Exploring Sodium Percarbonate's Function in Academic Laboratory Cleaners
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate in Lab Cleaning: Background and Objectives
Sodium percarbonate has emerged as a significant component in academic laboratory cleaning solutions, revolutionizing the approach to maintaining sterile and safe research environments. This powerful oxidizing agent, with its chemical formula 2Na2CO3·3H2O2, has gained prominence due to its effectiveness in breaking down organic matter and its environmentally friendly nature.
The evolution of laboratory cleaning practices has been driven by the need for increasingly stringent hygiene standards and the growing awareness of environmental impacts. Traditional cleaning methods often relied on harsh chemicals that posed risks to both laboratory personnel and the environment. The introduction of sodium percarbonate marks a pivotal shift towards safer, more sustainable cleaning practices without compromising on efficacy.
Sodium percarbonate's unique properties stem from its ability to release hydrogen peroxide when dissolved in water. This controlled release mechanism provides a sustained cleaning action, effectively tackling stubborn stains, organic residues, and microbial contaminants commonly found in laboratory settings. Its versatility extends across various laboratory surfaces, including glassware, benchtops, and equipment, making it an invaluable tool in maintaining laboratory hygiene.
The primary objective of exploring sodium percarbonate's function in academic laboratory cleaners is to optimize its application for enhanced cleaning performance while minimizing potential drawbacks. This involves investigating its effectiveness against different types of laboratory contaminants, its compatibility with various laboratory materials, and its long-term impact on equipment integrity.
Furthermore, research aims to elucidate the optimal concentration and application methods for sodium percarbonate in different laboratory contexts. This includes studying its synergistic effects when combined with other cleaning agents and exploring potential modifications to enhance its stability and shelf life.
Another crucial aspect of this exploration is to assess the environmental footprint of sodium percarbonate-based cleaners compared to traditional cleaning agents. This encompasses evaluating its biodegradability, impact on wastewater treatment systems, and overall lifecycle analysis.
As academic institutions increasingly prioritize sustainability alongside research excellence, understanding the full potential of sodium percarbonate in laboratory cleaning becomes paramount. This research not only contributes to improving laboratory safety and cleanliness but also aligns with broader goals of reducing the environmental impact of scientific research activities.
The evolution of laboratory cleaning practices has been driven by the need for increasingly stringent hygiene standards and the growing awareness of environmental impacts. Traditional cleaning methods often relied on harsh chemicals that posed risks to both laboratory personnel and the environment. The introduction of sodium percarbonate marks a pivotal shift towards safer, more sustainable cleaning practices without compromising on efficacy.
Sodium percarbonate's unique properties stem from its ability to release hydrogen peroxide when dissolved in water. This controlled release mechanism provides a sustained cleaning action, effectively tackling stubborn stains, organic residues, and microbial contaminants commonly found in laboratory settings. Its versatility extends across various laboratory surfaces, including glassware, benchtops, and equipment, making it an invaluable tool in maintaining laboratory hygiene.
The primary objective of exploring sodium percarbonate's function in academic laboratory cleaners is to optimize its application for enhanced cleaning performance while minimizing potential drawbacks. This involves investigating its effectiveness against different types of laboratory contaminants, its compatibility with various laboratory materials, and its long-term impact on equipment integrity.
Furthermore, research aims to elucidate the optimal concentration and application methods for sodium percarbonate in different laboratory contexts. This includes studying its synergistic effects when combined with other cleaning agents and exploring potential modifications to enhance its stability and shelf life.
Another crucial aspect of this exploration is to assess the environmental footprint of sodium percarbonate-based cleaners compared to traditional cleaning agents. This encompasses evaluating its biodegradability, impact on wastewater treatment systems, and overall lifecycle analysis.
As academic institutions increasingly prioritize sustainability alongside research excellence, understanding the full potential of sodium percarbonate in laboratory cleaning becomes paramount. This research not only contributes to improving laboratory safety and cleanliness but also aligns with broader goals of reducing the environmental impact of scientific research activities.
