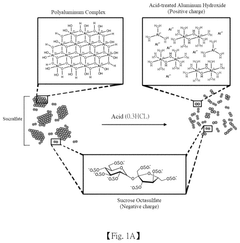
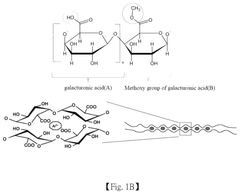
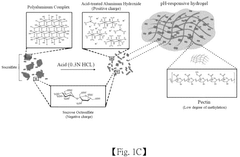
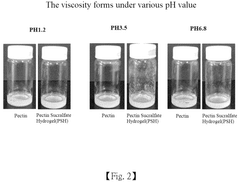
pH-Responsive Antimicrobial Hydrogel Systems
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
pH-Responsive Hydrogel Background and Objectives
pH-responsive hydrogels represent a significant advancement in smart materials science, evolving from simple polymer networks to sophisticated responsive systems over the past three decades. These materials undergo reversible volume changes in response to environmental pH variations, making them particularly valuable in biomedical applications. The fundamental mechanism involves ionizable functional groups within the polymer network that accept or release protons depending on the surrounding pH, causing swelling or contraction of the hydrogel structure.
The integration of antimicrobial properties with pH-responsive behavior has emerged as a promising direction in recent years, addressing the growing concerns of antimicrobial resistance and healthcare-associated infections. This technological convergence leverages the controlled release capabilities of pH-responsive systems to deliver antimicrobial agents precisely where and when needed, potentially reducing systemic toxicity while enhancing therapeutic efficacy.
Historical development of these systems can be traced through several key phases: initial discovery of pH-responsive polymers in the 1960s, development of basic hydrogel formulations in the 1980s, incorporation of antimicrobial agents in the early 2000s, and the current era of advanced multifunctional systems. Each evolutionary stage has contributed to expanding the application potential and performance characteristics of these materials.
The primary technical objectives for pH-responsive antimicrobial hydrogel research include enhancing pH sensitivity within physiologically relevant ranges (pH 5.0-7.4), improving antimicrobial efficacy against both Gram-positive and Gram-negative bacteria, extending the duration of antimicrobial activity, and ensuring biocompatibility with host tissues. Additionally, mechanical stability under varying pH conditions remains a critical challenge to overcome for practical applications.
Current research trends indicate growing interest in developing dual or multi-responsive systems that react not only to pH but also to temperature, light, or enzymatic activity. This multi-stimuli approach aims to create more sophisticated controlled release mechanisms that can respond to complex biological environments. Furthermore, the incorporation of natural antimicrobial peptides and sustainable, biodegradable polymers reflects the industry's shift toward environmentally friendly and biocompatible materials.
The technological trajectory suggests that pH-responsive antimicrobial hydrogels will play an increasingly important role in wound management, implantable devices, drug delivery systems, and preventative healthcare applications. As antimicrobial resistance continues to pose global health challenges, these advanced materials offer promising strategies for more effective and targeted antimicrobial interventions with reduced risk of resistance development.
The integration of antimicrobial properties with pH-responsive behavior has emerged as a promising direction in recent years, addressing the growing concerns of antimicrobial resistance and healthcare-associated infections. This technological convergence leverages the controlled release capabilities of pH-responsive systems to deliver antimicrobial agents precisely where and when needed, potentially reducing systemic toxicity while enhancing therapeutic efficacy.
Historical development of these systems can be traced through several key phases: initial discovery of pH-responsive polymers in the 1960s, development of basic hydrogel formulations in the 1980s, incorporation of antimicrobial agents in the early 2000s, and the current era of advanced multifunctional systems. Each evolutionary stage has contributed to expanding the application potential and performance characteristics of these materials.
