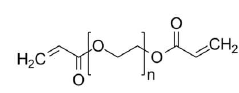
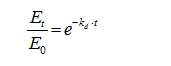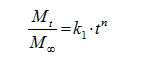Cross-Linking Density and Drug Release Kinetics in Hydrogels
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Cross-Linking Technology Background and Objectives
Hydrogels have emerged as a revolutionary class of biomaterials over the past several decades, evolving from simple cross-linked networks to sophisticated responsive systems. The fundamental concept of hydrogels dates back to the 1960s when the first synthetic hydrogels were developed for contact lens applications. Since then, the technology has undergone significant advancements, particularly in controlling the cross-linking density to achieve desired mechanical properties and drug release profiles.
The evolution of hydrogel technology has been marked by several key milestones, including the development of stimuli-responsive hydrogels in the 1980s, biodegradable hydrogels in the 1990s, and more recently, the integration of nanotechnology to create hybrid hydrogel systems. The cross-linking mechanisms have similarly evolved from simple chemical cross-linking to more sophisticated approaches including physical cross-linking, ionic interactions, and dynamic covalent chemistry.
Current technological trends in hydrogel cross-linking are moving toward precision engineering at the molecular level, allowing for unprecedented control over network architecture. This includes the development of double-network hydrogels, slide-ring hydrogels, and supramolecular hydrogels that exhibit enhanced mechanical properties while maintaining high water content. The relationship between cross-linking density and drug release kinetics has become a central focus, as it directly impacts the therapeutic efficacy of drug delivery systems.
The primary objective of research in this field is to establish quantitative relationships between cross-linking parameters and drug release profiles. This includes understanding how mesh size, cross-linking density, and network heterogeneity influence the diffusion of therapeutic molecules of varying sizes and chemical properties. Additionally, researchers aim to develop predictive models that can accurately forecast drug release kinetics based on hydrogel composition and cross-linking characteristics.
Another critical goal is to design hydrogels with programmable degradation profiles that synchronize with therapeutic requirements. This involves creating cross-linking strategies that respond to specific biological triggers or external stimuli, enabling precise temporal control over drug release. The ultimate aim is to develop "smart" hydrogel systems that can autonomously adjust their cross-linking density in response to physiological changes or disease markers.
From a translational perspective, the field is working toward standardizing cross-linking methodologies to improve manufacturing reproducibility and regulatory compliance. This includes developing robust analytical techniques for characterizing cross-linking density in situ and establishing correlations between manufacturing parameters and final product performance in terms of drug release kinetics.
The evolution of hydrogel technology has been marked by several key milestones, including the development of stimuli-responsive hydrogels in the 1980s, biodegradable hydrogels in the 1990s, and more recently, the integration of nanotechnology to create hybrid hydrogel systems. The cross-linking mechanisms have similarly evolved from simple chemical cross-linking to more sophisticated approaches including physical cross-linking, ionic interactions, and dynamic covalent chemistry.
Current technological trends in hydrogel cross-linking are moving toward precision engineering at the molecular level, allowing for unprecedented control over network architecture. This includes the development of double-network hydrogels, slide-ring hydrogels, and supramolecular hydrogels that exhibit enhanced mechanical properties while maintaining high water content. The relationship between cross-linking density and drug release kinetics has become a central focus, as it directly impacts the therapeutic efficacy of drug delivery systems.
The primary objective of research in this field is to establish quantitative relationships between cross-linking parameters and drug release profiles. This includes understanding how mesh size, cross-linking density, and network heterogeneity influence the diffusion of therapeutic molecules of varying sizes and chemical properties. Additionally, researchers aim to develop predictive models that can accurately forecast drug release kinetics based on hydrogel composition and cross-linking characteristics.
Another critical goal is to design hydrogels with programmable degradation profiles that synchronize with therapeutic requirements. This involves creating cross-linking strategies that respond to specific biological triggers or external stimuli, enabling precise temporal control over drug release. The ultimate aim is to develop "smart" hydrogel systems that can autonomously adjust their cross-linking density in response to physiological changes or disease markers.