Market Analysis for Academic Laboratory Cleaning Solutions
The academic laboratory cleaning solutions market has experienced significant growth in recent years, driven by the increasing focus on safety, hygiene, and environmental sustainability in educational institutions. This market segment is characterized by a diverse range of products designed to meet the specific cleaning needs of academic laboratories, including general-purpose cleaners, specialized surface disinfectants, and equipment-specific cleaning agents.
The demand for effective and safe cleaning solutions in academic laboratories has been further amplified by the global pandemic, which has heightened awareness of the importance of maintaining sterile environments. This has led to a surge in the adoption of advanced cleaning products and technologies, with a particular emphasis on those that offer both powerful cleaning capabilities and minimal environmental impact.
Sodium percarbonate, a key component in many academic laboratory cleaners, has gained prominence due to its dual functionality as both a cleaning agent and a disinfectant. Its ability to release hydrogen peroxide when dissolved in water makes it an effective oxidizing agent, capable of breaking down organic matter and eliminating bacteria and viruses. This property has made sodium percarbonate-based cleaners increasingly popular in academic settings, where they can address a wide range of cleaning challenges while minimizing the use of harsh chemicals.
The market for academic laboratory cleaning solutions is segmented by product type, application area, and geographic region. Product types include liquid cleaners, powders, wipes, and specialized formulations for specific laboratory equipment. Application areas span various scientific disciplines, including chemistry, biology, physics, and medical research laboratories, each with its unique cleaning requirements.
Geographically, North America and Europe currently dominate the market, owing to their well-established educational infrastructure and stringent safety regulations. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in education and research facilities across countries like China, India, and South Korea.
Key market trends include the growing preference for eco-friendly and biodegradable cleaning solutions, the integration of nanotechnology in cleaning products for enhanced efficacy, and the development of multi-functional cleaning agents that can address multiple cleaning needs simultaneously. The market is also seeing a shift towards automated cleaning systems and robotics, particularly in larger research institutions, to improve efficiency and consistency in laboratory maintenance.
The demand for effective and safe cleaning solutions in academic laboratories has been further amplified by the global pandemic, which has heightened awareness of the importance of maintaining sterile environments. This has led to a surge in the adoption of advanced cleaning products and technologies, with a particular emphasis on those that offer both powerful cleaning capabilities and minimal environmental impact.
Sodium percarbonate, a key component in many academic laboratory cleaners, has gained prominence due to its dual functionality as both a cleaning agent and a disinfectant. Its ability to release hydrogen peroxide when dissolved in water makes it an effective oxidizing agent, capable of breaking down organic matter and eliminating bacteria and viruses. This property has made sodium percarbonate-based cleaners increasingly popular in academic settings, where they can address a wide range of cleaning challenges while minimizing the use of harsh chemicals.
The market for academic laboratory cleaning solutions is segmented by product type, application area, and geographic region. Product types include liquid cleaners, powders, wipes, and specialized formulations for specific laboratory equipment. Application areas span various scientific disciplines, including chemistry, biology, physics, and medical research laboratories, each with its unique cleaning requirements.
Geographically, North America and Europe currently dominate the market, owing to their well-established educational infrastructure and stringent safety regulations. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in education and research facilities across countries like China, India, and South Korea.
Key market trends include the growing preference for eco-friendly and biodegradable cleaning solutions, the integration of nanotechnology in cleaning products for enhanced efficacy, and the development of multi-functional cleaning agents that can address multiple cleaning needs simultaneously. The market is also seeing a shift towards automated cleaning systems and robotics, particularly in larger research institutions, to improve efficiency and consistency in laboratory maintenance.
Current Challenges in Laboratory Cleaning Technologies
Laboratory cleaning technologies face several significant challenges in the current academic and research environment. One of the primary issues is the increasing complexity of contaminants encountered in modern laboratories. As research methodologies become more sophisticated, the range of chemicals, biological agents, and nanomaterials used in experiments expands, leading to more diverse and persistent forms of contamination. This diversity requires cleaning solutions that can effectively address a broad spectrum of substances without causing cross-reactivity or leaving residues that might interfere with subsequent experiments.
