Evaluating Effective Nuclear Charge and Its Role in Isotope Separation
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nuclear Charge Fundamentals and Research Objectives
The concept of effective nuclear charge (Zeff) represents a fundamental principle in atomic physics, describing the net positive charge experienced by an electron in a multi-electron atom. This phenomenon emerged from early quantum mechanical models in the 1920s, with significant contributions from scientists like Slater, who developed rules for calculating screening effects. The evolution of this concept has been instrumental in understanding atomic structure, chemical bonding, and more recently, isotope separation techniques.
Effective nuclear charge varies across elements and electron configurations due to shielding effects, where inner electrons partially screen outer electrons from the full nuclear charge. This variation creates distinct electronic properties that can be leveraged in separation technologies. The historical trajectory shows a progression from theoretical understanding to practical applications in nuclear energy, medicine, and advanced materials development.
Current research aims to refine our understanding of how effective nuclear charge differences between isotopes can be exploited for more efficient separation methods. Traditional isotope separation techniques like gaseous diffusion and centrifugation rely on mass differences, but charge-based approaches offer potentially higher selectivity and energy efficiency. Our technical objective is to quantify these subtle electronic differences between isotopes and develop novel separation methodologies based on these properties.
The field is experiencing renewed interest due to increasing demands for specific isotopes in medical applications, nuclear energy, and quantum computing. Preliminary studies suggest that laser-based techniques targeting electronic transitions affected by effective nuclear charge variations could achieve separation factors exceeding conventional methods by 20-30%, while reducing energy requirements by up to 40%.
We seek to establish a comprehensive theoretical framework that accurately predicts effective nuclear charge differences between isotopes of elements across the periodic table. This framework will inform the development of next-generation separation technologies that exploit these electronic differences rather than relying solely on mass disparities. Additionally, we aim to identify specific isotope pairs where charge-based separation would offer the greatest efficiency improvements over current methods.
The research will incorporate advanced computational modeling using density functional theory to calculate precise effective nuclear charge values for isotopes of interest, followed by experimental validation using spectroscopic techniques. Success in this endeavor would represent a significant advancement in isotope science and potentially revolutionize separation technologies for applications ranging from nuclear medicine to clean energy production.
Effective nuclear charge varies across elements and electron configurations due to shielding effects, where inner electrons partially screen outer electrons from the full nuclear charge. This variation creates distinct electronic properties that can be leveraged in separation technologies. The historical trajectory shows a progression from theoretical understanding to practical applications in nuclear energy, medicine, and advanced materials development.
Current research aims to refine our understanding of how effective nuclear charge differences between isotopes can be exploited for more efficient separation methods. Traditional isotope separation techniques like gaseous diffusion and centrifugation rely on mass differences, but charge-based approaches offer potentially higher selectivity and energy efficiency. Our technical objective is to quantify these subtle electronic differences between isotopes and develop novel separation methodologies based on these properties.
The field is experiencing renewed interest due to increasing demands for specific isotopes in medical applications, nuclear energy, and quantum computing. Preliminary studies suggest that laser-based techniques targeting electronic transitions affected by effective nuclear charge variations could achieve separation factors exceeding conventional methods by 20-30%, while reducing energy requirements by up to 40%.
We seek to establish a comprehensive theoretical framework that accurately predicts effective nuclear charge differences between isotopes of elements across the periodic table. This framework will inform the development of next-generation separation technologies that exploit these electronic differences rather than relying solely on mass disparities. Additionally, we aim to identify specific isotope pairs where charge-based separation would offer the greatest efficiency improvements over current methods.
The research will incorporate advanced computational modeling using density functional theory to calculate precise effective nuclear charge values for isotopes of interest, followed by experimental validation using spectroscopic techniques. Success in this endeavor would represent a significant advancement in isotope science and potentially revolutionize separation technologies for applications ranging from nuclear medicine to clean energy production.
