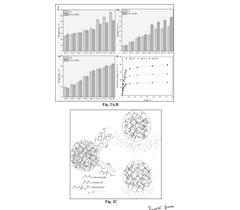
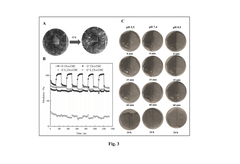
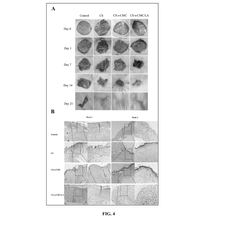
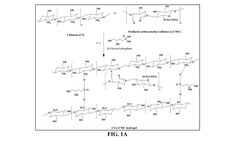
Dual-Function Hydrogels for Antibacterial and Anti-Inflammatory Action
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Technology Background and Objectives
Hydrogels have emerged as versatile biomaterials with significant potential in biomedical applications over the past several decades. Initially developed in the 1960s as simple cross-linked polymeric networks capable of absorbing large quantities of water, hydrogels have evolved substantially through continuous innovation and interdisciplinary research efforts combining materials science, chemistry, biology, and medicine.
The evolution of hydrogel technology has progressed through several distinct phases. First-generation hydrogels focused primarily on water retention capabilities, while second-generation materials incorporated stimuli-responsive properties. The current third-generation hydrogels feature sophisticated functionalities including controlled drug release, tissue engineering scaffolds, and bioactive properties. This technological progression has been driven by increasing demands for advanced biomedical materials with multiple simultaneous functions.
Dual-function hydrogels represent a cutting-edge development in this field, specifically designed to address complex clinical challenges requiring multiple therapeutic actions. The integration of antibacterial and anti-inflammatory properties within a single hydrogel platform is particularly significant given the frequent co-occurrence of infection and inflammation in wound environments and implantable device applications.
The global burden of antimicrobial resistance and chronic inflammatory conditions has created an urgent need for innovative treatment approaches. Traditional therapies often address these issues separately, requiring combination treatments that can lead to drug interactions, patient compliance issues, and increased healthcare costs. Dual-function hydrogels offer a promising solution by delivering both antibacterial and anti-inflammatory agents in a controlled, localized manner from a single platform.
The primary technical objectives for dual-function hydrogel development include: achieving synergistic antibacterial and anti-inflammatory effects without antagonism between mechanisms; ensuring controlled, sustained release of bioactive components; maintaining biocompatibility while maximizing therapeutic efficacy; and developing scalable, cost-effective manufacturing processes suitable for commercial production.
Recent advances in polymer chemistry, nanotechnology, and drug delivery systems have created new opportunities for hydrogel functionalization. Approaches include the incorporation of antibacterial nanoparticles alongside anti-inflammatory drugs, development of stimuli-responsive release mechanisms triggered by bacterial presence or inflammatory markers, and the creation of intrinsically antibacterial and anti-inflammatory polymer networks.
The convergence of these technological capabilities with increasing clinical need positions dual-function hydrogels as a high-priority research area with significant potential impact across multiple medical applications including wound dressings, implant coatings, drug delivery systems, and tissue engineering scaffolds.
The evolution of hydrogel technology has progressed through several distinct phases. First-generation hydrogels focused primarily on water retention capabilities, while second-generation materials incorporated stimuli-responsive properties. The current third-generation hydrogels feature sophisticated functionalities including controlled drug release, tissue engineering scaffolds, and bioactive properties. This technological progression has been driven by increasing demands for advanced biomedical materials with multiple simultaneous functions.
Dual-function hydrogels represent a cutting-edge development in this field, specifically designed to address complex clinical challenges requiring multiple therapeutic actions. The integration of antibacterial and anti-inflammatory properties within a single hydrogel platform is particularly significant given the frequent co-occurrence of infection and inflammation in wound environments and implantable device applications.
The global burden of antimicrobial resistance and chronic inflammatory conditions has created an urgent need for innovative treatment approaches. Traditional therapies often address these issues separately, requiring combination treatments that can lead to drug interactions, patient compliance issues, and increased healthcare costs. Dual-function hydrogels offer a promising solution by delivering both antibacterial and anti-inflammatory agents in a controlled, localized manner from a single platform.