Another challenge is the need for cleaning technologies that are both highly effective and environmentally friendly. Traditional cleaning agents often contain harsh chemicals that can be harmful to both laboratory personnel and the environment. There is a growing demand for green cleaning solutions that maintain high standards of cleanliness while minimizing ecological impact. This shift towards sustainability is complicated by the need to ensure that eco-friendly cleaners can match or exceed the performance of conventional products, particularly in sensitive research environments where even trace contaminants can compromise results.
The increasing focus on safety and health in laboratory settings also presents challenges for cleaning technologies. Cleaning products must not only remove visible contaminants but also effectively neutralize or remove potentially hazardous substances that may not be immediately apparent. This includes addressing concerns about microbial contamination, allergens, and volatile organic compounds (VOCs) that can affect air quality and worker health. Developing cleaning solutions that can achieve these goals while being safe for regular use by laboratory staff requires a delicate balance of efficacy and safety.
Furthermore, the automation of laboratory processes has created new demands for cleaning technologies. As more laboratories adopt robotic systems and high-throughput equipment, there is a need for cleaning solutions that can be integrated into automated workflows. This includes developing cleaners that are compatible with robotic handling systems and can be applied and removed without manual intervention. The challenge lies in creating cleaning technologies that can operate effectively within these automated systems while still addressing the full range of cleaning requirements.
Lastly, the economic pressures faced by many academic institutions pose a challenge for the adoption of advanced cleaning technologies. While there is a clear need for more effective and specialized cleaning solutions, budget constraints often limit the ability of laboratories to invest in new cleaning systems or products. This creates a demand for cost-effective cleaning technologies that can deliver superior performance without significantly increasing operational costs, a balance that is often difficult to achieve in practice.
Another challenge is the need for cleaning technologies that are both highly effective and environmentally friendly. Traditional cleaning agents often contain harsh chemicals that can be harmful to both laboratory personnel and the environment. There is a growing demand for green cleaning solutions that maintain high standards of cleanliness while minimizing ecological impact. This shift towards sustainability is complicated by the need to ensure that eco-friendly cleaners can match or exceed the performance of conventional products, particularly in sensitive research environments where even trace contaminants can compromise results.
The increasing focus on safety and health in laboratory settings also presents challenges for cleaning technologies. Cleaning products must not only remove visible contaminants but also effectively neutralize or remove potentially hazardous substances that may not be immediately apparent. This includes addressing concerns about microbial contamination, allergens, and volatile organic compounds (VOCs) that can affect air quality and worker health. Developing cleaning solutions that can achieve these goals while being safe for regular use by laboratory staff requires a delicate balance of efficacy and safety.
Furthermore, the automation of laboratory processes has created new demands for cleaning technologies. As more laboratories adopt robotic systems and high-throughput equipment, there is a need for cleaning solutions that can be integrated into automated workflows. This includes developing cleaners that are compatible with robotic handling systems and can be applied and removed without manual intervention. The challenge lies in creating cleaning technologies that can operate effectively within these automated systems while still addressing the full range of cleaning requirements.
Lastly, the economic pressures faced by many academic institutions pose a challenge for the adoption of advanced cleaning technologies. While there is a clear need for more effective and specialized cleaning solutions, budget constraints often limit the ability of laboratories to invest in new cleaning systems or products. This creates a demand for cost-effective cleaning technologies that can deliver superior performance without significantly increasing operational costs, a balance that is often difficult to achieve in practice.
Existing Sodium Percarbonate-based Cleaning Formulations
01 Oxidizing and bleaching properties
Sodium percarbonate is an effective cleaning agent due to its strong oxidizing and bleaching properties. When dissolved in water, it releases hydrogen peroxide, which acts as a powerful oxidizer capable of breaking down organic stains and killing bacteria. This makes it particularly useful for removing tough stains and disinfecting surfaces.- Oxidizing and bleaching properties: Sodium percarbonate is an effective cleaning agent due to its strong oxidizing and bleaching properties. When dissolved in water, it releases hydrogen peroxide, which can break down and remove tough stains, dirt, and organic matter. This makes it particularly useful for laundry applications and general household cleaning.
