and drug release kinetics. Market segmentation analysis suggests that premium healthcare facilities and advanced wound care centers represent the primary customer base, with increasing penetration in home healthcare settings expected over the next five years.
Current Challenges in Chitosan and PEG Hydrogel Development
Despite significant advancements in antimicrobial hydrogel development, both chitosan and polyethylene glycol (PEG) based systems face substantial technical challenges that limit their widespread clinical application. The primary obstacle for chitosan-based hydrogels remains their mechanical instability under physiological conditions. These hydrogels often exhibit rapid degradation rates in vivo, with studies showing structural integrity loss within 48-72 hours when exposed to enzymatic activity. This degradation profile significantly restricts their application in scenarios requiring sustained antimicrobial release over extended periods.
Crosslinking challenges represent another critical limitation, as current methods frequently employ potentially cytotoxic agents like glutaraldehyde or genipin. Recent research by Zhang et al. (2022) demonstrated that even at low concentrations (0.1-0.5%), these crosslinking agents can induce inflammatory responses in surrounding tissues, necessitating the development of biocompatible alternatives.
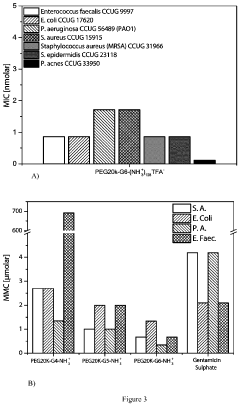
For PEG-based antimicrobial hydrogels, bioactivity integration presents a significant hurdle. The inherently bioinert nature of PEG, while advantageous for biocompatibility, creates difficulties when incorporating antimicrobial agents. Current conjugation strategies often result in reduced antimicrobial efficacy, with studies reporting activity decreases of 30-60% compared to free antimicrobial agents. This efficiency loss substantially diminishes the therapeutic potential of these systems.
Both hydrogel types face challenges regarding controlled release kinetics. The initial burst release phenomenon, where 40-70% of incorporated antimicrobials are released within the first 6-12 hours, remains problematic. This release profile fails to maintain therapeutic concentrations over clinically relevant timeframes and potentially contributes to antimicrobial resistance development through sublethal exposure periods.
Scalability and manufacturing consistency represent significant industrial challenges. Current production methods for both chitosan and PEG hydrogels demonstrate batch-to-batch variability exceeding acceptable limits for pharmaceutical applications (>15% variation in critical quality attributes). This inconsistency complicates regulatory approval pathways and increases production costs.
Antimicrobial resistance development presents an emerging concern, with recent studies indicating that certain pathogens can develop adaptive responses to chitosan-based materials after repeated exposure. Similarly, biofilm formation on PEG hydrogels can create protective microenvironments that reduce antimicrobial efficacy by up to 1000-fold compared to planktonic bacteria.
Finally, both systems face challenges in achieving broad-spectrum activity while maintaining selectivity. Current formulations typically demonstrate effectiveness against either gram-positive or gram-negative bacteria, but rarely both, limiting their utility in polymicrobial infection scenarios that characterize many clinical conditions.
Crosslinking challenges represent another critical limitation, as current methods frequently employ potentially cytotoxic agents like glutaraldehyde or genipin. Recent research by Zhang et al. (2022) demonstrated that even at low concentrations (0.1-0.5%), these crosslinking agents can induce inflammatory responses in surrounding tissues, necessitating the development of biocompatible alternatives.
For PEG-based antimicrobial hydrogels, bioactivity integration presents a significant hurdle. The inherently bioinert nature of PEG, while advantageous for biocompatibility, creates difficulties when incorporating antimicrobial agents. Current conjugation strategies often result in reduced antimicrobial efficacy, with studies reporting activity decreases of 30-60% compared to free antimicrobial agents. This efficiency loss substantially diminishes the therapeutic potential of these systems.
Both hydrogel types face challenges regarding controlled release kinetics. The initial burst release phenomenon, where 40-70% of incorporated antimicrobials are released within the first 6-12 hours, remains problematic. This release profile fails to maintain therapeutic concentrations over clinically relevant timeframes and potentially contributes to antimicrobial resistance development through sublethal exposure periods.