From a translational perspective, the field is working toward standardizing cross-linking methodologies to improve manufacturing reproducibility and regulatory compliance. This includes developing robust analytical techniques for characterizing cross-linking density in situ and establishing correlations between manufacturing parameters and final product performance in terms of drug release kinetics.
Market Analysis of Controlled Drug Delivery Systems
The global market for controlled drug delivery systems has experienced significant growth, reaching approximately $36.2 billion in 2022 and is projected to expand at a CAGR of 7.8% through 2028. Hydrogel-based delivery systems represent a rapidly growing segment within this market, currently valued at around $5.4 billion with expectations to reach $9.1 billion by 2027.
The pharmaceutical industry dominates the application landscape for cross-linked hydrogels, accounting for nearly 65% of market share. This dominance stems from the superior ability of hydrogels with optimized cross-linking density to provide sustained and controlled drug release profiles, addressing key challenges in therapeutic efficacy and patient compliance.
North America currently leads the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 9.3% annually, driven by expanding healthcare infrastructure, increasing chronic disease prevalence, and rising healthcare expenditure in countries like China and India.
Key market drivers include the growing prevalence of chronic diseases requiring long-term medication management, increasing demand for targeted drug delivery systems, and rising patient preference for minimally invasive therapeutic options. The aging global population further amplifies market potential, as elderly patients often require multiple medications with specific release profiles to manage comorbidities.
Market segmentation reveals distinct preferences across therapeutic areas. Oncology applications represent the largest segment (31%), followed by diabetes management (18%), cardiovascular treatments (15%), and pain management (12%). The remaining market share is distributed across various therapeutic applications including wound healing, tissue engineering, and ophthalmic treatments.
Competitive analysis indicates that major pharmaceutical companies are increasingly investing in hydrogel technology platforms with tunable cross-linking density. Strategic partnerships between material science companies and pharmaceutical manufacturers have increased by 35% over the past three years, highlighting industry recognition of the critical relationship between cross-linking parameters and drug release kinetics.
Regulatory considerations significantly impact market dynamics, with FDA and EMA approval pathways for novel hydrogel-based delivery systems typically requiring 3-5 years. Products demonstrating precise control over release kinetics through optimized cross-linking density generally experience expedited regulatory review, creating a competitive advantage for technically advanced solutions.
Market forecasts suggest that hydrogels with stimuli-responsive cross-linking mechanisms will experience the highest growth rate (11.2% CAGR) within the controlled release segment, reflecting increasing demand for "smart" delivery systems capable of responding to physiological or external triggers.
The pharmaceutical industry dominates the application landscape for cross-linked hydrogels, accounting for nearly 65% of market share. This dominance stems from the superior ability of hydrogels with optimized cross-linking density to provide sustained and controlled drug release profiles, addressing key challenges in therapeutic efficacy and patient compliance.
North America currently leads the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 9.3% annually, driven by expanding healthcare infrastructure, increasing chronic disease prevalence, and rising healthcare expenditure in countries like China and India.
Key market drivers include the growing prevalence of chronic diseases requiring long-term medication management, increasing demand for targeted drug delivery systems, and rising patient preference for minimally invasive therapeutic options. The aging global population further amplifies market potential, as elderly patients often require multiple medications with specific release profiles to manage comorbidities.
Market segmentation reveals distinct preferences across therapeutic areas. Oncology applications represent the largest segment (31%), followed by diabetes management (18%), cardiovascular treatments (15%), and pain management (12%). The remaining market share is distributed across various therapeutic applications including wound healing, tissue engineering, and ophthalmic treatments.
Competitive analysis indicates that major pharmaceutical companies are increasingly investing in hydrogel technology platforms with tunable cross-linking density. Strategic partnerships between material science companies and pharmaceutical manufacturers have increased by 35% over the past three years, highlighting industry recognition of the critical relationship between cross-linking parameters and drug release kinetics.
Regulatory considerations significantly impact market dynamics, with FDA and EMA approval pathways for novel hydrogel-based delivery systems typically requiring 3-5 years. Products demonstrating precise control over release kinetics through optimized cross-linking density generally experience expedited regulatory review, creating a competitive advantage for technically advanced solutions.