The primary technical objectives for dual-function hydrogel development include: achieving synergistic antibacterial and anti-inflammatory effects without antagonism between mechanisms; ensuring controlled, sustained release of bioactive components; maintaining biocompatibility while maximizing therapeutic efficacy; and developing scalable, cost-effective manufacturing processes suitable for commercial production.
Recent advances in polymer chemistry, nanotechnology, and drug delivery systems have created new opportunities for hydrogel functionalization. Approaches include the incorporation of antibacterial nanoparticles alongside anti-inflammatory drugs, development of stimuli-responsive release mechanisms triggered by bacterial presence or inflammatory markers, and the creation of intrinsically antibacterial and anti-inflammatory polymer networks.
The convergence of these technological capabilities with increasing clinical need positions dual-function hydrogels as a high-priority research area with significant potential impact across multiple medical applications including wound dressings, implant coatings, drug delivery systems, and tissue engineering scaffolds.
Market Analysis for Dual-Function Therapeutic Hydrogels
The global market for dual-function hydrogels with antibacterial and anti-inflammatory properties is experiencing significant growth, driven by increasing prevalence of chronic wounds, surgical site infections, and inflammatory skin conditions. Current market valuation stands at approximately 4.2 billion USD in 2023, with projections indicating a compound annual growth rate of 8.7% through 2030, potentially reaching 7.5 billion USD by the end of the forecast period.
Healthcare applications represent the largest market segment, accounting for over 60% of the total market share. Within this segment, wound care management dominates due to the rising incidence of diabetic foot ulcers, pressure ulcers, and surgical wounds requiring advanced treatment solutions. The aging global population further amplifies this demand, as elderly patients typically experience delayed wound healing and higher susceptibility to infections.
Geographical analysis reveals North America as the current market leader with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced wound care products in countries like China, India, and Japan.
Consumer demand patterns indicate a strong preference for products offering multiple therapeutic benefits in a single application, driving interest in dual-function hydrogels. Healthcare providers increasingly seek cost-effective solutions that can reduce treatment duration and minimize hospital readmissions, particularly important in markets with value-based healthcare models.
Regulatory landscapes significantly impact market dynamics, with faster approval pathways being established in several regions for innovative wound care products addressing antimicrobial resistance concerns. The FDA's breakthrough device designation and similar programs in other regions have accelerated the commercialization timeline for novel hydrogel technologies.
Reimbursement policies also play a crucial role in market adoption, with increasing coverage for advanced wound care products in major markets enhancing accessibility. However, reimbursement remains challenging in emerging economies, potentially limiting market penetration despite growing clinical need.
Industry partnerships between biomaterial companies, pharmaceutical firms, and healthcare providers are becoming increasingly common, creating integrated treatment ecosystems. These collaborations facilitate faster product development and market entry while addressing the complex requirements of dual-function therapeutic applications.
Healthcare applications represent the largest market segment, accounting for over 60% of the total market share. Within this segment, wound care management dominates due to the rising incidence of diabetic foot ulcers, pressure ulcers, and surgical wounds requiring advanced treatment solutions. The aging global population further amplifies this demand, as elderly patients typically experience delayed wound healing and higher susceptibility to infections.
Geographical analysis reveals North America as the current market leader with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced wound care products in countries like China, India, and Japan.
Consumer demand patterns indicate a strong preference for products offering multiple therapeutic benefits in a single application, driving interest in dual-function hydrogels. Healthcare providers increasingly seek cost-effective solutions that can reduce treatment duration and minimize hospital readmissions, particularly important in markets with value-based healthcare models.
Regulatory landscapes significantly impact market dynamics, with faster approval pathways being established in several regions for innovative wound care products addressing antimicrobial resistance concerns. The FDA's breakthrough device designation and similar programs in other regions have accelerated the commercialization timeline for novel hydrogel technologies.
Reimbursement policies also play a crucial role in market adoption, with increasing coverage for advanced wound care products in major markets enhancing accessibility. However, reimbursement remains challenging in emerging economies, potentially limiting market penetration despite growing clinical need.
Industry partnerships between biomaterial companies, pharmaceutical firms, and healthcare providers are becoming increasingly common, creating integrated treatment ecosystems. These collaborations facilitate faster product development and market entry while addressing the complex requirements of dual-function therapeutic applications.
