Phenolphthalein's Role in Pharmaceutical Quality Control
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein QC Background and Objectives
Phenolphthalein, a chemical compound discovered in the late 19th century, has played a significant role in pharmaceutical quality control for over a century. Initially used as a laxative, its application in the pharmaceutical industry has evolved dramatically, particularly in the realm of quality assurance and control processes.
The historical development of phenolphthalein in pharmaceutical quality control can be traced back to its early use as an acid-base indicator. Its unique property of changing color in response to pH levels made it an invaluable tool for titration processes, which are crucial in determining the purity and concentration of pharmaceutical compounds.
As the pharmaceutical industry progressed, so did the applications of phenolphthalein. It became an essential component in various analytical methods, including colorimetric assays and spectrophotometric techniques. These methods have been instrumental in ensuring the quality, safety, and efficacy of pharmaceutical products throughout the manufacturing process.
The evolution of phenolphthalein's role in quality control has been driven by the increasing complexity of pharmaceutical formulations and the growing demand for more precise and reliable analytical methods. Its versatility has allowed it to remain relevant even as new technologies have emerged, adapting to the changing landscape of pharmaceutical manufacturing and regulatory requirements.
In recent years, the focus on phenolphthalein in pharmaceutical quality control has shifted towards developing more sensitive and specific analytical methods. This shift is partly due to the need for detecting increasingly minute quantities of substances and the industry's move towards more complex drug formulations.
The primary objective of utilizing phenolphthalein in pharmaceutical quality control is to ensure the consistent production of high-quality, safe, and effective medications. This encompasses a range of specific goals, including accurate quantification of active pharmaceutical ingredients, detection of impurities, and verification of formulation consistency.
Another critical objective is to support compliance with regulatory standards set by bodies such as the FDA and EMA. Phenolphthalein-based methods often form part of the validated analytical procedures required for drug approval and ongoing quality assurance.
Looking ahead, the role of phenolphthalein in pharmaceutical quality control is expected to continue evolving. While traditional applications remain important, new frontiers are emerging, such as its potential use in novel drug delivery systems and personalized medicine. The ongoing research aims to enhance the sensitivity, specificity, and applicability of phenolphthalein-based methods to meet the future challenges of pharmaceutical quality control.
The historical development of phenolphthalein in pharmaceutical quality control can be traced back to its early use as an acid-base indicator. Its unique property of changing color in response to pH levels made it an invaluable tool for titration processes, which are crucial in determining the purity and concentration of pharmaceutical compounds.
As the pharmaceutical industry progressed, so did the applications of phenolphthalein. It became an essential component in various analytical methods, including colorimetric assays and spectrophotometric techniques. These methods have been instrumental in ensuring the quality, safety, and efficacy of pharmaceutical products throughout the manufacturing process.
The evolution of phenolphthalein's role in quality control has been driven by the increasing complexity of pharmaceutical formulations and the growing demand for more precise and reliable analytical methods. Its versatility has allowed it to remain relevant even as new technologies have emerged, adapting to the changing landscape of pharmaceutical manufacturing and regulatory requirements.
In recent years, the focus on phenolphthalein in pharmaceutical quality control has shifted towards developing more sensitive and specific analytical methods. This shift is partly due to the need for detecting increasingly minute quantities of substances and the industry's move towards more complex drug formulations.
The primary objective of utilizing phenolphthalein in pharmaceutical quality control is to ensure the consistent production of high-quality, safe, and effective medications. This encompasses a range of specific goals, including accurate quantification of active pharmaceutical ingredients, detection of impurities, and verification of formulation consistency.
Another critical objective is to support compliance with regulatory standards set by bodies such as the FDA and EMA. Phenolphthalein-based methods often form part of the validated analytical procedures required for drug approval and ongoing quality assurance.
Looking ahead, the role of phenolphthalein in pharmaceutical quality control is expected to continue evolving. While traditional applications remain important, new frontiers are emerging, such as its potential use in novel drug delivery systems and personalized medicine. The ongoing research aims to enhance the sensitivity, specificity, and applicability of phenolphthalein-based methods to meet the future challenges of pharmaceutical quality control.