Market forecasts suggest that hydrogels with stimuli-responsive cross-linking mechanisms will experience the highest growth rate (11.2% CAGR) within the controlled release segment, reflecting increasing demand for "smart" delivery systems capable of responding to physiological or external triggers.
Current Challenges in Cross-Linking Density Control
Despite significant advancements in hydrogel technology, controlling cross-linking density remains one of the most challenging aspects in the development of drug delivery systems. The primary difficulty lies in achieving precise and uniform cross-linking throughout the hydrogel matrix, as variations can lead to inconsistent drug release profiles. Current manufacturing processes often produce hydrogels with heterogeneous cross-linking density distributions, resulting in unpredictable drug release kinetics that compromise therapeutic efficacy.
The challenge of quantification presents another significant hurdle. Existing analytical methods for measuring cross-linking density, such as rheological testing, swelling studies, and mechanical property analysis, often provide only average values across the entire hydrogel structure. These techniques lack the spatial resolution necessary to detect localized variations in cross-linking density that can significantly impact drug diffusion pathways and release patterns.
Environmental sensitivity further complicates cross-linking density control. Hydrogels designed for in vivo applications must maintain their structural integrity and cross-linking characteristics across varying pH levels, temperatures, and ionic strengths. Current cross-linking strategies often fail to produce networks that can withstand these physiological fluctuations without undergoing significant structural changes that alter drug release profiles.
The balance between mechanical properties and drug release functionality presents another critical challenge. Increasing cross-linking density typically enhances mechanical strength but reduces mesh size, potentially restricting drug diffusion. Conversely, lower cross-linking density facilitates drug release but may compromise structural integrity. This inverse relationship creates a design paradox that limits the therapeutic window of hydrogel-based drug delivery systems.
Scalability and reproducibility issues further hinder progress in this field. Laboratory-scale methods for controlling cross-linking density often fail to translate effectively to industrial production settings. Batch-to-batch variations in cross-linking density remain problematic, leading to inconsistent drug release profiles among supposedly identical formulations.
Degradation kinetics add another layer of complexity. As hydrogels degrade in vivo, their cross-linking density changes dynamically, altering drug release rates over time. Current technologies struggle to predict and control this temporal evolution of cross-linking density, making it difficult to design systems with predetermined release profiles throughout their functional lifespan.
These challenges collectively highlight the need for innovative approaches to cross-linking density control that can overcome current limitations and enable the development of next-generation hydrogel-based drug delivery systems with predictable, tunable, and consistent release kinetics.
The challenge of quantification presents another significant hurdle. Existing analytical methods for measuring cross-linking density, such as rheological testing, swelling studies, and mechanical property analysis, often provide only average values across the entire hydrogel structure. These techniques lack the spatial resolution necessary to detect localized variations in cross-linking density that can significantly impact drug diffusion pathways and release patterns.
Environmental sensitivity further complicates cross-linking density control. Hydrogels designed for in vivo applications must maintain their structural integrity and cross-linking characteristics across varying pH levels, temperatures, and ionic strengths. Current cross-linking strategies often fail to produce networks that can withstand these physiological fluctuations without undergoing significant structural changes that alter drug release profiles.
The balance between mechanical properties and drug release functionality presents another critical challenge. Increasing cross-linking density typically enhances mechanical strength but reduces mesh size, potentially restricting drug diffusion. Conversely, lower cross-linking density facilitates drug release but may compromise structural integrity. This inverse relationship creates a design paradox that limits the therapeutic window of hydrogel-based drug delivery systems.
Scalability and reproducibility issues further hinder progress in this field. Laboratory-scale methods for controlling cross-linking density often fail to translate effectively to industrial production settings. Batch-to-batch variations in cross-linking density remain problematic, leading to inconsistent drug release profiles among supposedly identical formulations.
Degradation kinetics add another layer of complexity. As hydrogels degrade in vivo, their cross-linking density changes dynamically, altering drug release rates over time. Current technologies struggle to predict and control this temporal evolution of cross-linking density, making it difficult to design systems with predetermined release profiles throughout their functional lifespan.
These challenges collectively highlight the need for innovative approaches to cross-linking density control that can overcome current limitations and enable the development of next-generation hydrogel-based drug delivery systems with predictable, tunable, and consistent release kinetics.