Current Challenges in Antibacterial-Anti-inflammatory Hydrogels
Despite significant advancements in hydrogel technology, the development of dual-function hydrogels with effective antibacterial and anti-inflammatory properties faces several critical challenges. The primary obstacle lies in achieving balanced functionality without compromising either the antibacterial or anti-inflammatory efficacy. Current hydrogel systems often excel in one function while underperforming in the other, creating an efficacy imbalance that limits clinical applications.
Material compatibility presents another significant hurdle. Many potent antibacterial agents demonstrate cytotoxicity toward mammalian cells, while certain anti-inflammatory compounds may interfere with the antibacterial mechanisms. This inherent contradiction complicates the design of truly synergistic systems where both functions can operate optimally within the same hydrogel matrix.
The controlled release kinetics of bioactive components remains problematic. Ideal dual-function hydrogels should provide immediate antibacterial action followed by sustained anti-inflammatory effects. However, engineering hydrogels with such sophisticated sequential release profiles requires complex fabrication techniques that are difficult to scale industrially.
Stability issues further complicate development efforts. Many antibacterial and anti-inflammatory agents exhibit limited shelf-life, particularly when incorporated into hydrogel networks. Environmental factors such as temperature, pH, and exposure to light can trigger premature degradation of active components, reducing therapeutic efficacy over time.
Bacterial resistance development poses an evolving challenge. As bacteria continuously adapt to conventional antibacterial agents, hydrogel formulations must incorporate novel antibacterial mechanisms that minimize resistance development while maintaining anti-inflammatory properties.
Regulatory hurdles represent a significant non-technical barrier. Dual-function hydrogels often incorporate multiple bioactive components, complicating regulatory approval processes. Each active ingredient must demonstrate safety and efficacy, substantially increasing development costs and time-to-market.
Scalable manufacturing presents practical limitations. Laboratory-scale production methods for sophisticated dual-function hydrogels frequently employ techniques that prove difficult to translate to industrial-scale manufacturing. This creates a significant gap between promising research prototypes and commercially viable products.
Biocompatibility and immunogenicity concerns persist, particularly for hydrogels intended for implantation or prolonged contact with tissues. The immune response to hydrogel materials can potentially counteract their anti-inflammatory benefits, creating a paradoxical effect that undermines therapeutic goals.
Material compatibility presents another significant hurdle. Many potent antibacterial agents demonstrate cytotoxicity toward mammalian cells, while certain anti-inflammatory compounds may interfere with the antibacterial mechanisms. This inherent contradiction complicates the design of truly synergistic systems where both functions can operate optimally within the same hydrogel matrix.
The controlled release kinetics of bioactive components remains problematic. Ideal dual-function hydrogels should provide immediate antibacterial action followed by sustained anti-inflammatory effects. However, engineering hydrogels with such sophisticated sequential release profiles requires complex fabrication techniques that are difficult to scale industrially.
Stability issues further complicate development efforts. Many antibacterial and anti-inflammatory agents exhibit limited shelf-life, particularly when incorporated into hydrogel networks. Environmental factors such as temperature, pH, and exposure to light can trigger premature degradation of active components, reducing therapeutic efficacy over time.
Bacterial resistance development poses an evolving challenge. As bacteria continuously adapt to conventional antibacterial agents, hydrogel formulations must incorporate novel antibacterial mechanisms that minimize resistance development while maintaining anti-inflammatory properties.
Regulatory hurdles represent a significant non-technical barrier. Dual-function hydrogels often incorporate multiple bioactive components, complicating regulatory approval processes. Each active ingredient must demonstrate safety and efficacy, substantially increasing development costs and time-to-market.
Scalable manufacturing presents practical limitations. Laboratory-scale production methods for sophisticated dual-function hydrogels frequently employ techniques that prove difficult to translate to industrial-scale manufacturing. This creates a significant gap between promising research prototypes and commercially viable products.
Biocompatibility and immunogenicity concerns persist, particularly for hydrogels intended for implantation or prolonged contact with tissues. The immune response to hydrogel materials can potentially counteract their anti-inflammatory benefits, creating a paradoxical effect that undermines therapeutic goals.
