Hydrogel Degradation Behavior and Antimicrobial Lifetime Analysis
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Degradation Background and Research Objectives
Hydrogels have emerged as versatile biomaterials with significant applications in wound healing, drug delivery, tissue engineering, and antimicrobial surfaces. Since their initial development in the 1960s, these three-dimensional networks of hydrophilic polymers have evolved from simple water-retaining materials to sophisticated responsive systems. The evolution of hydrogels has been marked by transitions from conventional to smart hydrogels, and more recently to composite and nanocomposite formulations with enhanced properties.
The degradation behavior of hydrogels represents a critical aspect of their functionality, particularly in biomedical applications where controlled material breakdown is essential for therapeutic efficacy. Hydrogel degradation can occur through various mechanisms including hydrolysis, enzymatic degradation, dissolution, or physical disintegration. Understanding these mechanisms is paramount as they directly influence the release kinetics of antimicrobial agents and the overall effectiveness of hydrogel-based antimicrobial systems.
Recent advances in antimicrobial hydrogels have introduced innovative approaches incorporating silver nanoparticles, quaternary ammonium compounds, antimicrobial peptides, and natural antimicrobial agents. However, a significant challenge remains in predicting and controlling the degradation rates of these systems, which directly impacts their antimicrobial lifetime and efficacy in real-world applications.
The primary objective of this technical research is to establish a comprehensive framework for analyzing and predicting hydrogel degradation behavior in relation to antimicrobial lifetime. This includes developing mathematical models that can accurately correlate degradation kinetics with antimicrobial release profiles under various environmental conditions such as pH, temperature, ionic strength, and enzymatic activity.
Additionally, this research aims to investigate novel crosslinking strategies and polymer compositions that can provide tunable degradation rates, thereby enabling precise control over antimicrobial release. The development of standardized testing protocols for evaluating hydrogel degradation in simulated physiological environments represents another critical objective, addressing the current lack of uniformity in assessment methodologies.
Furthermore, this research seeks to explore the relationship between hydrogel microstructure and degradation patterns, utilizing advanced imaging techniques such as confocal microscopy, atomic force microscopy, and scanning electron microscopy to visualize degradation processes at the microscale. Understanding these structure-property relationships will facilitate the rational design of next-generation antimicrobial hydrogels with optimized degradation profiles.
The ultimate goal is to bridge the gap between theoretical understanding and practical application, providing industry stakeholders with actionable insights for developing hydrogel-based antimicrobial products with predictable lifetimes and performance characteristics across diverse application environments.
The degradation behavior of hydrogels represents a critical aspect of their functionality, particularly in biomedical applications where controlled material breakdown is essential for therapeutic efficacy. Hydrogel degradation can occur through various mechanisms including hydrolysis, enzymatic degradation, dissolution, or physical disintegration. Understanding these mechanisms is paramount as they directly influence the release kinetics of antimicrobial agents and the overall effectiveness of hydrogel-based antimicrobial systems.
Recent advances in antimicrobial hydrogels have introduced innovative approaches incorporating silver nanoparticles, quaternary ammonium compounds, antimicrobial peptides, and natural antimicrobial agents. However, a significant challenge remains in predicting and controlling the degradation rates of these systems, which directly impacts their antimicrobial lifetime and efficacy in real-world applications.
The primary objective of this technical research is to establish a comprehensive framework for analyzing and predicting hydrogel degradation behavior in relation to antimicrobial lifetime. This includes developing mathematical models that can accurately correlate degradation kinetics with antimicrobial release profiles under various environmental conditions such as pH, temperature, ionic strength, and enzymatic activity.
Additionally, this research aims to investigate novel crosslinking strategies and polymer compositions that can provide tunable degradation rates, thereby enabling precise control over antimicrobial release. The development of standardized testing protocols for evaluating hydrogel degradation in simulated physiological environments represents another critical objective, addressing the current lack of uniformity in assessment methodologies.
Furthermore, this research seeks to explore the relationship between hydrogel microstructure and degradation patterns, utilizing advanced imaging techniques such as confocal microscopy, atomic force microscopy, and scanning electron microscopy to visualize degradation processes at the microscale. Understanding these structure-property relationships will facilitate the rational design of next-generation antimicrobial hydrogels with optimized degradation profiles.
The ultimate goal is to bridge the gap between theoretical understanding and practical application, providing industry stakeholders with actionable insights for developing hydrogel-based antimicrobial products with predictable lifetimes and performance characteristics across diverse application environments.
Market Applications and Demand Analysis for Antimicrobial Hydrogels
The antimicrobial hydrogel market has experienced significant growth in recent years, driven primarily by increasing healthcare-associated infections and the rising demand for advanced wound care solutions. The global antimicrobial hydrogel market was valued at approximately $10.2 billion in 2022 and is projected to reach $16.5 billion by 2028, representing a compound annual growth rate of 8.3%.
Healthcare applications dominate the market landscape, accounting for over 65% of the total market share. Within this segment, wound dressing applications represent the largest sub-segment due to the critical need for infection control in chronic wounds such as diabetic ulcers, pressure sores, and surgical wounds. The aging population and rising prevalence of chronic diseases have substantially increased demand for these applications.
The personal care and cosmetics industry has emerged as the second-largest application area, with antimicrobial hydrogels being incorporated into various skincare products, hand sanitizers, and hygiene products. This segment has witnessed accelerated growth following the COVID-19 pandemic, which heightened consumer awareness regarding personal hygiene and antimicrobial products.