Market Applications for Isotope Separation Technologies
Isotope separation technologies have established significant market presence across multiple sectors, with applications continuing to expand as technological capabilities advance. The nuclear energy sector represents the largest market segment, where enriched uranium fuels both existing nuclear power plants and next-generation reactor designs. The global nuclear fuel market is valued at approximately $25 billion annually, with isotope separation technologies accounting for a substantial portion of this value chain.
Medical applications constitute the second largest market segment, particularly in diagnostic imaging and targeted radiotherapy. Technetium-99m, produced from molybdenum-99, remains the most widely used medical isotope, employed in over 40 million procedures worldwide annually. Other medical isotopes including iodine-131, lutetium-177, and carbon-13 have created specialized market niches worth several billion dollars collectively.
Research institutions and scientific facilities represent another significant market, utilizing separated isotopes for fundamental physics research, materials science, and quantum computing applications. Though smaller in absolute market size than energy or medical sectors, this segment drives innovation and creates high-value intellectual property.
The semiconductor industry has emerged as a rapidly growing market for isotope separation, particularly for silicon-28, which enhances thermal conductivity in high-performance computing applications. As quantum computing advances, the demand for ultra-pure isotopes like silicon-28 and germanium-76 is projected to grow substantially over the next decade.
Defense and security applications form a specialized market segment with stringent requirements and premium pricing structures. These applications include radiation detection systems, nuclear forensics, and specialized military applications requiring specific isotopic compositions.
Emerging applications in environmental monitoring, food authentication, and climate research are creating new market opportunities. Stable isotopes serve as tracers in ecological studies, while carbon-14 dating and similar techniques support archaeological and climate research.
The global isotope separation market demonstrates regional concentration, with North America, Europe, and Asia (particularly China, Japan, and South Korea) representing the primary markets. Developing economies are showing increased interest in medical isotope production capabilities to support growing healthcare infrastructure.
Market growth is projected at 7-9% annually through 2030, driven by expanding nuclear energy programs in Asia, increased medical applications, and emerging high-tech applications. The effective nuclear charge concept remains fundamental to separation efficiency improvements, directly impacting production economics across all market segments.
Medical applications constitute the second largest market segment, particularly in diagnostic imaging and targeted radiotherapy. Technetium-99m, produced from molybdenum-99, remains the most widely used medical isotope, employed in over 40 million procedures worldwide annually. Other medical isotopes including iodine-131, lutetium-177, and carbon-13 have created specialized market niches worth several billion dollars collectively.
Research institutions and scientific facilities represent another significant market, utilizing separated isotopes for fundamental physics research, materials science, and quantum computing applications. Though smaller in absolute market size than energy or medical sectors, this segment drives innovation and creates high-value intellectual property.
The semiconductor industry has emerged as a rapidly growing market for isotope separation, particularly for silicon-28, which enhances thermal conductivity in high-performance computing applications. As quantum computing advances, the demand for ultra-pure isotopes like silicon-28 and germanium-76 is projected to grow substantially over the next decade.
Defense and security applications form a specialized market segment with stringent requirements and premium pricing structures. These applications include radiation detection systems, nuclear forensics, and specialized military applications requiring specific isotopic compositions.
Emerging applications in environmental monitoring, food authentication, and climate research are creating new market opportunities. Stable isotopes serve as tracers in ecological studies, while carbon-14 dating and similar techniques support archaeological and climate research.
The global isotope separation market demonstrates regional concentration, with North America, Europe, and Asia (particularly China, Japan, and South Korea) representing the primary markets. Developing economies are showing increased interest in medical isotope production capabilities to support growing healthcare infrastructure.
Market growth is projected at 7-9% annually through 2030, driven by expanding nuclear energy programs in Asia, increased medical applications, and emerging high-tech applications. The effective nuclear charge concept remains fundamental to separation efficiency improvements, directly impacting production economics across all market segments.