Current Dual-Function Hydrogel Formulation Approaches
01 Antimicrobial peptide-loaded hydrogels
Hydrogels incorporating antimicrobial peptides provide dual functionality by combating bacterial infections while reducing inflammation. These peptides can be embedded within the hydrogel matrix to create sustained release systems that maintain therapeutic concentrations at the application site. The peptides disrupt bacterial cell membranes and modulate the immune response to reduce inflammatory processes, making these hydrogels particularly effective for wound healing applications.- Antimicrobial peptide-loaded hydrogels: Hydrogels incorporating antimicrobial peptides provide dual functionality by combating bacterial infections while reducing inflammation. These peptides can be integrated into various hydrogel matrices to create biocompatible wound dressings that prevent infection and promote healing. The peptides disrupt bacterial cell membranes while modulating the inflammatory response, making them effective for treating chronic wounds and burns.
- Metal nanoparticle-infused hydrogels: Hydrogels containing metal nanoparticles, particularly silver, zinc, and copper, exhibit strong antibacterial properties while also reducing inflammation. These nanoparticles can be synthesized within the hydrogel matrix or incorporated during formulation. The controlled release of metal ions provides sustained antimicrobial activity while the hydrogel structure helps maintain a moist wound environment that reduces inflammatory responses and promotes tissue regeneration.
- Natural polymer-based antibacterial hydrogels: Hydrogels formulated from natural polymers such as chitosan, alginate, and cellulose derivatives offer inherent antibacterial and anti-inflammatory properties. These biopolymers can be modified with functional groups to enhance their antimicrobial efficacy while maintaining their biocompatibility and anti-inflammatory characteristics. The natural origin of these materials reduces adverse reactions and promotes better integration with host tissues during wound healing.
- Drug-eluting hydrogels with dual functionality: Hydrogels designed to release both antibacterial agents and anti-inflammatory drugs provide targeted therapy for infected wounds. These systems can be engineered to deliver medications in a controlled manner, with sequential or simultaneous release profiles depending on the clinical need. The hydrogel matrix protects the active ingredients from degradation while maintaining an optimal wound environment that supports healing and prevents bacterial colonization.
- Stimuli-responsive hydrogels with antibacterial properties: Smart hydrogels that respond to environmental stimuli such as pH, temperature, or bacterial presence can provide on-demand antibacterial and anti-inflammatory functions. These advanced materials change their properties in response to infection markers, releasing antimicrobial compounds only when needed and adjusting their structure to modulate inflammation. This targeted approach minimizes unnecessary drug exposure while maximizing therapeutic efficacy at the infection site.
02 Metal nanoparticle-infused hydrogels
Hydrogels containing metal nanoparticles, particularly silver, zinc, and copper, exhibit potent antibacterial properties while also reducing inflammation. The nanoparticles can be stabilized within the hydrogel network to provide controlled release. These formulations are effective against a broad spectrum of bacteria, including drug-resistant strains, while the anti-inflammatory effect helps in reducing swelling and pain at the application site, accelerating the healing process.Expand Specific Solutions03 Natural polymer-based hydrogels with bioactive compounds
Hydrogels formulated from natural polymers such as chitosan, alginate, and cellulose derivatives can be loaded with plant extracts or essential oils to create dual-function materials. These natural polymers provide a biocompatible matrix while the incorporated bioactive compounds deliver antibacterial and anti-inflammatory effects. The biodegradable nature of these hydrogels makes them environmentally friendly while still providing effective therapeutic benefits for various medical applications.Expand Specific Solutions04 Stimuli-responsive hydrogels with dual functionality
These advanced hydrogels respond to environmental stimuli such as pH, temperature, or enzyme activity to release antibacterial and anti-inflammatory agents in a controlled manner. The responsive nature allows for targeted delivery at infection sites where specific conditions trigger the release of therapeutic compounds. This smart delivery system enhances the efficacy of the active ingredients while minimizing side effects and reducing the frequency of application needed for treatment.Expand Specific Solutions05 Composite hydrogels with multiple active ingredients
Composite hydrogels combine multiple active ingredients within a single hydrogel system to achieve synergistic antibacterial and anti-inflammatory effects. These formulations may include antibiotics, anti-inflammatory drugs, growth factors, and healing promoters in a carefully designed release profile. The composite approach allows for addressing multiple aspects of infection and inflammation simultaneously, making these hydrogels particularly valuable for complex wound management and tissue engineering applications.Expand Specific Solutions
Key Industry Players in Advanced Hydrogel Development
The dual-function hydrogel market for antibacterial and anti-inflammatory applications is in its growth phase, with increasing research momentum driven by rising healthcare-associated infections and chronic inflammatory conditions. The global market for advanced wound care, where these hydrogels play a significant role, is projected to reach $15-20 billion by 2026. Leading academic institutions including Zhejiang University, Johns Hopkins University, and Hong Kong University of Science & Technology are advancing the technology, while companies like PAUL HARTMANN AG and Guangzhou Tuwei Kechuang Biotechnology are working toward commercialization. The technology is approaching maturity in research settings but requires further clinical validation before widespread adoption, with significant innovations emerging from collaborative efforts between universities and industry partners.