The primary technical objectives for pH-responsive antimicrobial hydrogel research include enhancing pH sensitivity within physiologically relevant ranges (pH 5.0-7.4), improving antimicrobial efficacy against both Gram-positive and Gram-negative bacteria, extending the duration of antimicrobial activity, and ensuring biocompatibility with host tissues. Additionally, mechanical stability under varying pH conditions remains a critical challenge to overcome for practical applications.
Current research trends indicate growing interest in developing dual or multi-responsive systems that react not only to pH but also to temperature, light, or enzymatic activity. This multi-stimuli approach aims to create more sophisticated controlled release mechanisms that can respond to complex biological environments. Furthermore, the incorporation of natural antimicrobial peptides and sustainable, biodegradable polymers reflects the industry's shift toward environmentally friendly and biocompatible materials.
The technological trajectory suggests that pH-responsive antimicrobial hydrogels will play an increasingly important role in wound management, implantable devices, drug delivery systems, and preventative healthcare applications. As antimicrobial resistance continues to pose global health challenges, these advanced materials offer promising strategies for more effective and targeted antimicrobial interventions with reduced risk of resistance development.
Market Analysis for Antimicrobial Hydrogel Applications
The global market for antimicrobial hydrogels has witnessed substantial growth in recent years, driven primarily by increasing healthcare-associated infections and growing awareness about wound care management. The market for pH-responsive antimicrobial hydrogels specifically is emerging as a high-potential segment within this broader category, with applications spanning wound dressings, implant coatings, drug delivery systems, and personal care products.
Healthcare applications currently dominate the antimicrobial hydrogel market, accounting for the largest revenue share. Within this sector, advanced wound care represents the most significant application area, valued at approximately 3.2 billion USD in 2022 and projected to grow at a compound annual growth rate of 8.7% through 2030. This growth is fueled by the rising prevalence of chronic wounds, diabetic ulcers, and surgical site infections globally.
The pharmaceutical and biomedical sectors present substantial opportunities for pH-responsive antimicrobial hydrogels. These materials offer targeted drug release capabilities in response to the acidic microenvironment often found in infected tissues, providing more effective treatment with reduced side effects. This application segment is expected to witness the fastest growth rate in the coming years.
Regional analysis indicates North America currently leads the market with the highest revenue share, followed by Europe. However, the Asia-Pacific region is anticipated to register the highest growth rate during the forecast period, attributed to improving healthcare infrastructure, increasing healthcare expenditure, and rising awareness about advanced wound care products in countries like China, India, and Japan.
Consumer demand trends show increasing preference for products with dual functionality - antimicrobial properties combined with biocompatibility and biodegradability. This shift is particularly evident in the wound care segment, where patients and healthcare providers seek solutions that not only prevent infection but also promote healing and reduce the frequency of dressing changes.
Key market drivers include the rising incidence of chronic wounds and surgical site infections, growing geriatric population, increasing prevalence of diabetes, and heightened focus on infection prevention strategies in healthcare settings. Additionally, the COVID-19 pandemic has accelerated interest in antimicrobial technologies across various sectors, creating new market opportunities.
Challenges limiting market expansion include high production costs, regulatory hurdles, and limited awareness among end-users in developing regions. The complex manufacturing processes and specialized expertise required for developing pH-responsive systems further constrain market growth in certain regions.
Healthcare applications currently dominate the antimicrobial hydrogel market, accounting for the largest revenue share. Within this sector, advanced wound care represents the most significant application area, valued at approximately 3.2 billion USD in 2022 and projected to grow at a compound annual growth rate of 8.7% through 2030. This growth is fueled by the rising prevalence of chronic wounds, diabetic ulcers, and surgical site infections globally.
The pharmaceutical and biomedical sectors present substantial opportunities for pH-responsive antimicrobial hydrogels. These materials offer targeted drug release capabilities in response to the acidic microenvironment often found in infected tissues, providing more effective treatment with reduced side effects. This application segment is expected to witness the fastest growth rate in the coming years.