Regionally, North America holds the largest market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. The Asia-Pacific region is expected to witness the fastest growth rate due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced wound care products.
A significant market trend is the increasing demand for sustainable and biodegradable antimicrobial hydrogels. Environmental concerns and regulatory pressures have pushed manufacturers to develop eco-friendly formulations that maintain antimicrobial efficacy while reducing environmental impact. This trend aligns with the broader shift toward sustainable healthcare products.
Another emerging application area is drug delivery systems, where antimicrobial hydrogels serve as carriers for controlled release of antibiotics and other therapeutic agents. This application is expected to grow at 10.2% annually, outpacing the overall market growth rate.
The industrial sector represents a smaller but rapidly growing application area, with antimicrobial hydrogels being used in water treatment, food packaging, and textile industries. These applications leverage the material's ability to prevent biofilm formation and microbial contamination in various industrial processes.
Market challenges include the high cost of advanced antimicrobial hydrogels, regulatory hurdles for approval, and the need for extended antimicrobial lifetime without compromising biocompatibility. These challenges present opportunities for innovation in controlled degradation mechanisms that can optimize the release of antimicrobial agents over extended periods.
Healthcare applications dominate the market landscape, accounting for over 65% of the total market share. Within this segment, wound dressing applications represent the largest sub-segment due to the critical need for infection control in chronic wounds such as diabetic ulcers, pressure sores, and surgical wounds. The aging population and rising prevalence of chronic diseases have substantially increased demand for these applications.
The personal care and cosmetics industry has emerged as the second-largest application area, with antimicrobial hydrogels being incorporated into various skincare products, hand sanitizers, and hygiene products. This segment has witnessed accelerated growth following the COVID-19 pandemic, which heightened consumer awareness regarding personal hygiene and antimicrobial products.
Regionally, North America holds the largest market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. The Asia-Pacific region is expected to witness the fastest growth rate due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced wound care products.
A significant market trend is the increasing demand for sustainable and biodegradable antimicrobial hydrogels. Environmental concerns and regulatory pressures have pushed manufacturers to develop eco-friendly formulations that maintain antimicrobial efficacy while reducing environmental impact. This trend aligns with the broader shift toward sustainable healthcare products.
Another emerging application area is drug delivery systems, where antimicrobial hydrogels serve as carriers for controlled release of antibiotics and other therapeutic agents. This application is expected to grow at 10.2% annually, outpacing the overall market growth rate.
The industrial sector represents a smaller but rapidly growing application area, with antimicrobial hydrogels being used in water treatment, food packaging, and textile industries. These applications leverage the material's ability to prevent biofilm formation and microbial contamination in various industrial processes.
Market challenges include the high cost of advanced antimicrobial hydrogels, regulatory hurdles for approval, and the need for extended antimicrobial lifetime without compromising biocompatibility. These challenges present opportunities for innovation in controlled degradation mechanisms that can optimize the release of antimicrobial agents over extended periods.
Current Challenges in Hydrogel Stability and Antimicrobial Persistence
Despite significant advancements in hydrogel technology, several persistent challenges continue to impede the widespread application of antimicrobial hydrogels in clinical settings. The primary challenge remains the unpredictable degradation behavior of hydrogels in complex biological environments. When exposed to enzymatic activity, varying pH levels, and mechanical stresses in vivo, hydrogels often exhibit accelerated degradation rates that deviate significantly from laboratory predictions, resulting in premature structural failure.
The antimicrobial efficacy of hydrogels faces a critical durability issue, with most current formulations demonstrating a rapid decline in antimicrobial activity within 48-72 hours post-application. This short therapeutic window severely limits their utility in treating persistent infections or providing long-term prophylactic protection. The burst release phenomenon, where a significant portion of the antimicrobial payload is discharged within the initial hours, further exacerbates this limitation.
Cross-linking density presents a complex optimization challenge. Higher cross-linking improves mechanical stability but often restricts antimicrobial agent diffusion and release kinetics. Conversely, lower cross-linking enhances antimicrobial release but compromises structural integrity. This fundamental trade-off has yet to be satisfactorily resolved in commercial formulations.
Environmental factors such as temperature fluctuations, ionic strength variations, and protein adsorption significantly alter hydrogel performance in unpredictable ways. These factors can trigger premature degradation, antimicrobial deactivation, or unintended release profiles that compromise therapeutic outcomes. Current mathematical models fail to accurately predict these complex interactions, limiting our ability to design robust systems.
Antimicrobial resistance development presents another significant challenge. Suboptimal release profiles can create concentration gradients below the minimum inhibitory concentration, potentially fostering resistance development. The scientific community has documented several cases where antimicrobial hydrogels have contributed to the emergence of resistant strains, particularly in prolonged application scenarios.
Manufacturing scalability and shelf-life stability remain substantial hurdles. Many promising laboratory formulations encounter significant challenges during scale-up, with batch-to-batch variability in degradation rates and antimicrobial release profiles. Additionally, most current formulations demonstrate limited shelf stability, with significant changes in mechanical properties and antimicrobial potency occurring within 6-12 months of production.