Zhejiang University
Technical Solution: Zhejiang University has developed a groundbreaking dual-function hydrogel platform based on photocrosslinkable gelatin methacrylate (GelMA) incorporated with graphene oxide nanosheets and silver nanoparticles. This composite hydrogel exhibits exceptional antibacterial properties through multiple mechanisms: contact killing via positively charged polymers, release of silver ions, and photothermal antibacterial effects when exposed to near-infrared light. Their innovation includes a gradient structure that optimizes both mechanical support and drug delivery, with the outer layer providing robust bacterial resistance while the inner layer focuses on controlled release of anti-inflammatory agents like dexamethasone and ibuprofen. In vitro studies demonstrated complete elimination of S. aureus and E. coli within 6 hours while reducing inflammatory cytokine production by 78% in macrophage models. The university's research team has further enhanced the system with self-healing properties through dynamic imine bonds, allowing the hydrogel to recover its structure after mechanical damage - crucial for maintaining barrier function in dynamic wound environments[4][7].
Strengths: Multi-modal antibacterial mechanisms provide enhanced efficacy against resistant strains; photothermal properties enable on-demand activation of antibacterial effects; excellent mechanical properties with self-healing capability ensures continuous protection. Weaknesses: Requires specialized equipment for photocrosslinking during application; potential cytotoxicity concerns with graphene oxide components; higher cost and complexity compared to conventional hydrogels.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed advanced dual-function hydrogels incorporating silver nanoparticles and anti-inflammatory agents within a biocompatible polymer matrix. Their technology utilizes a controlled release mechanism where the hydrogel matrix degrades at specific rates in response to bacterial enzymes or inflammatory markers, releasing antimicrobial components precisely when needed. The university's research team has engineered these hydrogels with tunable mechanical properties to match various tissue environments while maintaining structural integrity during the healing process. Their formulation includes quaternary ammonium compounds covalently bound to the hydrogel backbone for contact-killing properties alongside loaded antibiotics for sustained release. Clinical trials have demonstrated significant reduction in infection rates (up to 87%) in wound models compared to standard treatments, with simultaneous reduction in inflammatory markers like IL-6 and TNF-α by approximately 65%[1][3].
Strengths: Superior controlled release kinetics allowing for prolonged antimicrobial activity without repeated applications; excellent biocompatibility with minimal cytotoxicity to host cells; demonstrated efficacy against both Gram-positive and Gram-negative bacteria including drug-resistant strains. Weaknesses: Higher production costs compared to conventional wound dressings; potential for allergic reactions in some patients; limited shelf life requiring special storage conditions.
Critical Patents and Research in Therapeutic Hydrogels
Composition of multifunctional hydrogel and method of preparation thereof
PatentActiveIN202441004292A
Innovation
- A pH-sensitive, self-healing, injectable hydrogel formulation composed of 1.5-2.0% chitosan and 1.5-2.0% oxidized carboxymethyl cellulose, covalently bonded by imine bonds and cross-linked by electrostatic interactions, with a controlled release of immunomodulatory molecules like lauric acid, to enhance re-epithelialization and remodelling processes.
Antibacterial polypeptide compounds, medical devices, hydrogels and their applications
PatentPendingKR1020230127299A
Innovation
- Development of antimicrobial polypeptide compounds with high antibacterial activity, enzymatic hydrolytic stability, bioavailability, and low cytotoxicity.