Regional analysis indicates North America currently leads the market with the highest revenue share, followed by Europe. However, the Asia-Pacific region is anticipated to register the highest growth rate during the forecast period, attributed to improving healthcare infrastructure, increasing healthcare expenditure, and rising awareness about advanced wound care products in countries like China, India, and Japan.
Consumer demand trends show increasing preference for products with dual functionality - antimicrobial properties combined with biocompatibility and biodegradability. This shift is particularly evident in the wound care segment, where patients and healthcare providers seek solutions that not only prevent infection but also promote healing and reduce the frequency of dressing changes.
Key market drivers include the rising incidence of chronic wounds and surgical site infections, growing geriatric population, increasing prevalence of diabetes, and heightened focus on infection prevention strategies in healthcare settings. Additionally, the COVID-19 pandemic has accelerated interest in antimicrobial technologies across various sectors, creating new market opportunities.
Challenges limiting market expansion include high production costs, regulatory hurdles, and limited awareness among end-users in developing regions. The complex manufacturing processes and specialized expertise required for developing pH-responsive systems further constrain market growth in certain regions.
Current Status and Technical Barriers in pH-Responsive Systems
The global landscape of pH-responsive antimicrobial hydrogel systems has witnessed significant advancements in recent years, with research institutions across North America, Europe, and Asia making substantial contributions. Current systems predominantly utilize either natural polymers (chitosan, alginate) or synthetic polymers (polyacrylic acid, poly-N-isopropylacrylamide) as base materials, with antimicrobial components integrated through various mechanisms including physical entrapment, chemical conjugation, or in-situ formation.
The state-of-the-art pH-responsive systems demonstrate sensitivity within physiologically relevant pH ranges (4.5-7.4), allowing targeted antimicrobial release at infection sites where pH typically deviates from normal tissue conditions. Advanced systems exhibit response times ranging from minutes to hours, with release kinetics that can be sustained over periods from 24 hours to several weeks depending on the application requirements.
Despite these advancements, several technical barriers persist in the development of clinically viable pH-responsive antimicrobial hydrogels. Foremost among these is the challenge of achieving precise pH-responsiveness within narrow pH ranges relevant to specific infection microenvironments. Current systems often exhibit broad response profiles that may trigger premature release or insufficient response at target sites.
Another significant limitation is the inconsistent correlation between in vitro and in vivo performance. Many systems demonstrating excellent pH-responsive behavior in controlled laboratory settings fail to maintain this functionality in complex biological environments where competing ions, proteins, and enzymatic activities can interfere with the responsive mechanisms.
Stability issues represent another major technical hurdle. Many pH-responsive hydrogels suffer from structural degradation over time, particularly in extreme pH conditions, limiting their shelf-life and clinical applicability. Additionally, the mechanical properties of these systems often compromise between flexibility and structural integrity, presenting challenges for applications requiring specific mechanical characteristics.
Biocompatibility concerns also persist, particularly with synthetic polymer-based systems that may release potentially toxic degradation products or unreacted monomers. Furthermore, achieving controlled and predictable release kinetics remains challenging, with many systems exhibiting initial burst release followed by suboptimal sustained delivery.
From a manufacturing perspective, scalability and reproducibility issues continue to impede commercial translation. Complex synthesis procedures, multiple fabrication steps, and difficulties in quality control during production represent significant barriers to widespread adoption of these technologies in clinical settings.
The state-of-the-art pH-responsive systems demonstrate sensitivity within physiologically relevant pH ranges (4.5-7.4), allowing targeted antimicrobial release at infection sites where pH typically deviates from normal tissue conditions. Advanced systems exhibit response times ranging from minutes to hours, with release kinetics that can be sustained over periods from 24 hours to several weeks depending on the application requirements.
Despite these advancements, several technical barriers persist in the development of clinically viable pH-responsive antimicrobial hydrogels. Foremost among these is the challenge of achieving precise pH-responsiveness within narrow pH ranges relevant to specific infection microenvironments. Current systems often exhibit broad response profiles that may trigger premature release or insufficient response at target sites.