Regulatory pathways for antimicrobial hydrogels remain complex and poorly defined, particularly for novel formulations incorporating multiple active ingredients or degradation-responsive elements. The lack of standardized testing protocols for evaluating long-term degradation behavior and antimicrobial efficacy further complicates the development and approval process for these promising therapeutic platforms.
The antimicrobial efficacy of hydrogels faces a critical durability issue, with most current formulations demonstrating a rapid decline in antimicrobial activity within 48-72 hours post-application. This short therapeutic window severely limits their utility in treating persistent infections or providing long-term prophylactic protection. The burst release phenomenon, where a significant portion of the antimicrobial payload is discharged within the initial hours, further exacerbates this limitation.
Cross-linking density presents a complex optimization challenge. Higher cross-linking improves mechanical stability but often restricts antimicrobial agent diffusion and release kinetics. Conversely, lower cross-linking enhances antimicrobial release but compromises structural integrity. This fundamental trade-off has yet to be satisfactorily resolved in commercial formulations.
Environmental factors such as temperature fluctuations, ionic strength variations, and protein adsorption significantly alter hydrogel performance in unpredictable ways. These factors can trigger premature degradation, antimicrobial deactivation, or unintended release profiles that compromise therapeutic outcomes. Current mathematical models fail to accurately predict these complex interactions, limiting our ability to design robust systems.
Antimicrobial resistance development presents another significant challenge. Suboptimal release profiles can create concentration gradients below the minimum inhibitory concentration, potentially fostering resistance development. The scientific community has documented several cases where antimicrobial hydrogels have contributed to the emergence of resistant strains, particularly in prolonged application scenarios.
Manufacturing scalability and shelf-life stability remain substantial hurdles. Many promising laboratory formulations encounter significant challenges during scale-up, with batch-to-batch variability in degradation rates and antimicrobial release profiles. Additionally, most current formulations demonstrate limited shelf stability, with significant changes in mechanical properties and antimicrobial potency occurring within 6-12 months of production.
Regulatory pathways for antimicrobial hydrogels remain complex and poorly defined, particularly for novel formulations incorporating multiple active ingredients or degradation-responsive elements. The lack of standardized testing protocols for evaluating long-term degradation behavior and antimicrobial efficacy further complicates the development and approval process for these promising therapeutic platforms.
Established Methodologies for Hydrogel Degradation Assessment
01 Hydrogel degradation mechanisms and control strategies
Hydrogels can be designed with controlled degradation profiles through various mechanisms including hydrolytic degradation, enzymatic degradation, and pH-responsive degradation. The degradation behavior can be tailored by modifying crosslinking density, incorporating degradable linkages, or using specific polymers with predetermined degradation rates. These strategies allow for the development of hydrogels with predictable lifespans suitable for various biomedical applications including drug delivery and tissue engineering.- Hydrogel degradation mechanisms and control methods: Various mechanisms influence hydrogel degradation behavior, including hydrolytic degradation, enzymatic breakdown, and environmental factors such as pH and temperature. Controlling these mechanisms through crosslinking density, polymer composition, and incorporation of stabilizing agents can extend the functional lifetime of hydrogels. Degradation profiles can be engineered to match specific application requirements, allowing for predictable material performance over time.
- Antimicrobial agents incorporation and release kinetics: Antimicrobial agents can be incorporated into hydrogels through various methods including physical entrapment, chemical bonding, or nanoparticle loading. The release kinetics of these agents determine their effective lifetime and can be modulated through polymer matrix design, diffusion barriers, and triggered release mechanisms. Sustained release formulations can significantly extend antimicrobial activity duration while reducing the need for frequent reapplication or replacement.
- Biodegradable hydrogels with controlled antimicrobial properties: Biodegradable hydrogels can be designed with controlled degradation rates that synchronize with antimicrobial release profiles. These systems often utilize natural polymers or synthetic biodegradable materials that break down through hydrolysis or enzymatic action. The degradation products can be engineered to be non-toxic and even beneficial in certain applications. This approach allows for temporary antimicrobial protection that naturally dissipates when no longer needed.
- Environmental responsiveness affecting degradation and antimicrobial efficacy: Environmentally responsive hydrogels can change their degradation behavior and antimicrobial release in response to specific stimuli such as temperature, pH, ionic strength, or the presence of specific biomolecules. These smart materials can extend antimicrobial lifetime by releasing active compounds only when needed or by changing their structure to enhance antimicrobial activity. This targeted approach improves efficiency and reduces the potential for antimicrobial resistance development.
- Novel composite hydrogels with enhanced stability and antimicrobial duration: Composite hydrogels incorporating nanomaterials, reinforcing agents, or multiple polymer networks demonstrate enhanced mechanical stability and prolonged antimicrobial activity. These advanced materials often combine the benefits of different components to overcome the limitations of traditional hydrogels. Innovations include self-healing properties, gradient structures, and interpenetrating networks that maintain structural integrity while providing sustained antimicrobial effects over extended periods.