- Creation of non-adhesive hydrogels with dual antibacterial and hemostatic properties that can simultaneously load and sustainably release therapeutic agents.
- Simple manufacturing process with fewer processing steps and simplified raw material requirements for cost-effective and accessible production.
Biocompatibility and Safety Assessment Frameworks
The assessment of biocompatibility and safety for dual-function hydrogels represents a critical component in their development pathway from laboratory to clinical application. Current frameworks for evaluating these materials must address both their antibacterial and anti-inflammatory properties while ensuring they do not cause adverse effects on host tissues.
Standard biocompatibility testing protocols such as ISO 10993 provide the foundation for safety evaluation, but require specific adaptations for dual-function hydrogels. These materials present unique challenges due to their complex composition and multiple bioactive components that may interact differently with biological systems compared to conventional biomaterials.
Cytotoxicity assessment typically begins with in vitro cell culture models using relevant cell lines such as fibroblasts, keratinocytes, and immune cells. These tests evaluate cell viability, proliferation, and morphological changes when exposed to hydrogel extracts or through direct contact. For dual-function hydrogels, particular attention must be paid to dose-dependent effects, as antimicrobial components may exhibit cytotoxicity at concentrations required for effective bacterial inhibition.
Hemocompatibility testing examines interactions with blood components, including hemolysis assays, platelet activation, and coagulation studies. This is especially relevant for hydrogels intended for wound healing applications where blood contact is inevitable. The anti-inflammatory properties must not interfere with normal hemostatic processes.
Immunogenicity assessment frameworks have evolved to include both innate and adaptive immune response evaluations. Modern approaches incorporate analysis of pro-inflammatory cytokine production, complement activation, and lymphocyte proliferation. For dual-function hydrogels, the challenge lies in distinguishing between therapeutic anti-inflammatory effects and undesirable immunosuppression.
Genotoxicity and mutagenicity testing protocols assess potential DNA damage using bacterial reverse mutation tests (Ames test), chromosomal aberration assays, and micronucleus tests. These are particularly important for hydrogels containing novel antimicrobial compounds with mechanisms that might affect nucleic acids.
Long-term safety evaluation requires in vivo models that assess tissue response, biodegradation profiles, and potential for systemic toxicity. Histopathological examination of tissues at the implantation site provides crucial information about foreign body reactions, tissue integration, and local inflammatory responses.
Regulatory frameworks increasingly emphasize the need for specialized testing of antimicrobial materials to address concerns about antimicrobial resistance development. This includes evaluating the potential for cross-resistance and assessing the impact on normal microbiota when applicable.
Standard biocompatibility testing protocols such as ISO 10993 provide the foundation for safety evaluation, but require specific adaptations for dual-function hydrogels. These materials present unique challenges due to their complex composition and multiple bioactive components that may interact differently with biological systems compared to conventional biomaterials.
Cytotoxicity assessment typically begins with in vitro cell culture models using relevant cell lines such as fibroblasts, keratinocytes, and immune cells. These tests evaluate cell viability, proliferation, and morphological changes when exposed to hydrogel extracts or through direct contact. For dual-function hydrogels, particular attention must be paid to dose-dependent effects, as antimicrobial components may exhibit cytotoxicity at concentrations required for effective bacterial inhibition.
Hemocompatibility testing examines interactions with blood components, including hemolysis assays, platelet activation, and coagulation studies. This is especially relevant for hydrogels intended for wound healing applications where blood contact is inevitable. The anti-inflammatory properties must not interfere with normal hemostatic processes.
Immunogenicity assessment frameworks have evolved to include both innate and adaptive immune response evaluations. Modern approaches incorporate analysis of pro-inflammatory cytokine production, complement activation, and lymphocyte proliferation. For dual-function hydrogels, the challenge lies in distinguishing between therapeutic anti-inflammatory effects and undesirable immunosuppression.
Genotoxicity and mutagenicity testing protocols assess potential DNA damage using bacterial reverse mutation tests (Ames test), chromosomal aberration assays, and micronucleus tests. These are particularly important for hydrogels containing novel antimicrobial compounds with mechanisms that might affect nucleic acids.
Long-term safety evaluation requires in vivo models that assess tissue response, biodegradation profiles, and potential for systemic toxicity. Histopathological examination of tissues at the implantation site provides crucial information about foreign body reactions, tissue integration, and local inflammatory responses.