- Stability and storage improvements: Various methods have been developed to improve the stability and storage properties of sodium percarbonate. These include coating the particles with protective layers, adding stabilizing agents, and controlling moisture content. Enhanced stability ensures that the cleaning effectiveness of sodium percarbonate is maintained over time, even under challenging storage conditions.
- Synergistic effects with other cleaning agents: Sodium percarbonate's cleaning effectiveness can be enhanced when combined with other cleaning agents such as surfactants, enzymes, or activators. These combinations can lead to improved stain removal, better soil lifting, and increased overall cleaning performance across various applications.
- Environmental and safety considerations: Sodium percarbonate is considered an environmentally friendly cleaning agent as it breaks down into harmless byproducts (water, oxygen, and sodium carbonate). Its effectiveness in cold water cleaning and ability to replace harsher chemicals make it a preferred choice for eco-friendly cleaning formulations. Safety considerations include proper handling and storage to prevent decomposition and potential reactivity with certain materials.
- Application-specific formulations: The cleaning effectiveness of sodium percarbonate can be optimized for specific applications through tailored formulations. This includes adjusting particle size, incorporating specific additives, or developing controlled-release mechanisms. Such formulations can enhance the performance of sodium percarbonate in various cleaning scenarios, from laundry detergents to industrial cleaning processes.
02 Stability and storage improvements
Various methods have been developed to improve the stability and storage properties of sodium percarbonate. These include coating the particles with protective layers, adding stabilizing agents, and controlling moisture content. Enhanced stability ensures that the cleaning effectiveness of sodium percarbonate is maintained over time, even under challenging storage conditions.Expand Specific Solutions03 Formulation in cleaning products
Sodium percarbonate is commonly formulated into various cleaning products, including laundry detergents, dishwashing agents, and all-purpose cleaners. Its effectiveness can be enhanced by combining it with other cleaning agents, surfactants, and enzymes. These formulations can be tailored for specific cleaning applications, improving overall cleaning performance.Expand Specific Solutions04 Environmental and safety considerations
Sodium percarbonate is considered an environmentally friendly cleaning agent as it breaks down into harmless byproducts (water, oxygen, and sodium carbonate). It is less corrosive and safer to handle compared to some other oxidizing agents. These properties make it a preferred choice for eco-friendly cleaning products and applications where safety is a concern.Expand Specific Solutions05 Application-specific effectiveness
The cleaning effectiveness of sodium percarbonate varies depending on the specific application. It has shown particular efficacy in removing organic stains, whitening fabrics, and disinfecting surfaces. Research has been conducted to optimize its performance in various scenarios, such as low-temperature cleaning, hard water conditions, and specific types of soils or contaminants.Expand Specific Solutions
Key Manufacturers and Suppliers in Laboratory Cleaning Industry
The exploration of sodium percarbonate's function in academic laboratory cleaners is currently in a growth phase, with increasing market demand driven by the need for effective and environmentally friendly cleaning solutions. The global market for laboratory cleaners is expanding, with sodium percarbonate gaining traction due to its dual cleaning and disinfecting properties. Technologically, the field is moderately mature, with established players like Solvay SA, Evonik Operations GmbH, and Henkel AG & Co. KGaA leading innovation. However, there's room for advancement in formulation efficiency and application-specific solutions. Emerging companies such as Zhejiang Jinke Daily Chemical Co. Ltd. and Puyang Hongye Environment Protection New Materials Co., Ltd. are contributing to market diversification and technological improvements, indicating a competitive and evolving landscape in this sector.