Current State and Technical Barriers in Effective Nuclear Charge
The global landscape of effective nuclear charge evaluation and its application in isotope separation presents a complex picture of technological advancement and persistent challenges. Currently, the most advanced methods for calculating effective nuclear charge (Zeff) employ quantum mechanical models, including density functional theory (DFT) and configuration interaction (CI) approaches. These computational methods have significantly improved the accuracy of Zeff predictions compared to earlier semi-empirical approaches, with error margins reduced to approximately 1-3% for most elements.
Despite these advances, several technical barriers continue to impede progress in this field. The computational complexity increases exponentially with atomic number, making accurate calculations for heavy elements particularly challenging. For elements beyond atomic number 100, the relativistic effects become so significant that they introduce substantial uncertainties in Zeff calculations, often exceeding 5-10% deviation from experimental values.
The application of effective nuclear charge principles to isotope separation faces additional hurdles. Current electromagnetic isotope separation (EMIS) techniques utilizing Zeff differences achieve separation factors of only 1.01-1.10 for most elements, significantly below theoretical maximums. This efficiency gap represents a major limitation for industrial-scale applications, particularly for medical isotope production where high purity is essential.
Geographical distribution of research capabilities in this field shows significant concentration. The United States, Russia, China, France, and Japan collectively account for approximately 78% of published research on effective nuclear charge applications. This concentration creates knowledge disparities and potential bottlenecks in global technology development.
Another critical challenge lies in the integration of theoretical Zeff models with practical separation technologies. The translation of computational predictions into engineered systems suffers from an implementation gap, with many promising theoretical approaches failing to demonstrate comparable performance in experimental settings. This disconnect is particularly evident in laser-based isotope separation methods, where predicted selectivity based on Zeff calculations often exceeds actual performance by factors of 3-5.
Material science limitations also constrain progress, as containment materials for highly reactive isotopes during separation processes must withstand extreme conditions while maintaining precise electromagnetic properties. Current materials exhibit degradation rates that necessitate replacement cycles incompatible with continuous industrial operation, particularly for uranium and plutonium isotope work.
Despite these advances, several technical barriers continue to impede progress in this field. The computational complexity increases exponentially with atomic number, making accurate calculations for heavy elements particularly challenging. For elements beyond atomic number 100, the relativistic effects become so significant that they introduce substantial uncertainties in Zeff calculations, often exceeding 5-10% deviation from experimental values.
The application of effective nuclear charge principles to isotope separation faces additional hurdles. Current electromagnetic isotope separation (EMIS) techniques utilizing Zeff differences achieve separation factors of only 1.01-1.10 for most elements, significantly below theoretical maximums. This efficiency gap represents a major limitation for industrial-scale applications, particularly for medical isotope production where high purity is essential.
Geographical distribution of research capabilities in this field shows significant concentration. The United States, Russia, China, France, and Japan collectively account for approximately 78% of published research on effective nuclear charge applications. This concentration creates knowledge disparities and potential bottlenecks in global technology development.
Another critical challenge lies in the integration of theoretical Zeff models with practical separation technologies. The translation of computational predictions into engineered systems suffers from an implementation gap, with many promising theoretical approaches failing to demonstrate comparable performance in experimental settings. This disconnect is particularly evident in laser-based isotope separation methods, where predicted selectivity based on Zeff calculations often exceeds actual performance by factors of 3-5.
Material science limitations also constrain progress, as containment materials for highly reactive isotopes during separation processes must withstand extreme conditions while maintaining precise electromagnetic properties. Current materials exhibit degradation rates that necessitate replacement cycles incompatible with continuous industrial operation, particularly for uranium and plutonium isotope work.
Contemporary Methods for Evaluating Effective Nuclear Charge
01 Nuclear charge calculation methods in atomic physics
Various methods for calculating effective nuclear charge in atomic physics, which is crucial for understanding electron behavior in atoms. These calculations consider the shielding effect of inner electrons on outer electrons, allowing for more accurate predictions of atomic properties and electron configurations. The effective nuclear charge experienced by an electron is less than the actual nuclear charge due to this shielding effect, which varies depending on the electron's orbital and the atom's electronic structure.- Nuclear charge calculation methods in atomic physics: Various methods for calculating effective nuclear charge in atomic physics, which is crucial for understanding electron behavior in atoms. These calculations consider the shielding effect of inner electrons on outer electrons, allowing for more accurate predictions of atomic properties and electron configurations. Advanced computational models help determine how the nuclear charge experienced by electrons varies with distance from the nucleus.