Current Approaches to Modulating Drug Release Kinetics
01 Hydrogel composition for controlled drug release
Specific hydrogel compositions can be engineered to control drug release kinetics. These compositions may include various polymers, cross-linking agents, and additives that affect the network structure and swelling behavior of the hydrogel. By manipulating the composition, researchers can achieve desired release profiles ranging from immediate to sustained release, allowing for precise control over drug delivery rates and durations.- Hydrogel composition for controlled drug release: Specific hydrogel compositions can be formulated to control drug release kinetics. These compositions may include various polymers, cross-linking agents, and additives that affect the network structure and swelling behavior of the hydrogel. By manipulating the composition, the release rate of drugs can be tailored for specific therapeutic applications, allowing for sustained or pulsatile release profiles.
- Stimuli-responsive hydrogels for drug delivery: Stimuli-responsive hydrogels can change their drug release kinetics in response to environmental triggers such as pH, temperature, light, or specific biomolecules. These smart hydrogels can provide on-demand drug release at targeted sites or under specific physiological conditions. The responsive behavior allows for precise control over the timing and location of drug delivery, improving therapeutic efficacy and reducing side effects.
- Mathematical modeling of drug release kinetics: Mathematical models are developed to predict and analyze drug release kinetics from hydrogel systems. These models incorporate various parameters such as diffusion coefficients, degradation rates, and swelling ratios to simulate the release behavior under different conditions. By applying these models, researchers can design hydrogel systems with optimized release profiles and better understand the mechanisms governing drug release from hydrogels.
- Nanocomposite hydrogels for enhanced drug delivery: Nanocomposite hydrogels incorporate nanomaterials such as nanoparticles, nanofibers, or nanoclays into the hydrogel matrix to modify drug release kinetics. These nanomaterials can create additional diffusion barriers, provide binding sites for drugs, or introduce new functionalities that affect release behavior. Nanocomposite hydrogels often exhibit improved mechanical properties and more sophisticated drug release profiles compared to conventional hydrogels.
- Biodegradable hydrogels for sustained drug release: Biodegradable hydrogels are designed to degrade over time in the body, providing an additional mechanism for controlling drug release kinetics. The degradation rate can be tuned by modifying the chemical structure of the polymers, the cross-linking density, or incorporating enzyme-sensitive linkages. As the hydrogel degrades, entrapped drugs are gradually released, allowing for sustained delivery over extended periods without the need for removal of the delivery system.
02 Environmental responsive hydrogels for drug delivery
Stimuli-responsive hydrogels can alter their drug release kinetics in response to environmental changes such as pH, temperature, or specific biomolecules. These smart hydrogels undergo structural changes when exposed to specific stimuli, which can trigger or modify drug release rates. This approach enables targeted drug delivery to specific sites in the body and allows for on-demand release profiles that can be tailored to therapeutic needs.Expand Specific Solutions03 Mathematical modeling of drug release from hydrogels
Mathematical models are developed to predict and analyze drug release kinetics from hydrogel systems. These models incorporate various parameters such as diffusion coefficients, degradation rates, and swelling ratios to simulate drug release behavior. By applying these mathematical approaches, researchers can design hydrogel systems with predetermined release profiles and optimize formulations without extensive experimental testing.Expand Specific Solutions04 Nanocomposite hydrogels for enhanced drug release control
Incorporating nanomaterials into hydrogels creates nanocomposite systems with enhanced control over drug release kinetics. Nanomaterials such as nanoparticles, nanofibers, or nanoclays can modify the network structure, create additional diffusion barriers, or provide alternative release mechanisms. These nanocomposite hydrogels offer improved mechanical properties and more precise control over release rates compared to conventional hydrogels.Expand Specific Solutions05 Biodegradable hydrogels for programmed drug release
Biodegradable hydrogels provide programmed drug release through controlled matrix degradation. These systems release drugs through a combination of diffusion and matrix erosion mechanisms, allowing for tailored release profiles. By adjusting the degradation rate of the hydrogel matrix through polymer selection and cross-linking density, the drug release kinetics can be precisely controlled to match therapeutic requirements while ensuring complete biodegradation after drug delivery.Expand Specific Solutions
Leading Research Groups and Companies in Hydrogel Technology
The hydrogel cross-linking density and drug release kinetics market is in a growth phase, with increasing applications in controlled drug delivery systems. The market size is expanding due to rising demand for advanced drug delivery technologies in pharmaceutical and biomedical sectors. Technologically, this field shows moderate maturity with ongoing innovations. Leading players include Ocular Therapeutix, which specializes in ophthalmic hydrogel applications, and Nektar Therapeutics, focusing on advanced drug delivery systems. Ethicon and Boston Scientific Scimed represent established medical device manufacturers incorporating hydrogel technologies. Academic institutions like Rutgers University and University of Washington contribute significant research, while specialized companies such as SupraPolix and Prolynx develop proprietary cross-linking technologies for controlled release applications, indicating a competitive landscape balanced between established corporations and innovative startups.