Solvay SA
Technical Solution: Solvay SA has developed advanced sodium percarbonate formulations specifically tailored for academic laboratory cleaners. Their technology focuses on enhancing the stability and efficacy of sodium percarbonate in cleaning solutions. They have implemented a proprietary coating process that protects the sodium percarbonate particles from moisture, increasing shelf life and maintaining its cleaning power over time[1]. Solvay's formulation also includes synergistic additives that boost the oxidizing potential of sodium percarbonate, making it more effective in removing organic residues and stains commonly found in laboratory environments[2]. Additionally, they have optimized the particle size distribution to ensure rapid dissolution and uniform dispersion in cleaning solutions, improving overall cleaning performance[3].
Strengths: Enhanced stability and longevity of sodium percarbonate, improved cleaning efficacy through synergistic additives, and optimized dissolution properties. Weaknesses: Potentially higher production costs due to specialized coating and formulation processes, which may impact product pricing.
Evonik Operations GmbH
Technical Solution: Evonik Operations GmbH has developed an innovative approach to incorporating sodium percarbonate in academic laboratory cleaners. Their technology focuses on creating a controlled-release mechanism for sodium percarbonate, allowing for sustained cleaning action over extended periods. This is achieved through a sophisticated encapsulation technique that gradually releases the active oxygen from sodium percarbonate as it comes into contact with water[1]. Evonik has also engineered a pH-responsive release system, ensuring that the sodium percarbonate's cleaning power is activated at the optimal pH for various laboratory cleaning applications[2]. Furthermore, their formulation includes stabilizers that prevent premature decomposition of sodium percarbonate in the presence of metal ions, which are common in laboratory environments[3].
Strengths: Controlled-release mechanism for sustained cleaning action, pH-responsive activation for versatile applications, and improved stability in the presence of metal ions. Weaknesses: Complex formulation may lead to higher production costs and potential challenges in scaling up manufacturing.
Innovative Applications of Sodium Percarbonate in Lab Cleaning
Apparatus and method for generating oxygen from sodium percarbonate and water, including seawater
PatentActiveUS12258267B2
Innovation
- An apparatus and method using sodium percarbonate, water, and potassium iodide, with optional sodium sulfate decahydrate, to generate oxygen gas through a chemical reaction, where the potassium iodide acts as a catalyst and sodium sulfate decahydrate can moderate temperature, allowing for compact and unpressurized transportation and use of seawater or deionized water to produce oxygen.
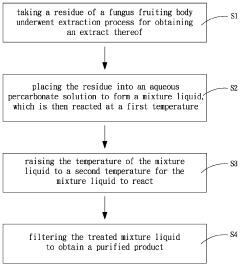
Purification method of fungal cell wall composition
PatentActiveUS20230310529A1
Innovation
- An aqueous percarbonate solution is used for decolorization and digestion, replacing the two-stage treatment with sodium hydroxide and hypochlorite or hydrogen peroxide, allowing for simultaneous decolorization and decomposition at controlled temperatures, reducing waste burden and increasing recovery rates.
Safety Regulations for Chemical Usage in Academic Laboratories
Safety regulations for chemical usage in academic laboratories are crucial for ensuring the well-being of researchers, students, and staff. These regulations typically encompass a wide range of aspects, from proper handling and storage of chemicals to emergency response procedures. In the context of sodium percarbonate's use in laboratory cleaners, specific safety measures must be implemented.
Firstly, proper personal protective equipment (PPE) is essential when handling sodium percarbonate or cleaners containing this compound. This includes wearing safety goggles, chemical-resistant gloves, and appropriate lab coats. The use of face shields may be necessary when dealing with larger quantities or during certain procedures.
Adequate ventilation is another critical safety requirement. Laboratories should be equipped with fume hoods or local exhaust ventilation systems to minimize exposure to potentially harmful vapors or dust particles. When using sodium percarbonate-based cleaners, it is important to work in well-ventilated areas to reduce the risk of inhalation.
Storage regulations for sodium percarbonate and related cleaning products are equally important. These chemicals should be stored in cool, dry areas away from direct sunlight and heat sources. Proper labeling of containers is mandatory, including clear identification of the chemical, hazard warnings, and any specific storage instructions.
Emergency response protocols must be in place and clearly communicated to all laboratory personnel. This includes the location and proper use of eyewash stations, safety showers, and fire extinguishers. Spill response kits specifically designed for sodium percarbonate should be readily available, and staff should be trained in their use.