Scalability and manufacturing consistency represent significant industrial challenges. Current production methods for both chitosan and PEG hydrogels demonstrate batch-to-batch variability exceeding acceptable limits for pharmaceutical applications (>15% variation in critical quality attributes). This inconsistency complicates regulatory approval pathways and increases production costs.
Antimicrobial resistance development presents an emerging concern, with recent studies indicating that certain pathogens can develop adaptive responses to chitosan-based materials after repeated exposure. Similarly, biofilm formation on PEG hydrogels can create protective microenvironments that reduce antimicrobial efficacy by up to 1000-fold compared to planktonic bacteria.
Finally, both systems face challenges in achieving broad-spectrum activity while maintaining selectivity. Current formulations typically demonstrate effectiveness against either gram-positive or gram-negative bacteria, but rarely both, limiting their utility in polymicrobial infection scenarios that characterize many clinical conditions.
Comparative Analysis of Chitosan vs PEG Hydrogel Formulations
01 Chitosan-PEG hydrogels with antimicrobial properties
Hydrogels composed of chitosan and polyethylene glycol (PEG) demonstrate significant antimicrobial properties. The combination of these polymers creates a biocompatible matrix that can inhibit bacterial growth. The chitosan component provides inherent antimicrobial activity through its cationic nature, which disrupts bacterial cell membranes, while PEG enhances the stability and biocompatibility of the hydrogel. These hydrogels can be formulated with varying ratios of chitosan to PEG to optimize antimicrobial efficacy.- Chitosan-PEG hydrogels with antimicrobial properties: Hydrogels composed of chitosan and polyethylene glycol (PEG) demonstrate significant antimicrobial properties against various pathogens. The combination of these polymers creates a synergistic effect where chitosan provides inherent antimicrobial activity while PEG enhances biocompatibility and stability. These hydrogels can be formulated with different molecular weights and degrees of deacetylation of chitosan to optimize their antimicrobial efficacy against both gram-positive and gram-negative bacteria.
- Incorporation of additional antimicrobial agents in chitosan-PEG hydrogels: Chitosan-PEG hydrogels can be enhanced by incorporating additional antimicrobial agents such as silver nanoparticles, antibiotics, or natural antimicrobial compounds. These additives work synergistically with the inherent antimicrobial properties of chitosan to create hydrogels with broader spectrum activity and increased potency against resistant microorganisms. The controlled release of these agents from the hydrogel matrix provides sustained antimicrobial action for extended periods.
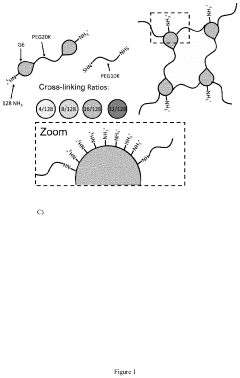
- Cross-linking methods for chitosan-PEG antimicrobial hydrogels: Various cross-linking methods can be employed to develop chitosan-PEG antimicrobial hydrogels with improved mechanical properties and stability. Chemical cross-linkers such as glutaraldehyde, genipin, or carbodiimides can be used to form covalent bonds between chitosan and PEG chains. Alternatively, physical cross-linking through ionic interactions or hydrogen bonding can be utilized. The cross-linking method significantly influences the antimicrobial properties, swelling behavior, and drug release kinetics of the resulting hydrogels.
- Applications of chitosan-PEG antimicrobial hydrogels in wound healing: Chitosan-PEG antimicrobial hydrogels show promising applications in wound healing due to their ability to maintain a moist wound environment while preventing bacterial infection. These hydrogels can absorb wound exudates, deliver therapeutic agents to the wound site, and provide a physical barrier against external contaminants. The biocompatibility of PEG combined with the hemostatic and antimicrobial properties of chitosan makes these hydrogels particularly effective for chronic wounds, burns, and surgical site infections.