Regulatory frameworks increasingly emphasize the need for specialized testing of antimicrobial materials to address concerns about antimicrobial resistance development. This includes evaluating the potential for cross-resistance and assessing the impact on normal microbiota when applicable.
Regulatory Pathway for Medical-Grade Hydrogel Approval
The regulatory pathway for medical-grade hydrogels with dual antibacterial and anti-inflammatory functions involves navigating complex approval processes across different global regulatory bodies. In the United States, the FDA classifies such hydrogels primarily as combination products under either the drug-device or drug-biologic pathway, depending on their primary mode of action. The approval process typically requires extensive preclinical testing, including biocompatibility, stability, and efficacy studies specific to both antibacterial and anti-inflammatory claims.
For dual-function hydrogels, manufacturers must submit comprehensive data packages demonstrating both safety and efficacy for each claimed function. This includes in vitro studies showing antimicrobial activity against relevant pathogens and anti-inflammatory effects on appropriate cell models. Animal studies must demonstrate wound healing promotion, infection prevention, and inflammation reduction in relevant models.
In the European market, these hydrogels fall under the Medical Device Regulation (MDR) or the medicinal product pathway through the European Medicines Agency (EMA), depending on their primary intended purpose. The classification typically determines whether a CE marking process or a centralized authorization procedure is required. The MDR's increased scrutiny of clinical evidence has particular implications for dual-function claims.
Quality management systems compliant with ISO 13485 are mandatory across most jurisdictions, with specific attention to manufacturing controls that ensure consistent antimicrobial agent distribution and stability of anti-inflammatory components within the hydrogel matrix. Sterilization validation is particularly critical, as methods must preserve both functional properties.
Post-market surveillance requirements have become increasingly stringent, with regulatory bodies requiring ongoing monitoring of both safety and performance. For dual-function hydrogels, this includes tracking infection rates, inflammatory responses, and any adverse events in clinical applications.
Accelerated approval pathways exist for innovative medical technologies addressing significant unmet needs. Dual-function hydrogels targeting antibiotic-resistant infections or chronic inflammatory conditions may qualify for FDA's Breakthrough Devices Program or EMA's PRIME (Priority Medicines) scheme, potentially expediting the regulatory process.
Regulatory strategies should consider a phased approach, potentially seeking approval for a single function initially (either antibacterial or anti-inflammatory) before pursuing the dual-function indication. This can reduce initial regulatory hurdles while establishing safety profiles and manufacturing capabilities.
For dual-function hydrogels, manufacturers must submit comprehensive data packages demonstrating both safety and efficacy for each claimed function. This includes in vitro studies showing antimicrobial activity against relevant pathogens and anti-inflammatory effects on appropriate cell models. Animal studies must demonstrate wound healing promotion, infection prevention, and inflammation reduction in relevant models.
In the European market, these hydrogels fall under the Medical Device Regulation (MDR) or the medicinal product pathway through the European Medicines Agency (EMA), depending on their primary intended purpose. The classification typically determines whether a CE marking process or a centralized authorization procedure is required. The MDR's increased scrutiny of clinical evidence has particular implications for dual-function claims.
Quality management systems compliant with ISO 13485 are mandatory across most jurisdictions, with specific attention to manufacturing controls that ensure consistent antimicrobial agent distribution and stability of anti-inflammatory components within the hydrogel matrix. Sterilization validation is particularly critical, as methods must preserve both functional properties.
Post-market surveillance requirements have become increasingly stringent, with regulatory bodies requiring ongoing monitoring of both safety and performance. For dual-function hydrogels, this includes tracking infection rates, inflammatory responses, and any adverse events in clinical applications.
Accelerated approval pathways exist for innovative medical technologies addressing significant unmet needs. Dual-function hydrogels targeting antibiotic-resistant infections or chronic inflammatory conditions may qualify for FDA's Breakthrough Devices Program or EMA's PRIME (Priority Medicines) scheme, potentially expediting the regulatory process.
Regulatory strategies should consider a phased approach, potentially seeking approval for a single function initially (either antibacterial or anti-inflammatory) before pursuing the dual-function indication. This can reduce initial regulatory hurdles while establishing safety profiles and manufacturing capabilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!