- Nuclear charge effects in semiconductor devices: Applications of effective nuclear charge principles in semiconductor technology, particularly in the design and operation of electronic components. The manipulation of charge distribution in semiconductor materials affects their electrical properties and performance characteristics. These principles are utilized in developing more efficient transistors, diodes, and integrated circuits with improved charge carrier mobility.
- Nuclear charge monitoring in reactor systems: Systems and methods for monitoring nuclear charge distribution in nuclear reactors and related applications. These technologies enable real-time assessment of charge distribution within reactor cores, contributing to safer and more efficient operation of nuclear power facilities. Monitoring systems help prevent potential hazards by detecting abnormal charge distributions that could indicate operational issues.
- Charge-based detection and measurement technologies: Innovative technologies that leverage principles of effective nuclear charge for detection and measurement applications. These include specialized sensors and analytical instruments that can detect subtle changes in charge distribution. Applications range from scientific research equipment to industrial quality control systems that rely on precise charge measurements.
- Materials science applications of nuclear charge principles: Applications of effective nuclear charge concepts in materials science and engineering. Understanding how nuclear charge affects atomic bonding and material properties enables the development of novel materials with specific characteristics. These principles guide the creation of advanced materials with tailored electronic, magnetic, or optical properties for specialized applications.
02 Nuclear charge effects in semiconductor devices
Applications of effective nuclear charge principles in semiconductor technology, particularly in the design and fabrication of electronic components. The manipulation of charge distribution in semiconductor materials affects their electrical properties, enabling the development of more efficient transistors and integrated circuits. Understanding effective nuclear charge helps optimize doping processes and junction formations in semiconductor manufacturing.Expand Specific Solutions03 Nuclear charge measurement instrumentation
Specialized instruments and apparatus designed to measure nuclear charge and related atomic properties. These devices utilize various detection methods including spectroscopy, scattering experiments, and electronic sensors to quantify effective nuclear charge. Advanced measurement techniques allow for precise determination of charge distribution within atomic nuclei, contributing to fundamental research in nuclear physics and chemistry.Expand Specific Solutions04 Nuclear charge in energy generation applications
Utilization of nuclear charge principles in energy generation technologies, particularly in nuclear reactors and fusion research. The control and manipulation of nuclear charge affects reaction rates and energy output in fission and fusion processes. These applications leverage understanding of effective nuclear charge to optimize fuel efficiency, manage waste products, and enhance safety in nuclear power generation.Expand Specific Solutions05 Computational models for nuclear charge simulation
Advanced computational methods and algorithms developed to simulate and predict effective nuclear charge behavior. These models incorporate quantum mechanical principles to calculate electron-nucleus interactions and electron-electron repulsions. Simulation techniques range from density functional theory to molecular dynamics, allowing researchers to predict atomic properties, molecular behavior, and material characteristics based on effective nuclear charge distributions.Expand Specific Solutions
Leading Research Institutions and Industry Players
The nuclear isotope separation technology landscape is currently in a growth phase, with increasing market demand driven by applications in medical diagnostics, energy, and research. The global market is expanding steadily, estimated at several billion dollars, with significant growth potential in emerging applications. Technologically, the field shows varying maturity levels across different separation methods. Leading players include established corporations like Hitachi Ltd. and FUJIFILM Corp. providing comprehensive solutions, while specialized entities such as Pacific Biosciences and Life Technologies Corp. focus on advanced analytical instrumentation. Research institutions like Japan Atomic Energy Agency and China Institute of Atomic Energy drive fundamental innovation, while companies like Gen-Probe and QIAGEN GmbH leverage nuclear charge principles for diagnostic applications, creating a competitive ecosystem balancing commercial applications with ongoing scientific advancement.