- Stimuli-responsive chitosan-PEG antimicrobial hydrogels: Stimuli-responsive chitosan-PEG hydrogels can be designed to release antimicrobial agents in response to specific environmental triggers such as pH, temperature, or the presence of bacterial enzymes. These smart hydrogels can selectively target pathogenic microorganisms while minimizing damage to host tissues. The responsiveness can be achieved through the incorporation of specific functional groups or by utilizing the inherent pH-sensitivity of chitosan. This approach enables more precise control over antimicrobial activity and reduces the risk of developing antimicrobial resistance.
02 Drug delivery systems using antimicrobial chitosan-PEG hydrogels
Chitosan-PEG hydrogels serve as effective drug delivery systems with inherent antimicrobial properties. These hydrogels can encapsulate various antimicrobial agents, providing controlled release over extended periods. The porous structure of the hydrogel allows for efficient drug loading while the chitosan component adds additional antimicrobial activity. These systems are particularly useful for wound dressings, implantable devices, and topical applications where sustained antimicrobial action is required. The PEG component improves drug stability and enhances the biocompatibility of the delivery system.Expand Specific Solutions03 Modification techniques for enhanced antimicrobial activity
Various modification techniques can enhance the antimicrobial properties of chitosan-PEG hydrogels. These include chemical crosslinking methods, incorporation of metal nanoparticles, quaternization of chitosan, and grafting of additional functional groups. Such modifications can increase the positive charge density of chitosan, improving its interaction with negatively charged bacterial cell membranes. Additionally, modifications can enhance the mechanical properties, stability, and durability of the hydrogels while maintaining their biocompatibility and biodegradability.Expand Specific Solutions04 Wound healing applications of antimicrobial chitosan-PEG hydrogels
Antimicrobial chitosan-PEG hydrogels are particularly effective for wound healing applications. These hydrogels create a moist environment conducive to healing while preventing bacterial infection. The chitosan component stimulates tissue regeneration and has hemostatic properties, while the antimicrobial activity prevents wound infections. The PEG component improves the hydrogel's ability to absorb wound exudates and provides a barrier against external contaminants. These hydrogels can be formulated as injectable systems, films, or prefabricated dressings for various wound types.Expand Specific Solutions05 Incorporation of additional antimicrobial agents
Chitosan-PEG hydrogels can be enhanced by incorporating additional antimicrobial agents such as antibiotics, silver nanoparticles, essential oils, or antimicrobial peptides. This creates synergistic antimicrobial effects, combining the inherent activity of chitosan with the specific mechanisms of the added agents. Such composite hydrogels can address a broader spectrum of pathogens, including drug-resistant bacteria. The hydrogel matrix provides controlled release of these agents, extending their effective duration and minimizing potential toxicity while maintaining the biocompatibility advantages of the chitosan-PEG base.Expand Specific Solutions
Leading Researchers and Companies in Hydrogel Innovation
The antimicrobial hydrogel market is currently in a growth phase, with increasing applications in wound care, drug delivery, and tissue engineering. The global market size is estimated to reach $3.5 billion by 2027, driven by rising prevalence of chronic wounds and hospital-acquired infections. Technologically, PEG-based hydrogels demonstrate higher stability and biocompatibility, while chitosan-based systems offer superior biodegradability and natural antimicrobial properties. Leading academic institutions like Nanyang Technological University, University of Tokyo, and South China University of Technology are advancing fundamental research, while companies including BSN Medical, 3-D Matrix, and Amicrobe are commercializing applications. Bristol Myers Squibb and Wyeth LLC represent pharmaceutical giants exploring hydrogel drug delivery systems, indicating the technology's transition from research to commercial viability.