Market Demand for Pharmaceutical QC Indicators
The pharmaceutical industry's demand for quality control indicators has been steadily increasing, driven by stringent regulatory requirements and the growing complexity of drug formulations. Phenolphthalein, a long-standing indicator in pharmaceutical quality control, continues to play a crucial role in this expanding market. The global pharmaceutical quality control market is projected to reach significant growth in the coming years, with a substantial portion attributed to chemical indicators like phenolphthalein.
The demand for phenolphthalein in pharmaceutical quality control is primarily fueled by its versatility and reliability in acid-base titrations, a fundamental process in drug manufacturing and testing. As pharmaceutical companies expand their product portfolios and increase production volumes, the need for efficient and accurate quality control measures becomes paramount. This trend is particularly evident in emerging markets, where rapid industrialization and improving healthcare infrastructure are driving the growth of the pharmaceutical sector.
In developed markets, the focus on precision medicine and personalized drug formulations is creating new opportunities for advanced quality control methods. While this might suggest a shift towards more sophisticated technologies, the fundamental role of phenolphthalein in basic quality assurance processes remains strong. Its cost-effectiveness and well-established protocols make it an indispensable tool for both small-scale and large pharmaceutical manufacturers.
The increasing emphasis on drug safety and efficacy by regulatory bodies worldwide is another key factor driving the demand for reliable quality control indicators. Phenolphthalein's ability to provide clear, visible results in pH-sensitive reactions makes it valuable in ensuring the consistency and purity of pharmaceutical products. This is particularly important in the context of growing concerns over counterfeit drugs and the need for robust quality assurance measures throughout the supply chain.
Environmental and sustainability considerations are also shaping the market demand for pharmaceutical quality control indicators. While phenolphthalein remains widely used, there is a growing interest in eco-friendly alternatives. This trend is creating a dual market dynamic where traditional indicators like phenolphthalein coexist with newer, greener options, catering to different segments of the pharmaceutical industry.
The COVID-19 pandemic has further highlighted the importance of robust quality control in pharmaceutical manufacturing. The urgent need for vaccines and treatments has accelerated drug development timelines, placing greater emphasis on rapid yet reliable quality control methods. This scenario has reinforced the value of well-established indicators like phenolphthalein, which can be quickly deployed in various testing protocols.
The demand for phenolphthalein in pharmaceutical quality control is primarily fueled by its versatility and reliability in acid-base titrations, a fundamental process in drug manufacturing and testing. As pharmaceutical companies expand their product portfolios and increase production volumes, the need for efficient and accurate quality control measures becomes paramount. This trend is particularly evident in emerging markets, where rapid industrialization and improving healthcare infrastructure are driving the growth of the pharmaceutical sector.
In developed markets, the focus on precision medicine and personalized drug formulations is creating new opportunities for advanced quality control methods. While this might suggest a shift towards more sophisticated technologies, the fundamental role of phenolphthalein in basic quality assurance processes remains strong. Its cost-effectiveness and well-established protocols make it an indispensable tool for both small-scale and large pharmaceutical manufacturers.
The increasing emphasis on drug safety and efficacy by regulatory bodies worldwide is another key factor driving the demand for reliable quality control indicators. Phenolphthalein's ability to provide clear, visible results in pH-sensitive reactions makes it valuable in ensuring the consistency and purity of pharmaceutical products. This is particularly important in the context of growing concerns over counterfeit drugs and the need for robust quality assurance measures throughout the supply chain.
Environmental and sustainability considerations are also shaping the market demand for pharmaceutical quality control indicators. While phenolphthalein remains widely used, there is a growing interest in eco-friendly alternatives. This trend is creating a dual market dynamic where traditional indicators like phenolphthalein coexist with newer, greener options, catering to different segments of the pharmaceutical industry.
The COVID-19 pandemic has further highlighted the importance of robust quality control in pharmaceutical manufacturing. The urgent need for vaccines and treatments has accelerated drug development timelines, placing greater emphasis on rapid yet reliable quality control methods. This scenario has reinforced the value of well-established indicators like phenolphthalein, which can be quickly deployed in various testing protocols.