Regular safety training and education programs are essential components of laboratory safety regulations. These programs should cover the specific hazards associated with sodium percarbonate and other cleaning agents, proper handling techniques, and emergency procedures. Documentation of training sessions and periodic refresher courses are typically required to ensure ongoing compliance.
Risk assessment procedures should be implemented before introducing new chemicals or cleaning agents into the laboratory environment. This involves evaluating the potential hazards, determining appropriate control measures, and updating safety protocols as necessary.
Waste disposal regulations must also be adhered to when using sodium percarbonate-based cleaners. Proper disposal methods should be clearly outlined, and laboratory personnel should be trained in the correct procedures for handling and disposing of chemical waste.
Lastly, regular inspections and audits of laboratory safety practices are crucial for maintaining a safe working environment. These assessments help identify potential hazards, ensure compliance with safety regulations, and provide opportunities for continuous improvement in laboratory safety protocols.
Firstly, proper personal protective equipment (PPE) is essential when handling sodium percarbonate or cleaners containing this compound. This includes wearing safety goggles, chemical-resistant gloves, and appropriate lab coats. The use of face shields may be necessary when dealing with larger quantities or during certain procedures.
Adequate ventilation is another critical safety requirement. Laboratories should be equipped with fume hoods or local exhaust ventilation systems to minimize exposure to potentially harmful vapors or dust particles. When using sodium percarbonate-based cleaners, it is important to work in well-ventilated areas to reduce the risk of inhalation.
Storage regulations for sodium percarbonate and related cleaning products are equally important. These chemicals should be stored in cool, dry areas away from direct sunlight and heat sources. Proper labeling of containers is mandatory, including clear identification of the chemical, hazard warnings, and any specific storage instructions.
Emergency response protocols must be in place and clearly communicated to all laboratory personnel. This includes the location and proper use of eyewash stations, safety showers, and fire extinguishers. Spill response kits specifically designed for sodium percarbonate should be readily available, and staff should be trained in their use.
Regular safety training and education programs are essential components of laboratory safety regulations. These programs should cover the specific hazards associated with sodium percarbonate and other cleaning agents, proper handling techniques, and emergency procedures. Documentation of training sessions and periodic refresher courses are typically required to ensure ongoing compliance.
Risk assessment procedures should be implemented before introducing new chemicals or cleaning agents into the laboratory environment. This involves evaluating the potential hazards, determining appropriate control measures, and updating safety protocols as necessary.
Waste disposal regulations must also be adhered to when using sodium percarbonate-based cleaners. Proper disposal methods should be clearly outlined, and laboratory personnel should be trained in the correct procedures for handling and disposing of chemical waste.
Lastly, regular inspections and audits of laboratory safety practices are crucial for maintaining a safe working environment. These assessments help identify potential hazards, ensure compliance with safety regulations, and provide opportunities for continuous improvement in laboratory safety protocols.
Environmental Impact of Sodium Percarbonate-based Cleaners
The environmental impact of sodium percarbonate-based cleaners is a crucial consideration in their application within academic laboratory settings. These cleaners, while effective in their primary function, have both positive and negative effects on the environment that warrant careful examination.
Sodium percarbonate, when dissolved in water, breaks down into hydrogen peroxide and sodium carbonate. This decomposition process is generally considered environmentally friendly, as it produces oxygen, water, and soda ash as byproducts. The oxygen released can aid in the oxidation of organic matter, potentially contributing to the natural cleaning processes in water systems.
However, the environmental impact of these cleaners extends beyond their immediate decomposition. The production of sodium percarbonate involves energy-intensive processes, which contribute to carbon emissions and resource depletion. Additionally, the mining and processing of raw materials for its manufacture can lead to habitat disruption and soil degradation.
In aquatic environments, sodium percarbonate-based cleaners can have mixed effects. While the oxygen released can be beneficial for aerobic organisms, an excess of these compounds can lead to eutrophication in water bodies. This process can result in algal blooms and subsequent oxygen depletion, potentially harming aquatic ecosystems.