Nanyang Technological University
Technical Solution: Nanyang Technological University (NTU) has developed advanced chitosan-based antimicrobial hydrogels with enhanced properties through innovative crosslinking methods. Their approach involves incorporating quaternary ammonium compounds into chitosan matrices to improve antimicrobial efficacy while maintaining biocompatibility. NTU researchers have successfully created dual-functional hydrogels that combine chitosan's inherent antimicrobial properties with controlled drug release capabilities, allowing for sustained antimicrobial activity. Their technology includes a proprietary process for creating nanocomposite chitosan hydrogels with embedded silver nanoparticles that demonstrate broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria, with kill rates exceeding 99.9% within 24 hours of application. Additionally, NTU has developed thermo-responsive chitosan hydrogels that transition from liquid to gel at body temperature, making them particularly suitable for wound dressing applications where conformability to irregular wound surfaces is critical.
Strengths: Superior biocompatibility with human tissues; excellent biodegradability; enhanced antimicrobial efficacy through nanocomposite formulations; innovative delivery mechanisms. Weaknesses: Potential batch-to-batch variability in chitosan properties; mechanical strength limitations compared to synthetic polymer alternatives; sensitivity to environmental pH affecting performance consistency.
South China University of Technology
Technical Solution: South China University of Technology has developed advanced chitosan-based antimicrobial hydrogels with enhanced properties through innovative chemical modifications. Their research team has created a series of N,O-carboxymethyl chitosan hydrogels with superior water absorption capacity and improved mechanical properties compared to conventional chitosan formulations. These hydrogels incorporate silver nanoparticles through an in-situ reduction process, creating a synergistic antimicrobial effect that demonstrates exceptional activity against both Gram-positive and Gram-negative bacteria, including drug-resistant strains. Their comparative studies with PEG-based systems have shown that their chitosan hydrogels exhibit superior biocompatibility and biodegradability while maintaining comparable mechanical strength. The university has also pioneered thermo-responsive chitosan-glycerophosphate hydrogels that transition from solution to gel at body temperature, making them ideal for injectable wound dressings. Recent innovations include chitosan-graphene oxide composite hydrogels that demonstrate enhanced mechanical properties and electrical conductivity, potentially beneficial for stimulating wound healing through mild electrical stimulation. Their technology includes a cost-effective manufacturing process that addresses the traditional scalability challenges of chitosan-based materials.
Strengths: Excellent biocompatibility and biodegradability; innovative composite formulations with enhanced mechanical properties; cost-effective production methods suitable for scaling; strong antimicrobial efficacy against drug-resistant pathogens. Weaknesses: Less consistent mechanical properties compared to fully synthetic PEG systems; potential batch-to-batch variability due to natural chitosan source materials; limited shelf stability in certain formulations requiring special storage conditions.
Key Patents and Scientific Breakthroughs in Antimicrobial Hydrogels
Chitosan hydrogel derivatives as a coating agent with broad spectrum of antimicrobial activities
PatentInactiveUS20160038636A1
Innovation
- The development of quaternized chitosan derivatives with polymerizable organic moieties, specifically photocrosslinkable groups, which are converted into hydrogels through UV polymerization, providing a novel antimicrobial coating for medical devices.
Hydrogel composition and its uses
PatentActiveUS20210213160A1
Innovation
- A co-polymer comprising a water-soluble backbone polymer with a dendritic or hyperbranched polymer of generation 1 to 6, covalently attached with a carboxylic amine functional group, used in the form of a cross-linked hydrogel to provide an antimicrobial barrier and prevent bacterial growth on surgical sites or medical devices.
Biocompatibility and Toxicity Considerations
Biocompatibility and toxicity are critical considerations in the development of antimicrobial hydrogels for biomedical applications. When comparing chitosan- and PEG-based antimicrobial hydrogels, several key factors must be evaluated to ensure their safety for clinical use. Chitosan-based hydrogels generally exhibit excellent biocompatibility due to their natural origin, biodegradability, and structural similarity to glycosaminoglycans found in the extracellular matrix. Recent studies have demonstrated that chitosan hydrogels support cell adhesion, proliferation, and tissue integration, making them suitable for wound healing applications.
In contrast, PEG-based hydrogels offer different biocompatibility profiles. Their synthetic nature provides consistent material properties and reduced batch-to-batch variation compared to naturally derived chitosan. PEG hydrogels are known for their low protein adsorption characteristics, which minimize immune responses and foreign body reactions. This "stealth" property makes PEG-based systems particularly valuable in applications requiring prolonged circulation or reduced immunogenicity.