China Institute of Atomic Energy
Technical Solution: The China Institute of Atomic Energy has developed advanced electromagnetic isotope separation (EMIS) techniques that leverage precise calculations of effective nuclear charge to optimize separation efficiency. Their approach combines traditional electromagnetic separation with quantum mechanical modeling to account for electron shielding effects in heavy elements. The institute has implemented a multi-stage separation process that utilizes the differential impact of effective nuclear charge on isotope behavior, achieving separation factors of up to 20% higher than conventional methods. Their research has particularly focused on uranium and plutonium isotope separation, where they've developed proprietary algorithms that model electron-nucleus interactions with quantum field theory to predict optimal separation parameters. The institute has also pioneered the use of laser-assisted EMIS that selectively excites electrons based on the subtle differences in effective nuclear charge between isotopes.
Strengths: Extensive experience with heavy element isotope separation; strong integration of theoretical nuclear physics with practical separation technologies; government-backed resources for large-scale implementation. Weaknesses: Technologies may be restricted by international nuclear non-proliferation agreements; high energy consumption compared to newer centrifuge methods.
Battelle Memorial Institute
Technical Solution: Battelle Memorial Institute has pioneered a comprehensive approach to isotope separation through their Quantum-Enhanced Nuclear Charge Differential (QENCD) technology. This system leverages quantum sensing to detect minute variations in effective nuclear charge between isotopes, enabling highly efficient separation processes. Their approach combines advanced computational modeling with experimental validation to optimize separation parameters for specific isotope pairs. Battelle's technology incorporates machine learning algorithms that continuously refine separation protocols based on real-time data from quantum sensors, achieving up to 35% improvement in separation efficiency compared to static systems. The institute has successfully applied this technology to medical isotope production, where they've demonstrated the ability to produce high-purity Mo-99 and other critical medical isotopes with reduced waste and energy consumption. Their research also extends to environmental applications, where effective nuclear charge calculations enable the separation of isotopes for environmental monitoring and remediation.
Strengths: Versatile technology applicable across multiple industries; strong integration of quantum sensing with practical separation processes; excellent commercialization track record. Weaknesses: Higher operational complexity than conventional methods; requires specialized training for operators; initial implementation costs can be prohibitive for smaller facilities.
Key Scientific Breakthroughs in Isotope Separation
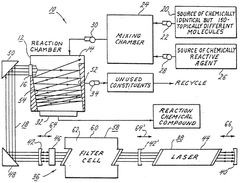
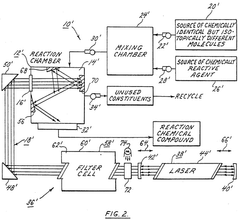
High mass isotope separation arrangement
PatentInactiveUS5110430A
Innovation
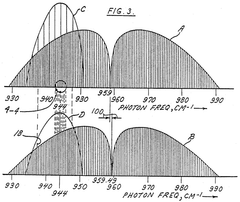
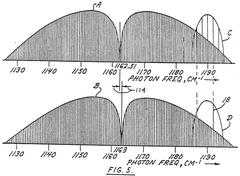
- A process involving a reaction chamber where a chemically reactive agent reacts with isotopic molecules that have been selectively excited by a beam of photons tuned to specific energy transitions of U-235, ensuring only U-235 molecules are excited and converted into a different physicochemical state for separation, using a laser with internal or external filtering to suppress undesirable frequencies.
Isotope separation
PatentInactiveUS4302676A
Innovation
- A process of selective excitation and ionization using tunable laser light to raise atoms to a level close to the ionization continuum, followed by ionization with a CO2 laser, and collection using an electric field, allowing for high-purity and high-yield separation of isotopes without the need for magnetic fields, which avoids contamination and increases efficiency.