Another significant limitation is the inconsistent correlation between in vitro and in vivo performance. Many systems demonstrating excellent pH-responsive behavior in controlled laboratory settings fail to maintain this functionality in complex biological environments where competing ions, proteins, and enzymatic activities can interfere with the responsive mechanisms.
Stability issues represent another major technical hurdle. Many pH-responsive hydrogels suffer from structural degradation over time, particularly in extreme pH conditions, limiting their shelf-life and clinical applicability. Additionally, the mechanical properties of these systems often compromise between flexibility and structural integrity, presenting challenges for applications requiring specific mechanical characteristics.
Biocompatibility concerns also persist, particularly with synthetic polymer-based systems that may release potentially toxic degradation products or unreacted monomers. Furthermore, achieving controlled and predictable release kinetics remains challenging, with many systems exhibiting initial burst release followed by suboptimal sustained delivery.
From a manufacturing perspective, scalability and reproducibility issues continue to impede commercial translation. Complex synthesis procedures, multiple fabrication steps, and difficulties in quality control during production represent significant barriers to widespread adoption of these technologies in clinical settings.
Existing pH-Responsive Antimicrobial Hydrogel Solutions
01 pH-responsive hydrogel design principles
pH-responsive hydrogels are designed with functional groups that can accept or donate protons in response to environmental pH changes. These systems typically incorporate acidic (carboxylic, sulfonic) or basic (amine) groups that undergo ionization state changes at specific pH thresholds, causing the hydrogel network to swell or contract. This responsiveness can be tuned by selecting appropriate polymers and adjusting crosslinking density to create materials that respond to physiological or pathological pH conditions, making them valuable for targeted antimicrobial delivery.- pH-responsive hydrogel design principles: pH-responsive hydrogels are designed with specific polymeric structures that can undergo conformational changes in response to environmental pH variations. These hydrogels typically incorporate ionizable functional groups such as carboxylic acids, amines, or phosphates that can accept or donate protons depending on the surrounding pH. This responsiveness allows for controlled swelling, drug release, and antimicrobial activity at targeted pH values, making them particularly useful for applications in wound healing and infection control where pH fluctuations are common.
- Antimicrobial agent incorporation mechanisms: Various methods are employed to incorporate antimicrobial agents into pH-responsive hydrogels. These include physical entrapment, chemical conjugation, and in-situ formation of antimicrobial nanoparticles within the hydrogel matrix. The release of these antimicrobial agents can be triggered by pH changes, allowing for targeted delivery at infection sites where pH typically deviates from normal physiological conditions. Common antimicrobial agents incorporated include silver nanoparticles, quaternary ammonium compounds, antibiotics, and natural antimicrobial peptides.
- Dual-responsive hydrogel systems: Advanced hydrogel systems that respond to both pH and additional stimuli such as temperature, light, or redox conditions offer enhanced control over antimicrobial activity. These dual-responsive systems can provide more precise targeting and controlled release profiles. For example, hydrogels that respond to both pH and temperature can maintain structural integrity at body temperature while releasing antimicrobial agents in response to the acidic environment of an infection site. This multi-responsive approach improves therapeutic efficacy and reduces potential side effects.
- Biodegradable pH-responsive antimicrobial hydrogels: Biodegradable pH-responsive hydrogels offer the advantage of eliminating the need for removal after treatment. These systems are designed with hydrolytically or enzymatically degradable crosslinks that break down over time or in response to specific biological conditions. Natural polymers such as chitosan, alginate, and hyaluronic acid are commonly used as base materials for these biodegradable systems. The degradation rate can be tuned to match the healing process, and the degradation products can be designed to be non-toxic and easily metabolized by the body.