02 Antimicrobial hydrogels with sustained release properties
Antimicrobial hydrogels can be formulated to provide sustained release of active agents over extended periods. These systems incorporate antimicrobial compounds through physical entrapment, chemical conjugation, or in-situ formation. The release kinetics can be controlled through matrix design, allowing for prolonged antimicrobial activity. This sustained release approach helps maintain effective antimicrobial concentrations while reducing the frequency of application or replacement, making them suitable for wound dressings and implantable devices.Expand Specific Solutions03 Environmental factors affecting hydrogel stability and antimicrobial efficacy
Environmental conditions significantly impact hydrogel degradation behavior and antimicrobial performance. Factors such as temperature, pH, ionic strength, and presence of enzymes can accelerate or decelerate degradation rates. Additionally, these factors influence the release and activity of antimicrobial agents. Understanding these environmental influences is crucial for predicting the lifetime of antimicrobial hydrogels in specific applications and ensuring consistent performance across varying conditions.Expand Specific Solutions04 Novel polymer compositions for extended antimicrobial activity
Advanced polymer compositions have been developed to extend the antimicrobial lifetime of hydrogels. These include nanocomposite hydrogels, interpenetrating polymer networks, and hybrid materials combining synthetic and natural polymers. Such compositions can enhance mechanical stability while providing controlled degradation profiles. Additionally, incorporating secondary mechanisms such as self-healing properties or stimuli-responsive elements can further extend functional lifetime, maintaining antimicrobial efficacy over longer periods.Expand Specific Solutions05 Monitoring and predicting hydrogel degradation and antimicrobial performance
Methods for monitoring and predicting hydrogel degradation and antimicrobial performance have been developed to ensure reliable functionality throughout the intended lifetime. These include real-time monitoring systems, predictive mathematical models, and accelerated aging tests. Such approaches enable the assessment of degradation kinetics, antimicrobial release profiles, and long-term stability. This information is valuable for quality control, regulatory compliance, and optimizing formulations to achieve desired performance characteristics.Expand Specific Solutions
Leading Research Groups and Commercial Entities in Antimicrobial Hydrogels
The hydrogel degradation behavior and antimicrobial lifetime analysis market is currently in a growth phase, with increasing applications in wound care, drug delivery, and biomedical devices. The global market size is estimated to reach $5-7 billion by 2025, driven by rising antibiotic resistance concerns and growing demand for advanced wound management solutions. Technologically, the field is moderately mature but rapidly evolving, with academic institutions like Zhejiang University, Nanyang Technological University, and Brown University leading fundamental research, while companies such as Baxter International, Amferia AB, and Pykus Therapeutics are commercializing applications. Industry players like BASF and Covestro are developing advanced polymer formulations, while specialized firms like Bio-Tec Environmental focus on biodegradable solutions, creating a competitive landscape balanced between established corporations and innovative startups.
Baxter International, Inc.
Technical Solution: Baxter International has developed advanced hydrogel technologies with controlled degradation profiles specifically designed for medical applications. Their approach involves cross-linked polymer networks with hydrolytically degradable linkages that can be fine-tuned to provide predictable degradation rates ranging from days to months. The company has pioneered antimicrobial hydrogels incorporating silver nanoparticles and chlorhexidine that maintain effective antimicrobial activity throughout the degradation process. Their proprietary technology enables the creation of dual-function hydrogels that combine controlled drug release with antimicrobial properties, particularly valuable in wound care applications. Baxter's hydrogels undergo rigorous lifetime analysis using accelerated aging studies and mathematical modeling to predict long-term performance in various physiological conditions, ensuring consistent antimicrobial efficacy throughout the intended therapeutic window.
Strengths: Extensive clinical validation in medical settings; sophisticated degradation control mechanisms; integration with existing medical delivery systems. Weaknesses: Higher production costs compared to conventional materials; potential regulatory hurdles for novel antimicrobial agents; limited application outside healthcare settings.
BASF Corp.
Technical Solution: BASF has developed a comprehensive platform for hydrogel degradation control and antimicrobial functionality through their EcoGel™ technology. This system utilizes biodegradable polymers derived from renewable resources combined with precisely engineered cross-linking agents to create hydrogels with predictable degradation profiles. Their approach incorporates environmentally responsive triggers (pH, temperature, enzymatic) that can accelerate or decelerate degradation based on application requirements. BASF's antimicrobial hydrogels feature proprietary encapsulation technology that releases active ingredients at controlled rates, maintaining effective antimicrobial concentrations throughout the material's lifetime. Their analytical framework combines real-time monitoring techniques with advanced computational models to predict antimicrobial efficacy over time under various environmental conditions, enabling precise lifetime predictions for different applications ranging from agricultural products to consumer goods packaging.
Strengths: Extensive polymer chemistry expertise; scalable manufacturing capabilities; broad application potential across multiple industries. Weaknesses: Less specialized in medical applications compared to healthcare-focused competitors; environmental impact concerns with some antimicrobial agents; higher cost compared to conventional materials.