The alkaline nature of sodium carbonate, a byproduct of sodium percarbonate decomposition, can affect the pH balance of water systems. This pH shift may impact sensitive aquatic organisms and alter ecosystem dynamics. However, in most cases, the buffering capacity of natural water systems can mitigate these effects, especially when the cleaners are used in moderation.
From a waste management perspective, sodium percarbonate-based cleaners offer advantages over many traditional cleaning agents. Their biodegradability reduces the long-term environmental burden associated with persistent chemical pollutants. This characteristic aligns well with the growing emphasis on sustainable laboratory practices in academic institutions.
The use of these cleaners can also indirectly benefit the environment by reducing the need for more harmful cleaning agents. Many conventional laboratory cleaners contain chlorine or other toxic compounds that pose significant environmental risks. By substituting these with sodium percarbonate-based alternatives, laboratories can minimize their ecological footprint.
However, it is essential to consider the potential for misuse or overuse of these cleaners. Excessive application can lead to unnecessary chemical runoff, potentially overwhelming local water treatment facilities and contributing to water pollution. Proper training and guidelines for laboratory staff are crucial to ensure responsible use and disposal practices.
In conclusion, while sodium percarbonate-based cleaners offer several environmental advantages, their impact is not entirely benign. Balancing their benefits against potential risks requires careful management and consideration of local environmental conditions. As academic laboratories strive for sustainability, the judicious use of these cleaners, coupled with ongoing research into their long-term environmental effects, will be key to optimizing their role in eco-friendly laboratory practices.
Sodium percarbonate, when dissolved in water, breaks down into hydrogen peroxide and sodium carbonate. This decomposition process is generally considered environmentally friendly, as it produces oxygen, water, and soda ash as byproducts. The oxygen released can aid in the oxidation of organic matter, potentially contributing to the natural cleaning processes in water systems.
However, the environmental impact of these cleaners extends beyond their immediate decomposition. The production of sodium percarbonate involves energy-intensive processes, which contribute to carbon emissions and resource depletion. Additionally, the mining and processing of raw materials for its manufacture can lead to habitat disruption and soil degradation.
In aquatic environments, sodium percarbonate-based cleaners can have mixed effects. While the oxygen released can be beneficial for aerobic organisms, an excess of these compounds can lead to eutrophication in water bodies. This process can result in algal blooms and subsequent oxygen depletion, potentially harming aquatic ecosystems.
The alkaline nature of sodium carbonate, a byproduct of sodium percarbonate decomposition, can affect the pH balance of water systems. This pH shift may impact sensitive aquatic organisms and alter ecosystem dynamics. However, in most cases, the buffering capacity of natural water systems can mitigate these effects, especially when the cleaners are used in moderation.
From a waste management perspective, sodium percarbonate-based cleaners offer advantages over many traditional cleaning agents. Their biodegradability reduces the long-term environmental burden associated with persistent chemical pollutants. This characteristic aligns well with the growing emphasis on sustainable laboratory practices in academic institutions.
The use of these cleaners can also indirectly benefit the environment by reducing the need for more harmful cleaning agents. Many conventional laboratory cleaners contain chlorine or other toxic compounds that pose significant environmental risks. By substituting these with sodium percarbonate-based alternatives, laboratories can minimize their ecological footprint.
However, it is essential to consider the potential for misuse or overuse of these cleaners. Excessive application can lead to unnecessary chemical runoff, potentially overwhelming local water treatment facilities and contributing to water pollution. Proper training and guidelines for laboratory staff are crucial to ensure responsible use and disposal practices.
In conclusion, while sodium percarbonate-based cleaners offer several environmental advantages, their impact is not entirely benign. Balancing their benefits against potential risks requires careful management and consideration of local environmental conditions. As academic laboratories strive for sustainability, the judicious use of these cleaners, coupled with ongoing research into their long-term environmental effects, will be key to optimizing their role in eco-friendly laboratory practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!