Ascendis Pharma GmbH
Technical Solution: Ascendis Pharma has developed the TransCon™ hydrogel technology platform that utilizes precisely controlled cross-linking density to achieve predictable drug release kinetics. Their approach employs a unique "prodrug" concept where therapeutic molecules are temporarily bound to the hydrogel matrix via cleavable linkers with predetermined release rates[1]. The company's technology creates hydrogels with a hierarchical cross-linking structure: primary structural cross-links maintain the overall hydrogel integrity, while secondary cross-links containing drug molecules degrade at controlled rates to release the therapeutic payload[2]. This dual cross-linking approach enables independent control of mechanical properties and drug release kinetics. Ascendis has pioneered the use of enzymatically cleavable cross-linkers that respond to specific biological environments, allowing for site-specific drug release. Their hydrogels demonstrate remarkable versatility in accommodating both small molecules and large proteins while maintaining their biological activity throughout the release period[3]. The technology has been successfully applied to create long-acting formulations of growth hormone, PTH, and CNP with precisely controlled release profiles.
Strengths: Clinically validated technology with products in late-stage development; ability to maintain protein stability during release; customizable release profiles for different therapeutic applications. Weaknesses: Complex manufacturing requirements; potential immunogenicity concerns with some linker chemistries; higher development costs compared to conventional delivery systems.
Ocular Therapeutix, Inc.
Technical Solution: Ocular Therapeutix has pioneered hydrogel technology specifically designed for ophthalmic drug delivery with precise cross-linking density control. Their flagship RESURE® Sealant and DEXTENZA® products utilize a proprietary polyethylene glycol (PEG) hydrogel technology with tailored cross-linking density to achieve sustained drug release profiles[1]. The company's innovative approach involves a dual-stage cross-linking process: an initial photo-initiated cross-linking followed by a secondary hydrolytically degradable cross-linking mechanism[2]. This creates hydrogels with a core-shell architecture where cross-linking density gradually decreases from the center outward, enabling zero-order drug release kinetics. Their technology incorporates specialized cross-linkers with hydrolytically labile bonds that degrade at predetermined rates, allowing for programmable drug release over periods ranging from days to months[3]. The hydrogels are engineered to maintain structural integrity in the ocular environment while gradually releasing therapeutic agents at the target site.
Strengths: Specialized expertise in ocular applications; proven clinical efficacy with FDA-approved products; ability to achieve extended release periods in challenging environments. Weaknesses: Limited application outside ophthalmology; potential for initial burst release in some formulations; relatively higher cost compared to conventional delivery systems.
Key Patents and Literature on Cross-Linking Density Manipulation
Dual-mode drug release hydrogel and method of fabricating the same
PatentActiveKR1020200020423A
Innovation
- A hydrogel composition comprising poly(ethylene glycol) diacrylate (PEGDA) and polyethyleneimine (PEI) is cross-linked to form a dual-mode drug release system, allowing for in situ formation and controlled drug release through swelling and degradation mechanisms.
Biocompatibility and Degradation Considerations
The biocompatibility of hydrogel systems represents a critical factor in their application for drug delivery, particularly when considering the relationship between cross-linking density and drug release kinetics. Hydrogels with varying cross-linking densities exhibit different interactions with biological tissues, potentially triggering immune responses or inflammation if not properly engineered. Materials commonly used in biocompatible hydrogels include natural polymers such as alginate, chitosan, and hyaluronic acid, as well as synthetic polymers like poly(ethylene glycol) (PEG) and poly(vinyl alcohol) (PVA).