Toxicity assessments reveal important distinctions between these materials. Chitosan degradation products are generally recognized as safe, metabolized through natural pathways in the body. However, the degree of deacetylation and molecular weight of chitosan can significantly influence cellular responses and potential inflammatory reactions. Higher molecular weight chitosan derivatives have demonstrated increased cytotoxicity in some in vitro studies, necessitating careful optimization.
PEG-based systems present different toxicity concerns. While the PEG backbone itself is considered biologically inert, certain crosslinking agents and photoinitiators used in PEG hydrogel formation may introduce toxicity. Additionally, incomplete polymerization can result in leaching of unreacted components, potentially causing adverse effects in surrounding tissues. Recent advances in "click chemistry" approaches have addressed some of these concerns by enabling more efficient crosslinking under physiological conditions.
The incorporation of antimicrobial agents introduces additional biocompatibility considerations. Both chitosan and PEG hydrogels can be loaded with various antimicrobial compounds, including antibiotics, metal nanoparticles, and antimicrobial peptides. The controlled release profiles of these agents differ between the two systems, with chitosan often demonstrating stronger electrostatic interactions with negatively charged antimicrobials, potentially affecting both efficacy and toxicity profiles.
Long-term implantation studies have revealed that chitosan-based hydrogels may trigger mild inflammatory responses in some cases, particularly those with higher degrees of deacetylation. PEG-based systems generally demonstrate excellent long-term biocompatibility but may face challenges related to degradation rates and mechanical stability over time. The balance between antimicrobial efficacy and biocompatibility remains a central challenge in the development of both hydrogel systems.
In contrast, PEG-based hydrogels offer different biocompatibility profiles. Their synthetic nature provides consistent material properties and reduced batch-to-batch variation compared to naturally derived chitosan. PEG hydrogels are known for their low protein adsorption characteristics, which minimize immune responses and foreign body reactions. This "stealth" property makes PEG-based systems particularly valuable in applications requiring prolonged circulation or reduced immunogenicity.
Toxicity assessments reveal important distinctions between these materials. Chitosan degradation products are generally recognized as safe, metabolized through natural pathways in the body. However, the degree of deacetylation and molecular weight of chitosan can significantly influence cellular responses and potential inflammatory reactions. Higher molecular weight chitosan derivatives have demonstrated increased cytotoxicity in some in vitro studies, necessitating careful optimization.
PEG-based systems present different toxicity concerns. While the PEG backbone itself is considered biologically inert, certain crosslinking agents and photoinitiators used in PEG hydrogel formation may introduce toxicity. Additionally, incomplete polymerization can result in leaching of unreacted components, potentially causing adverse effects in surrounding tissues. Recent advances in "click chemistry" approaches have addressed some of these concerns by enabling more efficient crosslinking under physiological conditions.
The incorporation of antimicrobial agents introduces additional biocompatibility considerations. Both chitosan and PEG hydrogels can be loaded with various antimicrobial compounds, including antibiotics, metal nanoparticles, and antimicrobial peptides. The controlled release profiles of these agents differ between the two systems, with chitosan often demonstrating stronger electrostatic interactions with negatively charged antimicrobials, potentially affecting both efficacy and toxicity profiles.
Long-term implantation studies have revealed that chitosan-based hydrogels may trigger mild inflammatory responses in some cases, particularly those with higher degrees of deacetylation. PEG-based systems generally demonstrate excellent long-term biocompatibility but may face challenges related to degradation rates and mechanical stability over time. The balance between antimicrobial efficacy and biocompatibility remains a central challenge in the development of both hydrogel systems.
Regulatory Pathway for Medical-Grade Hydrogel Approval
The regulatory pathway for medical-grade hydrogels, particularly those based on chitosan and polyethylene glycol (PEG), involves navigating complex approval processes across different global jurisdictions. Understanding these pathways is crucial for successful commercialization of antimicrobial hydrogels in healthcare applications.