- Applications in wound healing and infection control: pH-responsive antimicrobial hydrogels find extensive applications in wound healing and infection control due to their ability to respond to the pH changes associated with wound environments. Chronic wounds often exhibit elevated pH levels compared to healthy skin, while bacterial infections can create acidic microenvironments. These hydrogels can be designed to release antimicrobial agents specifically under these conditions, providing targeted therapy. Additionally, some systems incorporate pH indicators that change color in response to infection, allowing for visual monitoring of wound status and treatment efficacy.
02 Antimicrobial agent incorporation strategies
Various strategies exist for incorporating antimicrobial agents into pH-responsive hydrogels. These include physical entrapment within the polymer network, covalent attachment to the polymer backbone, layer-by-layer assembly techniques, and in-situ formation of antimicrobial nanoparticles. The release kinetics of these agents can be controlled by the hydrogel's pH-dependent swelling behavior, allowing for targeted delivery at infection sites where pH changes occur. Common antimicrobial agents incorporated include silver nanoparticles, quaternary ammonium compounds, antibiotics, and natural antimicrobial peptides.Expand Specific Solutions03 Smart delivery systems for infection sites
pH-responsive antimicrobial hydrogels can function as smart delivery systems that respond to the acidic environment typically found at infection sites. When bacteria colonize tissues, they often produce acidic metabolites that lower the local pH. Hydrogels designed to expand or degrade in acidic conditions can release their antimicrobial payload specifically at these locations, increasing therapeutic efficacy while reducing systemic exposure. This targeted approach helps overcome antimicrobial resistance by maintaining high local drug concentrations while minimizing exposure to sub-inhibitory concentrations elsewhere in the body.Expand Specific Solutions04 Biocompatible materials for medical applications
Biocompatible materials are essential for developing pH-responsive antimicrobial hydrogels for medical applications. Natural polymers like chitosan, alginate, and hyaluronic acid are frequently used due to their inherent biocompatibility and biodegradability. Synthetic polymers such as poly(acrylic acid), poly(methacrylic acid), and poly(ethylene glycol) offer tunable properties and reproducibility. Hybrid systems combining natural and synthetic components can leverage the advantages of both material types. These biocompatible hydrogels can be applied as wound dressings, implant coatings, or injectable systems for treating localized infections.Expand Specific Solutions05 Dual-responsive and multi-functional systems
Advanced pH-responsive antimicrobial hydrogels often incorporate dual or multi-responsive mechanisms to enhance their performance. These systems may respond to additional stimuli such as temperature, light, or enzyme activity alongside pH changes. Multi-functional hydrogels can combine antimicrobial activity with other beneficial properties like promoting wound healing, reducing inflammation, or supporting tissue regeneration. Some systems also incorporate diagnostic capabilities, such as colorimetric pH indicators that signal infection presence through visible color changes, creating theranostic platforms that simultaneously treat infections and monitor healing progress.Expand Specific Solutions
Leading Research Groups and Commercial Entities
The pH-responsive antimicrobial hydrogel systems market is currently in a growth phase, with increasing research interest driven by healthcare applications and antimicrobial resistance concerns. The global market for advanced wound care products, which includes antimicrobial hydrogels, is projected to reach $15-20 billion by 2026. Academic institutions dominate the research landscape, with Northwestern University, Zhejiang University, and Tianjin University leading fundamental research, while companies like IBM and Ecolab are developing commercial applications. Research organizations such as CNRS, Fraunhofer-Gesellschaft, and A*STAR are bridging the gap between academic research and industrial implementation. The technology is advancing from proof-of-concept to early commercialization, with increasing focus on biocompatibility, controlled drug release mechanisms, and scale-up manufacturing processes.