Cross-linking density significantly impacts the foreign body response to implanted hydrogels. Higher cross-linking densities typically result in stiffer materials that may cause increased mechanical irritation to surrounding tissues, while lower cross-linking densities produce softer materials that better mimic natural tissue mechanics. However, this relationship must be balanced against drug release requirements, as lower cross-linking densities generally facilitate faster drug release rates.
Degradation kinetics of hydrogels present another crucial consideration that directly influences drug release profiles. Hydrogels can be designed with varying degradation timeframes—from hours to months—depending on the therapeutic application. The degradation mechanism may be hydrolytic, enzymatic, or a combination of both, with cross-linking density serving as a primary determinant of degradation rate. Highly cross-linked networks typically demonstrate slower degradation due to increased resistance to hydrolytic or enzymatic attack.
Recent advances have focused on developing "smart" degradable hydrogels where degradation can be triggered by specific biological stimuli such as pH changes, enzyme concentrations, or redox conditions. These systems allow for more precise control over both degradation and subsequent drug release, creating opportunities for targeted delivery to specific tissues or cellular compartments.
The byproducts of hydrogel degradation must also be carefully evaluated for toxicity. Ideal degradation products should be easily metabolized or excreted without accumulation in tissues or organs. For instance, PEG-based hydrogels typically degrade into components that can be cleared renally, while natural polymer-based hydrogels often break down into naturally occurring metabolites.
Regulatory considerations for biocompatible hydrogels have become increasingly stringent, requiring comprehensive testing of both the initial material and its degradation products. ISO 10993 standards provide guidelines for evaluating biocompatibility, including cytotoxicity, sensitization, irritation, and systemic toxicity assessments. These regulatory frameworks have driven innovation toward hydrogel systems with predictable degradation profiles and well-characterized degradation products.
Cross-linking density significantly impacts the foreign body response to implanted hydrogels. Higher cross-linking densities typically result in stiffer materials that may cause increased mechanical irritation to surrounding tissues, while lower cross-linking densities produce softer materials that better mimic natural tissue mechanics. However, this relationship must be balanced against drug release requirements, as lower cross-linking densities generally facilitate faster drug release rates.
Degradation kinetics of hydrogels present another crucial consideration that directly influences drug release profiles. Hydrogels can be designed with varying degradation timeframes—from hours to months—depending on the therapeutic application. The degradation mechanism may be hydrolytic, enzymatic, or a combination of both, with cross-linking density serving as a primary determinant of degradation rate. Highly cross-linked networks typically demonstrate slower degradation due to increased resistance to hydrolytic or enzymatic attack.
Recent advances have focused on developing "smart" degradable hydrogels where degradation can be triggered by specific biological stimuli such as pH changes, enzyme concentrations, or redox conditions. These systems allow for more precise control over both degradation and subsequent drug release, creating opportunities for targeted delivery to specific tissues or cellular compartments.
The byproducts of hydrogel degradation must also be carefully evaluated for toxicity. Ideal degradation products should be easily metabolized or excreted without accumulation in tissues or organs. For instance, PEG-based hydrogels typically degrade into components that can be cleared renally, while natural polymer-based hydrogels often break down into naturally occurring metabolites.
Regulatory considerations for biocompatible hydrogels have become increasingly stringent, requiring comprehensive testing of both the initial material and its degradation products. ISO 10993 standards provide guidelines for evaluating biocompatibility, including cytotoxicity, sensitization, irritation, and systemic toxicity assessments. These regulatory frameworks have driven innovation toward hydrogel systems with predictable degradation profiles and well-characterized degradation products.
Regulatory Pathway for Hydrogel-Based Drug Delivery Systems
The regulatory landscape for hydrogel-based drug delivery systems presents a complex framework that developers must navigate to bring their products to market. For hydrogels with varying cross-linking densities that affect drug release kinetics, regulatory considerations are particularly nuanced due to the direct impact these parameters have on therapeutic efficacy and safety profiles.