In the United States, the Food and Drug Administration (FDA) classifies most hydrogels as medical devices or combination products, depending on their primary mode of action. Chitosan-based antimicrobial hydrogels typically follow the 510(k) clearance pathway if substantially equivalent to predicate devices. However, novel formulations with unique antimicrobial properties may require the more rigorous Premarket Approval (PMA) process, including extensive clinical trials demonstrating safety and efficacy.
The European Union's regulatory framework under the Medical Device Regulation (MDR) categorizes antimicrobial hydrogels based on their intended use and risk profile. Most wound care hydrogels fall under Class IIb or III, requiring Notified Body assessment and CE marking. The MDR's emphasis on clinical evidence and post-market surveillance presents additional challenges for manufacturers of chitosan- and PEG-based hydrogels.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the "Sakigake" designation for innovative medical technologies, potentially expediting approval for novel antimicrobial hydrogels. This pathway could be particularly relevant for advanced chitosan-PEG composite hydrogels with enhanced antimicrobial properties.
Quality management systems compliant with ISO 13485 are universally required across major markets. For antimicrobial hydrogels, additional standards such as ISO 10993 for biocompatibility testing and ASTM F2900 for antimicrobial susceptibility testing are essential components of the regulatory submission package.
Environmental considerations are increasingly important in regulatory assessments. Chitosan's biodegradability offers advantages over synthetic PEG in this regard, potentially streamlining certain aspects of environmental impact assessments required by regulatory bodies.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually reducing regulatory divergence, though significant differences remain. Manufacturers of antimicrobial hydrogels must develop comprehensive regulatory strategies accounting for these variations, particularly regarding antimicrobial efficacy claims and resistance monitoring requirements.
The regulatory timeline for novel antimicrobial hydrogels typically spans 3-5 years from initial development to market approval, with costs ranging from $1-10 million depending on the complexity of the formulation and intended applications. Early engagement with regulatory authorities through pre-submission consultations can significantly optimize this pathway.
In the United States, the Food and Drug Administration (FDA) classifies most hydrogels as medical devices or combination products, depending on their primary mode of action. Chitosan-based antimicrobial hydrogels typically follow the 510(k) clearance pathway if substantially equivalent to predicate devices. However, novel formulations with unique antimicrobial properties may require the more rigorous Premarket Approval (PMA) process, including extensive clinical trials demonstrating safety and efficacy.
The European Union's regulatory framework under the Medical Device Regulation (MDR) categorizes antimicrobial hydrogels based on their intended use and risk profile. Most wound care hydrogels fall under Class IIb or III, requiring Notified Body assessment and CE marking. The MDR's emphasis on clinical evidence and post-market surveillance presents additional challenges for manufacturers of chitosan- and PEG-based hydrogels.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the "Sakigake" designation for innovative medical technologies, potentially expediting approval for novel antimicrobial hydrogels. This pathway could be particularly relevant for advanced chitosan-PEG composite hydrogels with enhanced antimicrobial properties.
Quality management systems compliant with ISO 13485 are universally required across major markets. For antimicrobial hydrogels, additional standards such as ISO 10993 for biocompatibility testing and ASTM F2900 for antimicrobial susceptibility testing are essential components of the regulatory submission package.
Environmental considerations are increasingly important in regulatory assessments. Chitosan's biodegradability offers advantages over synthetic PEG in this regard, potentially streamlining certain aspects of environmental impact assessments required by regulatory bodies.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually reducing regulatory divergence, though significant differences remain. Manufacturers of antimicrobial hydrogels must develop comprehensive regulatory strategies accounting for these variations, particularly regarding antimicrobial efficacy claims and resistance monitoring requirements.
The regulatory timeline for novel antimicrobial hydrogels typically spans 3-5 years from initial development to market approval, with costs ranging from $1-10 million depending on the complexity of the formulation and intended applications. Early engagement with regulatory authorities through pre-submission consultations can significantly optimize this pathway.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!