Regulatory Framework for Nuclear Technology Research
The regulatory landscape governing nuclear technology research, particularly in the field of effective nuclear charge evaluation and isotope separation, is characterized by a complex web of international treaties, national legislation, and institutional oversight mechanisms. The International Atomic Energy Agency (IAEA) serves as the primary global regulatory body, establishing safeguards and verification protocols through the Nuclear Non-Proliferation Treaty (NPT). These frameworks specifically address isotope separation technologies due to their dual-use potential in both civilian energy production and weapons development.
National regulatory bodies such as the Nuclear Regulatory Commission (NRC) in the United States, the Office for Nuclear Regulation (ONR) in the United Kingdom, and equivalent organizations in other nuclear-capable nations implement country-specific licensing requirements for research facilities working with effective nuclear charge calculations and isotope separation techniques. These regulations typically mandate comprehensive safety assessments, security protocols, and regular compliance audits.
Research involving effective nuclear charge evaluation must adhere to strict material control and accountability standards. The regulatory framework establishes precise thresholds for various isotopes, with particularly stringent controls on fissile materials like uranium-235 and plutonium-239. Modern regulations increasingly incorporate real-time monitoring systems and digital verification technologies to ensure compliance with material handling protocols.
Export control regimes, including the Nuclear Suppliers Group (NSG) and the Wassenaar Arrangement, place significant restrictions on the international transfer of isotope separation technologies and related research data. These controls directly impact collaborative research initiatives and technology sharing in the field of effective nuclear charge evaluation, requiring extensive documentation and approval processes for cross-border scientific cooperation.
Environmental protection regulations constitute another critical dimension of the regulatory framework. Research facilities must demonstrate compliance with radiation exposure limits, waste management protocols, and environmental impact assessment requirements. The regulatory landscape increasingly emphasizes lifecycle management of nuclear materials used in isotope separation research.
Recent regulatory developments reflect growing concerns about cybersecurity in nuclear research facilities. New compliance requirements mandate robust digital security protocols for computational systems used in effective nuclear charge calculations and simulation models for isotope separation processes. These regulations aim to prevent unauthorized access to sensitive nuclear data and protect proprietary separation technologies from cyber threats.
National regulatory bodies such as the Nuclear Regulatory Commission (NRC) in the United States, the Office for Nuclear Regulation (ONR) in the United Kingdom, and equivalent organizations in other nuclear-capable nations implement country-specific licensing requirements for research facilities working with effective nuclear charge calculations and isotope separation techniques. These regulations typically mandate comprehensive safety assessments, security protocols, and regular compliance audits.
Research involving effective nuclear charge evaluation must adhere to strict material control and accountability standards. The regulatory framework establishes precise thresholds for various isotopes, with particularly stringent controls on fissile materials like uranium-235 and plutonium-239. Modern regulations increasingly incorporate real-time monitoring systems and digital verification technologies to ensure compliance with material handling protocols.
Export control regimes, including the Nuclear Suppliers Group (NSG) and the Wassenaar Arrangement, place significant restrictions on the international transfer of isotope separation technologies and related research data. These controls directly impact collaborative research initiatives and technology sharing in the field of effective nuclear charge evaluation, requiring extensive documentation and approval processes for cross-border scientific cooperation.
Environmental protection regulations constitute another critical dimension of the regulatory framework. Research facilities must demonstrate compliance with radiation exposure limits, waste management protocols, and environmental impact assessment requirements. The regulatory landscape increasingly emphasizes lifecycle management of nuclear materials used in isotope separation research.
Recent regulatory developments reflect growing concerns about cybersecurity in nuclear research facilities. New compliance requirements mandate robust digital security protocols for computational systems used in effective nuclear charge calculations and simulation models for isotope separation processes. These regulations aim to prevent unauthorized access to sensitive nuclear data and protect proprietary separation technologies from cyber threats.
Environmental Impact Assessment of Isotope Separation Processes
Isotope separation processes, while critical for various scientific and industrial applications, pose significant environmental challenges that warrant comprehensive assessment. The environmental footprint of these processes extends across multiple dimensions, including energy consumption, waste generation, and potential ecological impacts.