Northwestern University
Technical Solution: Northwestern University has developed advanced pH-responsive antimicrobial hydrogel systems utilizing self-assembling peptide amphiphiles that form nanofiber networks. Their technology incorporates antimicrobial peptides (AMPs) that become active in acidic environments typical of infection sites. The hydrogels maintain stability at physiological pH (7.4) but undergo conformational changes at lower pH values (5.5-6.5), triggering the release of antimicrobial agents[1]. Their platform employs β-hairpin peptides with histidine residues that become protonated in acidic conditions, causing electrostatic repulsion and gel swelling for controlled drug release[2]. Northwestern's research has demonstrated effective killing of both Gram-positive and Gram-negative bacteria, including drug-resistant strains, with minimal cytotoxicity to mammalian cells. The hydrogels also show promising results in wound healing applications, where they can adapt to the changing pH environment during the healing process[3].
Strengths: Highly specific pH-triggered antimicrobial activity with minimal off-target effects; biocompatible and biodegradable materials; effective against drug-resistant bacteria. Weaknesses: Potential challenges in scaling up peptide-based systems; higher production costs compared to conventional antimicrobials; possible stability issues during long-term storage.
Jiangnan University
Technical Solution: Jiangnan University has pioneered pH-responsive antimicrobial hydrogel systems based on chitosan derivatives and polyelectrolyte complexes. Their approach utilizes modified chitosan backbones with grafted pH-sensitive side chains that undergo reversible protonation in acidic environments. The hydrogels incorporate quaternary ammonium compounds and zinc oxide nanoparticles for enhanced antimicrobial efficacy[1]. A key innovation is their dual-responsive system that combines pH sensitivity with temperature responsiveness, allowing precise control over antimicrobial release in different physiological conditions[2]. Their hydrogels demonstrate excellent mucoadhesive properties, making them particularly suitable for oral and gastrointestinal applications where pH variations can be leveraged for targeted delivery. Recent developments include injectable formulations that transition from sol to gel states in situ, conforming to irregular wound surfaces while maintaining pH-responsive antimicrobial properties[3]. The technology has shown particular efficacy against food-borne pathogens, positioning it for food safety applications.
Strengths: Cost-effective production using abundant natural polymers; excellent biocompatibility and biodegradability; versatile formulation options including injectable and sprayable formats. Weaknesses: Potential batch-to-batch variation in natural chitosan sources; limited mechanical strength compared to synthetic alternatives; possible reduced efficacy against certain fungal species.
Key Patents and Scientific Breakthroughs
PH-responsive hydrogel and manufacturing method thereof
PatentActiveUS12208115B2
Innovation
- A pH-responsive hydrogel is developed, comprising an aqueous solution of pectin and acid-treated sucralfate, which forms a transient barrier on the gastrointestinal tract, mimicking the nutrient absorption reduction effect of gastric bypass without invasive procedures.
Ph-responsive hydrogel biocarrier and application
PatentPendingUS20250303020A1
Innovation
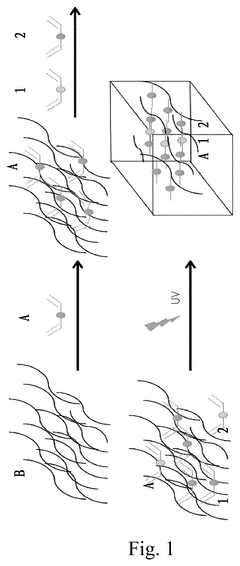
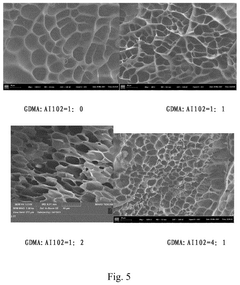
- A pH-responsive hydrogel biocarrier is developed using methacrylic anhydride hyaluronic acid, crosslinked with GDMA and AI102, to encapsulate PDGF-BB, ensuring controlled release and stability through ester bond degradation responsive to alkaline pH.
Biocompatibility and Safety Considerations
The biocompatibility and safety profile of pH-responsive antimicrobial hydrogels represent critical considerations for their clinical translation and commercial viability. These advanced materials must demonstrate not only efficacy against pathogens but also compatibility with human tissues and physiological systems. The safety assessment framework for these hydrogels encompasses multiple dimensions including cytotoxicity, immunogenicity, hemocompatibility, and long-term tissue response.