Key Scientific Advances in Controlling Antimicrobial Release Kinetics
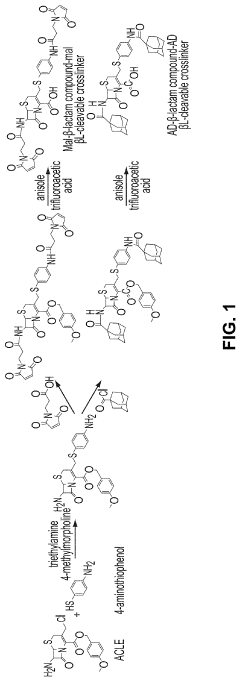
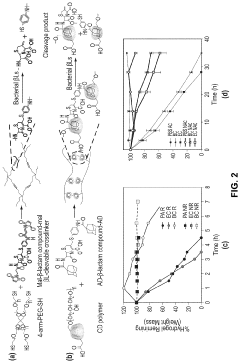
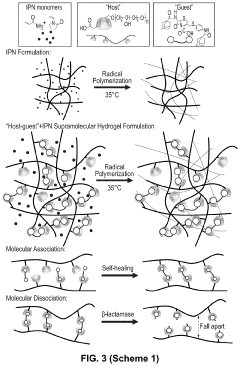
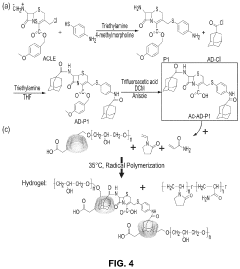
Bacterial beta-lactamase responsive hydrogels
PatentPendingUS20230029034A1
Innovation
- Development of β-lactamase-responsive supramolecular hydrogels that degrade specifically in the presence of β-lactamase enzymes or β-lactamase-producing bacteria, allowing for targeted drug delivery and potential use in infection treatment and diagnostics.
Hydrogel formulations
PatentActiveUS7968085B2
Innovation
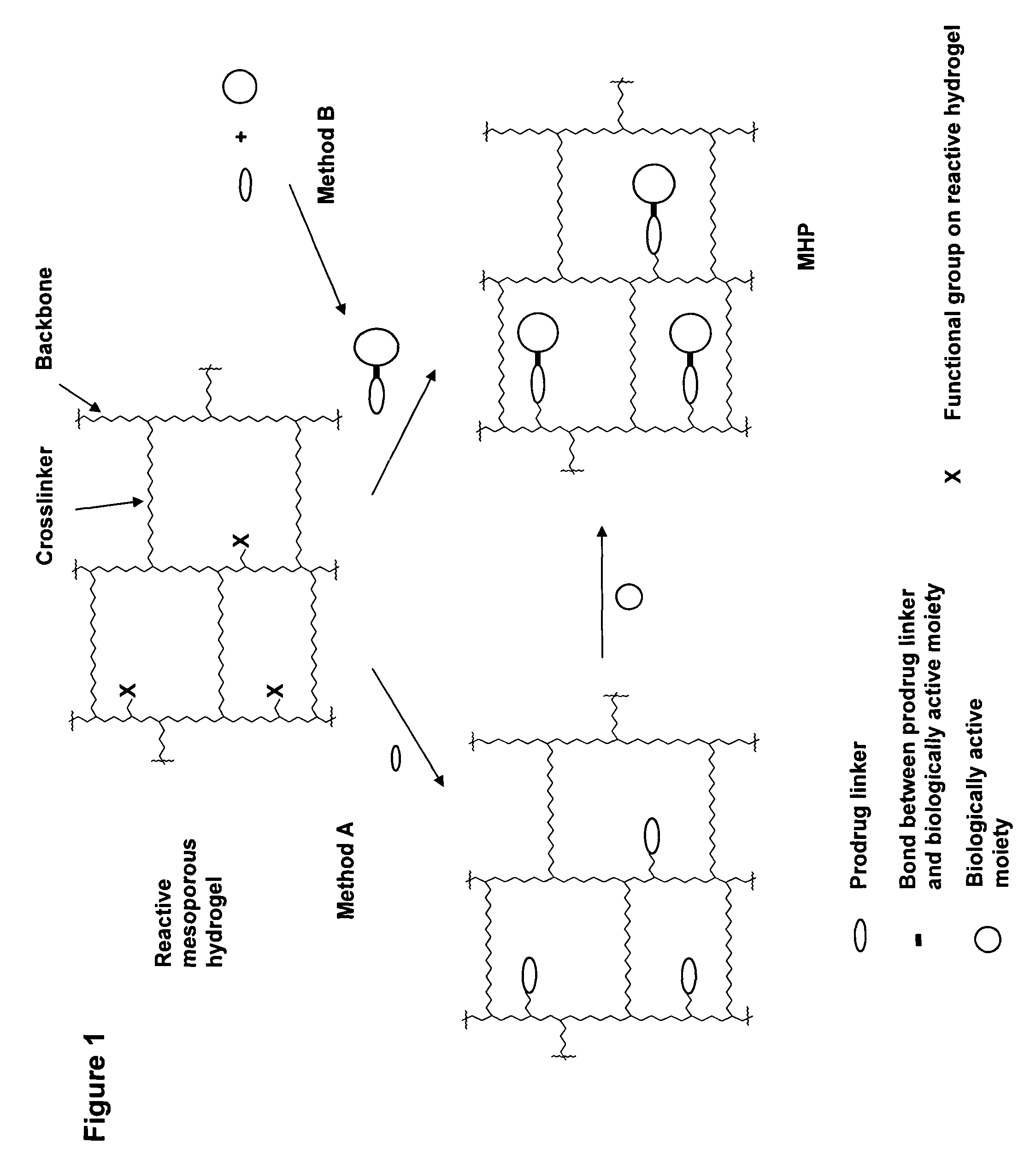
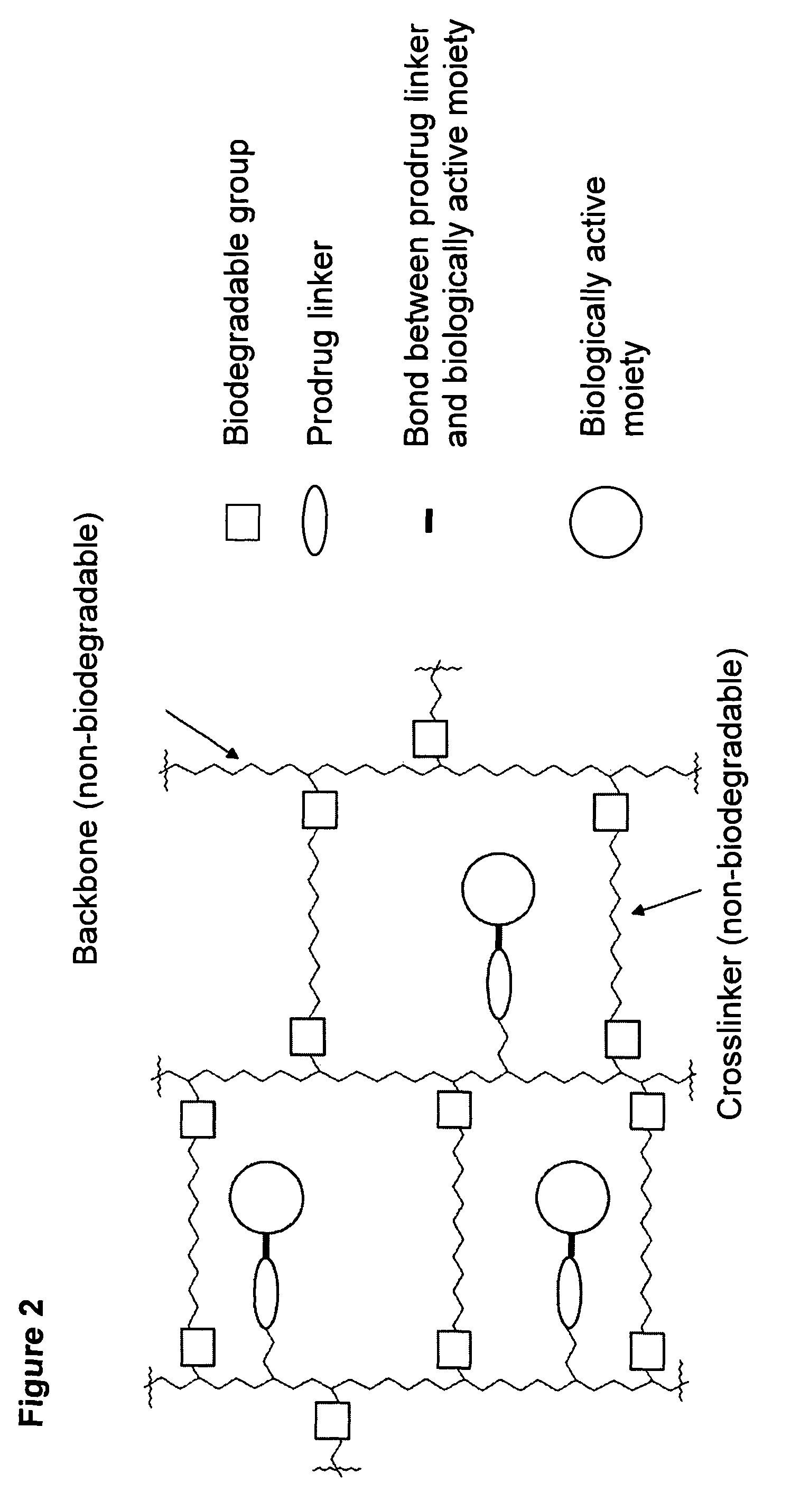
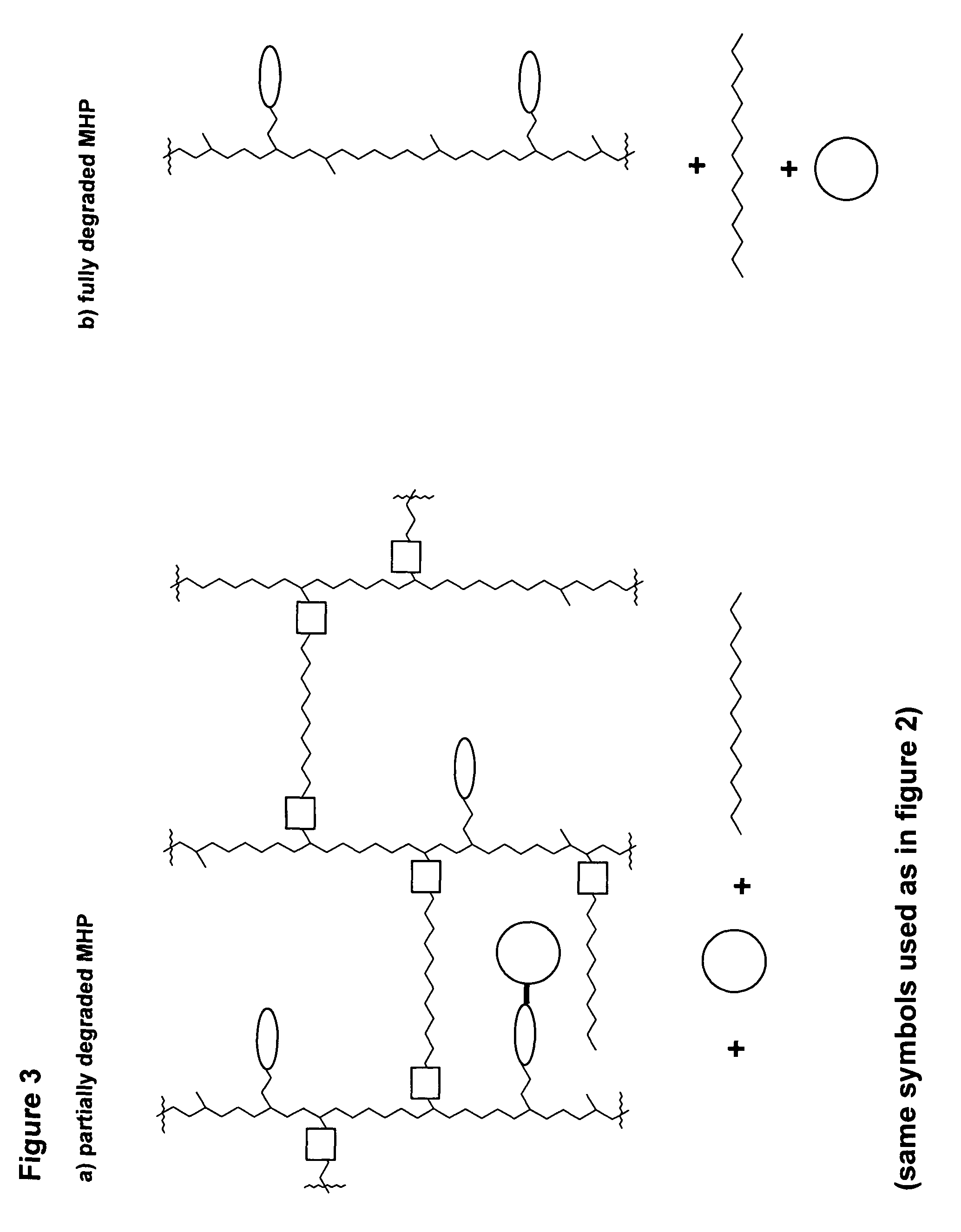
- The development of mesoporous hydrogel prodrugs (MHPs) where biologically active moieties are reversibly linked to a mesoporous hydrogel using a prodrug system, allowing for sustained release independent of the hydrogel's degradation, with controlled release kinetics and minimal modification of the active moiety.
Biocompatibility and Toxicity Considerations
The biocompatibility of hydrogels represents a critical factor in their application as antimicrobial materials in medical settings. When evaluating hydrogel systems designed for controlled degradation and antimicrobial release, comprehensive toxicity assessments must be conducted across multiple biological levels. These evaluations typically include cytotoxicity testing with relevant cell lines, hemolysis assays for blood-contacting applications, and inflammatory response measurements to ensure minimal host tissue reaction.