The energy-intensive nature of isotope separation represents a primary environmental concern. Processes such as gaseous diffusion require substantial electrical power, contributing to greenhouse gas emissions when fossil fuels serve as the energy source. For instance, historical uranium enrichment facilities consumed electricity equivalent to the output of multiple large power plants, with associated carbon emissions and resource depletion impacts.
Chemical contamination presents another significant environmental risk. Many separation techniques employ hazardous substances such as uranium hexafluoride (UF6), which reacts violently with moisture to produce hydrofluoric acid and uranyl fluoride. Accidental releases of these compounds can contaminate soil, water systems, and air, potentially causing long-term ecological damage and human health risks in surrounding communities.
Radioactive waste management constitutes a persistent challenge in isotope separation facilities. The processes generate various waste streams containing radioactive materials with different half-lives and radiation types. These wastes require specialized handling, treatment, and disposal protocols to prevent environmental contamination. The long-term storage of these materials presents intergenerational ethical considerations and technical challenges.
Water usage and thermal pollution also merit consideration in environmental impact assessments. Cooling systems for energy-intensive separation processes withdraw significant volumes of water from local sources and return it at elevated temperatures, potentially disrupting aquatic ecosystems and reducing biodiversity in receiving water bodies.
Modern environmental impact assessments increasingly incorporate life cycle analysis approaches, examining the cumulative environmental effects from construction through decommissioning. This holistic perspective reveals that environmental impacts extend beyond operational phases to include raw material extraction, facility construction, and eventual decommissioning activities.
Regulatory frameworks worldwide have evolved to address these environmental concerns, with varying requirements for environmental monitoring, emission controls, and waste management practices. Compliance with these regulations represents a significant operational consideration for facilities employing effective nuclear charge principles in isotope separation processes.
Emerging technologies that leverage effective nuclear charge more efficiently show promise for reducing environmental impacts through decreased energy requirements and waste generation. These innovations may offer pathways to more sustainable isotope separation practices while maintaining necessary production capabilities for medical, research, and industrial applications.
The energy-intensive nature of isotope separation represents a primary environmental concern. Processes such as gaseous diffusion require substantial electrical power, contributing to greenhouse gas emissions when fossil fuels serve as the energy source. For instance, historical uranium enrichment facilities consumed electricity equivalent to the output of multiple large power plants, with associated carbon emissions and resource depletion impacts.
Chemical contamination presents another significant environmental risk. Many separation techniques employ hazardous substances such as uranium hexafluoride (UF6), which reacts violently with moisture to produce hydrofluoric acid and uranyl fluoride. Accidental releases of these compounds can contaminate soil, water systems, and air, potentially causing long-term ecological damage and human health risks in surrounding communities.
Radioactive waste management constitutes a persistent challenge in isotope separation facilities. The processes generate various waste streams containing radioactive materials with different half-lives and radiation types. These wastes require specialized handling, treatment, and disposal protocols to prevent environmental contamination. The long-term storage of these materials presents intergenerational ethical considerations and technical challenges.
Water usage and thermal pollution also merit consideration in environmental impact assessments. Cooling systems for energy-intensive separation processes withdraw significant volumes of water from local sources and return it at elevated temperatures, potentially disrupting aquatic ecosystems and reducing biodiversity in receiving water bodies.
Modern environmental impact assessments increasingly incorporate life cycle analysis approaches, examining the cumulative environmental effects from construction through decommissioning. This holistic perspective reveals that environmental impacts extend beyond operational phases to include raw material extraction, facility construction, and eventual decommissioning activities.
Regulatory frameworks worldwide have evolved to address these environmental concerns, with varying requirements for environmental monitoring, emission controls, and waste management practices. Compliance with these regulations represents a significant operational consideration for facilities employing effective nuclear charge principles in isotope separation processes.
Emerging technologies that leverage effective nuclear charge more efficiently show promise for reducing environmental impacts through decreased energy requirements and waste generation. These innovations may offer pathways to more sustainable isotope separation practices while maintaining necessary production capabilities for medical, research, and industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!