Primary cytotoxicity evaluations typically involve in vitro cell viability assays using relevant cell lines such as fibroblasts, keratinocytes, or specific tissue cells depending on the intended application. Research indicates that the cytotoxicity of pH-responsive antimicrobial hydrogels often correlates with their charge density, with highly cationic systems potentially disrupting mammalian cell membranes alongside their antimicrobial activity. This necessitates careful optimization of the charge balance to achieve selective toxicity toward microbes.
Immunological responses present another critical safety consideration. pH-responsive hydrogels may trigger inflammatory cascades through interaction with immune cells or complement activation. Studies have shown that hydrogel degradation products can potentially act as haptens or directly stimulate immune responses. Comprehensive immunological profiling including assessment of pro-inflammatory cytokine production, macrophage polarization, and lymphocyte activation provides essential safety data.
Hemocompatibility testing represents a mandatory evaluation for hydrogels intended for blood-contacting applications. This includes hemolysis assays, platelet activation studies, and coagulation cascade assessments. pH-responsive systems present unique challenges as their surface properties dynamically change with environmental pH, potentially altering their interaction with blood components under different physiological or pathological conditions.
The biodegradation profile of these hydrogels significantly impacts their safety profile. Ideal systems should degrade into non-toxic metabolites that can be cleared through natural physiological pathways. Current research focuses on incorporating enzymatically degradable linkages or hydrolytically labile bonds that respond to specific biological triggers, ensuring controlled degradation aligned with the healing process.
Regulatory considerations for pH-responsive antimicrobial hydrogels are complex and evolving. These materials often fall under combination product classifications, requiring comprehensive documentation of both the device aspects (the hydrogel matrix) and drug components (antimicrobial agents). Standardized testing protocols following ISO 10993 guidelines provide the foundation for safety evaluation, though additional specialized testing may be necessary to address the unique responsive properties of these systems.
Primary cytotoxicity evaluations typically involve in vitro cell viability assays using relevant cell lines such as fibroblasts, keratinocytes, or specific tissue cells depending on the intended application. Research indicates that the cytotoxicity of pH-responsive antimicrobial hydrogels often correlates with their charge density, with highly cationic systems potentially disrupting mammalian cell membranes alongside their antimicrobial activity. This necessitates careful optimization of the charge balance to achieve selective toxicity toward microbes.
Immunological responses present another critical safety consideration. pH-responsive hydrogels may trigger inflammatory cascades through interaction with immune cells or complement activation. Studies have shown that hydrogel degradation products can potentially act as haptens or directly stimulate immune responses. Comprehensive immunological profiling including assessment of pro-inflammatory cytokine production, macrophage polarization, and lymphocyte activation provides essential safety data.
Hemocompatibility testing represents a mandatory evaluation for hydrogels intended for blood-contacting applications. This includes hemolysis assays, platelet activation studies, and coagulation cascade assessments. pH-responsive systems present unique challenges as their surface properties dynamically change with environmental pH, potentially altering their interaction with blood components under different physiological or pathological conditions.
The biodegradation profile of these hydrogels significantly impacts their safety profile. Ideal systems should degrade into non-toxic metabolites that can be cleared through natural physiological pathways. Current research focuses on incorporating enzymatically degradable linkages or hydrolytically labile bonds that respond to specific biological triggers, ensuring controlled degradation aligned with the healing process.
Regulatory considerations for pH-responsive antimicrobial hydrogels are complex and evolving. These materials often fall under combination product classifications, requiring comprehensive documentation of both the device aspects (the hydrogel matrix) and drug components (antimicrobial agents). Standardized testing protocols following ISO 10993 guidelines provide the foundation for safety evaluation, though additional specialized testing may be necessary to address the unique responsive properties of these systems.