In the United States, the FDA typically classifies hydrogel drug delivery systems as combination products, requiring review by multiple centers within the agency. The Center for Drug Evaluation and Research (CDER) and the Center for Devices and Radiological Health (CDRH) often collaborate on these evaluations, with the primary mode of action determining the lead review center. For hydrogels where cross-linking density significantly impacts drug release, developers must provide comprehensive data demonstrating consistent manufacturing processes and reproducible release profiles.
European regulatory pathways through the European Medicines Agency (EMA) similarly emphasize the importance of characterization studies that correlate cross-linking density with drug release kinetics. The EMA's guidelines on quality aspects of drug-device combinations require manufacturers to establish design space parameters that ensure consistent performance across production batches, with particular attention to critical material attributes affecting drug release.
Regulatory submissions for these systems must include detailed information on the relationship between cross-linking methods, resulting network structures, and subsequent release profiles. Authorities typically require in vitro dissolution studies under various physiological conditions, stability data demonstrating consistent release kinetics throughout the product's shelf life, and in vivo correlation studies validating that laboratory findings translate to clinical performance.
Quality by Design (QbD) principles have become increasingly important in regulatory strategies for hydrogel-based systems. Developers must identify critical quality attributes related to cross-linking density and establish acceptable ranges that ensure consistent drug release. Process analytical technology (PAT) implementations that monitor cross-linking reactions in real-time can strengthen regulatory submissions by demonstrating robust manufacturing control.
For novel cross-linking chemistries or innovative hydrogel compositions, regulatory agencies may require additional safety assessments, particularly regarding degradation products and their potential biological effects. The biocompatibility testing framework outlined in ISO 10993 serves as a foundation for these evaluations, with specific attention to local tissue responses and systemic toxicity considerations.
International harmonization efforts through the International Council for Harmonisation (ICH) have established guidelines that impact hydrogel drug delivery systems, particularly ICH Q8 on pharmaceutical development and ICH Q9 on quality risk management. These frameworks help developers establish regulatory strategies that address the critical relationship between cross-linking density and drug release kinetics across global markets.
In the United States, the FDA typically classifies hydrogel drug delivery systems as combination products, requiring review by multiple centers within the agency. The Center for Drug Evaluation and Research (CDER) and the Center for Devices and Radiological Health (CDRH) often collaborate on these evaluations, with the primary mode of action determining the lead review center. For hydrogels where cross-linking density significantly impacts drug release, developers must provide comprehensive data demonstrating consistent manufacturing processes and reproducible release profiles.
European regulatory pathways through the European Medicines Agency (EMA) similarly emphasize the importance of characterization studies that correlate cross-linking density with drug release kinetics. The EMA's guidelines on quality aspects of drug-device combinations require manufacturers to establish design space parameters that ensure consistent performance across production batches, with particular attention to critical material attributes affecting drug release.
Regulatory submissions for these systems must include detailed information on the relationship between cross-linking methods, resulting network structures, and subsequent release profiles. Authorities typically require in vitro dissolution studies under various physiological conditions, stability data demonstrating consistent release kinetics throughout the product's shelf life, and in vivo correlation studies validating that laboratory findings translate to clinical performance.
Quality by Design (QbD) principles have become increasingly important in regulatory strategies for hydrogel-based systems. Developers must identify critical quality attributes related to cross-linking density and establish acceptable ranges that ensure consistent drug release. Process analytical technology (PAT) implementations that monitor cross-linking reactions in real-time can strengthen regulatory submissions by demonstrating robust manufacturing control.
For novel cross-linking chemistries or innovative hydrogel compositions, regulatory agencies may require additional safety assessments, particularly regarding degradation products and their potential biological effects. The biocompatibility testing framework outlined in ISO 10993 serves as a foundation for these evaluations, with specific attention to local tissue responses and systemic toxicity considerations.
International harmonization efforts through the International Council for Harmonisation (ICH) have established guidelines that impact hydrogel drug delivery systems, particularly ICH Q8 on pharmaceutical development and ICH Q9 on quality risk management. These frameworks help developers establish regulatory strategies that address the critical relationship between cross-linking density and drug release kinetics across global markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!