Current research indicates that the degradation products of antimicrobial hydrogels require particular scrutiny, as these compounds may exhibit different toxicity profiles compared to the intact material. For instance, antimicrobial peptide-loaded hydrogels may release fragments during degradation that interact with host cells differently than the original peptides. Studies have demonstrated that degradation rate significantly influences the local concentration of antimicrobial agents, directly affecting both efficacy and potential cytotoxicity.
The relationship between antimicrobial efficacy and biocompatibility often presents a challenging balance. Higher concentrations of antimicrobial agents typically provide enhanced pathogen elimination but may simultaneously increase toxicity to host tissues. Recent advances in hydrogel design have focused on developing systems with selective toxicity—materials that effectively target microbial cells while remaining benign to mammalian cells through mechanisms such as charge-based selectivity or specific binding domains.
Long-term exposure considerations represent another crucial aspect of biocompatibility assessment. Chronic implantation studies in appropriate animal models have revealed that some hydrogel systems may trigger delayed hypersensitivity reactions or foreign body responses not evident in short-term evaluations. These findings emphasize the importance of extended duration testing that matches the intended clinical application timeframe.
Regulatory frameworks worldwide have established specific guidelines for biocompatibility testing of degradable antimicrobial materials. ISO 10993 standards provide a structured approach to biological evaluation, while FDA guidance documents outline additional considerations for antimicrobial devices. Compliance with these standards requires systematic evaluation of genotoxicity, sensitization potential, and systemic toxicity beyond basic cytotoxicity screening.
Emerging research has begun exploring the integration of anti-inflammatory components within antimicrobial hydrogels to mitigate potential adverse tissue responses. These dual-function materials show promise in applications where infection control must be balanced with tissue healing, such as chronic wound management or implant coatings. The incorporation of anti-inflammatory agents has been shown to improve overall biocompatibility profiles while maintaining antimicrobial efficacy.
Current research indicates that the degradation products of antimicrobial hydrogels require particular scrutiny, as these compounds may exhibit different toxicity profiles compared to the intact material. For instance, antimicrobial peptide-loaded hydrogels may release fragments during degradation that interact with host cells differently than the original peptides. Studies have demonstrated that degradation rate significantly influences the local concentration of antimicrobial agents, directly affecting both efficacy and potential cytotoxicity.
The relationship between antimicrobial efficacy and biocompatibility often presents a challenging balance. Higher concentrations of antimicrobial agents typically provide enhanced pathogen elimination but may simultaneously increase toxicity to host tissues. Recent advances in hydrogel design have focused on developing systems with selective toxicity—materials that effectively target microbial cells while remaining benign to mammalian cells through mechanisms such as charge-based selectivity or specific binding domains.