Scalability and Manufacturing Challenges
The scalability and manufacturing of pH-responsive antimicrobial hydrogel systems present significant challenges that must be addressed for successful commercial implementation. Current laboratory-scale production methods often involve batch processes with limited throughput, creating a substantial gap between research prototypes and industrial-scale manufacturing requirements. The transition to large-scale production necessitates process optimization to maintain consistent material properties, antimicrobial efficacy, and pH-responsiveness across production batches.
Material sourcing represents another critical challenge, particularly for specialized components such as antimicrobial agents and pH-responsive polymers. The availability, cost, and quality consistency of these materials can fluctuate significantly, potentially affecting the final product performance and manufacturing economics. Additionally, regulatory considerations for antimicrobial materials add complexity to the supply chain management process.
Process standardization remains underdeveloped for these advanced hydrogel systems. The gelation kinetics, crosslinking density, and incorporation of antimicrobial agents must be precisely controlled during scale-up to ensure product uniformity. Current manufacturing technologies struggle with achieving homogeneous distribution of antimicrobial agents throughout larger hydrogel volumes, potentially leading to efficacy variations in the final product.
Quality control methodologies require significant advancement to accommodate industrial-scale production. Existing analytical techniques for characterizing pH-responsiveness and antimicrobial activity are often time-consuming and difficult to implement as in-line monitoring systems. This creates bottlenecks in production and increases quality assurance costs.
Sterilization processes present unique challenges for antimicrobial hydrogels. Traditional methods such as autoclaving or gamma irradiation may compromise the structural integrity of the hydrogel network or deactivate the antimicrobial components. Alternative sterilization approaches compatible with these sensitive materials need further development for industrial implementation.
Cost-effectiveness remains a significant barrier to widespread adoption. Current manufacturing approaches for specialized hydrogel systems typically result in high production costs that limit commercial viability. Process intensification strategies, such as continuous manufacturing technologies and automated production lines, offer potential solutions but require substantial investment in equipment and process development.
Shelf-life stability under various storage conditions represents another manufacturing challenge. pH-responsive antimicrobial hydrogels must maintain their functional properties throughout the product lifecycle, necessitating appropriate packaging solutions and stabilization strategies that can be implemented at industrial scale.
Material sourcing represents another critical challenge, particularly for specialized components such as antimicrobial agents and pH-responsive polymers. The availability, cost, and quality consistency of these materials can fluctuate significantly, potentially affecting the final product performance and manufacturing economics. Additionally, regulatory considerations for antimicrobial materials add complexity to the supply chain management process.
Process standardization remains underdeveloped for these advanced hydrogel systems. The gelation kinetics, crosslinking density, and incorporation of antimicrobial agents must be precisely controlled during scale-up to ensure product uniformity. Current manufacturing technologies struggle with achieving homogeneous distribution of antimicrobial agents throughout larger hydrogel volumes, potentially leading to efficacy variations in the final product.
Quality control methodologies require significant advancement to accommodate industrial-scale production. Existing analytical techniques for characterizing pH-responsiveness and antimicrobial activity are often time-consuming and difficult to implement as in-line monitoring systems. This creates bottlenecks in production and increases quality assurance costs.
Sterilization processes present unique challenges for antimicrobial hydrogels. Traditional methods such as autoclaving or gamma irradiation may compromise the structural integrity of the hydrogel network or deactivate the antimicrobial components. Alternative sterilization approaches compatible with these sensitive materials need further development for industrial implementation.
Cost-effectiveness remains a significant barrier to widespread adoption. Current manufacturing approaches for specialized hydrogel systems typically result in high production costs that limit commercial viability. Process intensification strategies, such as continuous manufacturing technologies and automated production lines, offer potential solutions but require substantial investment in equipment and process development.
Shelf-life stability under various storage conditions represents another manufacturing challenge. pH-responsive antimicrobial hydrogels must maintain their functional properties throughout the product lifecycle, necessitating appropriate packaging solutions and stabilization strategies that can be implemented at industrial scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!