Long-term exposure considerations represent another crucial aspect of biocompatibility assessment. Chronic implantation studies in appropriate animal models have revealed that some hydrogel systems may trigger delayed hypersensitivity reactions or foreign body responses not evident in short-term evaluations. These findings emphasize the importance of extended duration testing that matches the intended clinical application timeframe.
Regulatory frameworks worldwide have established specific guidelines for biocompatibility testing of degradable antimicrobial materials. ISO 10993 standards provide a structured approach to biological evaluation, while FDA guidance documents outline additional considerations for antimicrobial devices. Compliance with these standards requires systematic evaluation of genotoxicity, sensitization potential, and systemic toxicity beyond basic cytotoxicity screening.
Emerging research has begun exploring the integration of anti-inflammatory components within antimicrobial hydrogels to mitigate potential adverse tissue responses. These dual-function materials show promise in applications where infection control must be balanced with tissue healing, such as chronic wound management or implant coatings. The incorporation of anti-inflammatory agents has been shown to improve overall biocompatibility profiles while maintaining antimicrobial efficacy.
Standardization of Hydrogel Lifetime Testing Protocols
The standardization of hydrogel lifetime testing protocols represents a critical challenge in the advancement of hydrogel technology, particularly for antimicrobial applications. Current testing methodologies exhibit significant variability across research institutions and industries, leading to inconsistent results and difficulties in comparing performance data between different hydrogel formulations.
A comprehensive review of existing protocols reveals three primary areas requiring standardization: degradation measurement techniques, environmental condition controls, and antimicrobial efficacy assessment methods. The lack of unified approaches in these areas has hindered progress in translating promising laboratory results to commercial applications.
Degradation measurement techniques currently employed range from simple gravimetric analysis to sophisticated spectroscopic methods. While gravimetric analysis offers simplicity, it fails to capture the molecular-level changes occurring during degradation. Conversely, techniques such as FTIR spectroscopy provide detailed molecular information but require specialized equipment and expertise.
Environmental condition standardization presents another significant challenge. Hydrogel degradation behavior varies dramatically under different temperature, pH, mechanical stress, and biological exposure conditions. Research indicates that even minor variations in these parameters can lead to substantial differences in degradation rates and antimicrobial efficacy duration.
Several international organizations, including ASTM International and ISO, have begun developing standardized testing frameworks. The ASTM F2900 standard for hydrogel characterization represents a starting point, but requires expansion to specifically address degradation behavior and antimicrobial lifetime assessment.
Industry stakeholders have identified key parameters that should be included in standardized protocols: degradation kinetics under various physiological conditions, mechanical property changes during degradation, antimicrobial release profiles, and correlation between in vitro and in vivo performance. Establishing clear metrics for these parameters would enable meaningful comparison between different hydrogel systems.
Recent collaborative efforts between academic institutions and regulatory bodies have proposed a tiered testing approach. This framework begins with basic physicochemical characterization, followed by controlled degradation studies under standardized conditions, and culminates in application-specific performance testing. This approach balances the need for standardization with the flexibility required to address diverse hydrogel applications.
Implementation of standardized protocols would significantly accelerate development cycles by enabling more efficient screening of candidate materials and formulations. Furthermore, regulatory approval processes would benefit from consistent data generation methodologies, potentially reducing time-to-market for novel hydrogel-based antimicrobial products.
A comprehensive review of existing protocols reveals three primary areas requiring standardization: degradation measurement techniques, environmental condition controls, and antimicrobial efficacy assessment methods. The lack of unified approaches in these areas has hindered progress in translating promising laboratory results to commercial applications.
Degradation measurement techniques currently employed range from simple gravimetric analysis to sophisticated spectroscopic methods. While gravimetric analysis offers simplicity, it fails to capture the molecular-level changes occurring during degradation. Conversely, techniques such as FTIR spectroscopy provide detailed molecular information but require specialized equipment and expertise.
Environmental condition standardization presents another significant challenge. Hydrogel degradation behavior varies dramatically under different temperature, pH, mechanical stress, and biological exposure conditions. Research indicates that even minor variations in these parameters can lead to substantial differences in degradation rates and antimicrobial efficacy duration.
Several international organizations, including ASTM International and ISO, have begun developing standardized testing frameworks. The ASTM F2900 standard for hydrogel characterization represents a starting point, but requires expansion to specifically address degradation behavior and antimicrobial lifetime assessment.
Industry stakeholders have identified key parameters that should be included in standardized protocols: degradation kinetics under various physiological conditions, mechanical property changes during degradation, antimicrobial release profiles, and correlation between in vitro and in vivo performance. Establishing clear metrics for these parameters would enable meaningful comparison between different hydrogel systems.
Recent collaborative efforts between academic institutions and regulatory bodies have proposed a tiered testing approach. This framework begins with basic physicochemical characterization, followed by controlled degradation studies under standardized conditions, and culminates in application-specific performance testing. This approach balances the need for standardization with the flexibility required to address diverse hydrogel applications.
Implementation of standardized protocols would significantly accelerate development cycles by enabling more efficient screening of candidate materials and formulations. Furthermore, regulatory approval processes would benefit from consistent data generation methodologies, potentially reducing time-to-market for novel hydrogel-based antimicrobial products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!