Current Challenges in Phenolphthalein-based QC
Despite the widespread use of phenolphthalein in pharmaceutical quality control, several challenges persist in its application. One of the primary issues is the sensitivity of phenolphthalein to environmental factors, particularly pH and temperature fluctuations. These variations can significantly affect the accuracy and reliability of test results, leading to potential misinterpretations in quality assessments.
Another challenge lies in the stability of phenolphthalein solutions over time. The indicator's effectiveness can diminish with prolonged storage or exposure to light, necessitating frequent preparation of fresh solutions. This requirement not only increases operational costs but also introduces potential inconsistencies in test results across different batches of the indicator.
The specificity of phenolphthalein reactions presents another hurdle in pharmaceutical quality control. While the indicator is highly effective for certain pH-based analyses, it may not be suitable for detecting subtle changes in complex pharmaceutical formulations. This limitation can result in false negatives or positives, potentially compromising the overall quality assurance process.
Furthermore, the disposal of phenolphthalein-containing waste poses environmental concerns. As a potentially harmful substance, proper handling and disposal protocols must be strictly adhered to, adding complexity to laboratory operations and increasing the risk of non-compliance with environmental regulations.
The standardization of phenolphthalein-based quality control methods across different pharmaceutical manufacturing facilities and laboratories remains a challenge. Variations in testing protocols, equipment calibration, and interpretation of results can lead to inconsistencies in quality assessments between different facilities or even within the same organization.
Lastly, the increasing trend towards automation and high-throughput screening in pharmaceutical quality control presents challenges for traditional phenolphthalein-based methods. Integrating these manual techniques into automated systems requires careful consideration of factors such as sample preparation, reaction time, and result interpretation, which can be complex to standardize across different automated platforms.
Addressing these challenges requires ongoing research and development efforts to enhance the reliability, consistency, and applicability of phenolphthalein-based quality control methods in the pharmaceutical industry. Innovations in formulation stability, environmental control, and integration with modern analytical technologies are crucial for overcoming these obstacles and ensuring the continued relevance of phenolphthalein in pharmaceutical quality assurance.
Another challenge lies in the stability of phenolphthalein solutions over time. The indicator's effectiveness can diminish with prolonged storage or exposure to light, necessitating frequent preparation of fresh solutions. This requirement not only increases operational costs but also introduces potential inconsistencies in test results across different batches of the indicator.
The specificity of phenolphthalein reactions presents another hurdle in pharmaceutical quality control. While the indicator is highly effective for certain pH-based analyses, it may not be suitable for detecting subtle changes in complex pharmaceutical formulations. This limitation can result in false negatives or positives, potentially compromising the overall quality assurance process.
Furthermore, the disposal of phenolphthalein-containing waste poses environmental concerns. As a potentially harmful substance, proper handling and disposal protocols must be strictly adhered to, adding complexity to laboratory operations and increasing the risk of non-compliance with environmental regulations.
The standardization of phenolphthalein-based quality control methods across different pharmaceutical manufacturing facilities and laboratories remains a challenge. Variations in testing protocols, equipment calibration, and interpretation of results can lead to inconsistencies in quality assessments between different facilities or even within the same organization.
Lastly, the increasing trend towards automation and high-throughput screening in pharmaceutical quality control presents challenges for traditional phenolphthalein-based methods. Integrating these manual techniques into automated systems requires careful consideration of factors such as sample preparation, reaction time, and result interpretation, which can be complex to standardize across different automated platforms.
Addressing these challenges requires ongoing research and development efforts to enhance the reliability, consistency, and applicability of phenolphthalein-based quality control methods in the pharmaceutical industry. Innovations in formulation stability, environmental control, and integration with modern analytical technologies are crucial for overcoming these obstacles and ensuring the continued relevance of phenolphthalein in pharmaceutical quality assurance.
Existing Phenolphthalein QC Methodologies
01 Synthesis and production of phenolphthalein
Various methods and processes for synthesizing and producing phenolphthalein are described. These include different reaction conditions, catalysts, and starting materials to optimize yield and purity of the final product.- Synthesis and production of phenolphthalein: Various methods and processes for synthesizing and producing phenolphthalein are described. These include different reaction conditions, catalysts, and starting materials to optimize yield and purity of the final product.
- Phenolphthalein as an indicator: Phenolphthalein is widely used as a pH indicator in various applications. Its color-changing properties in different pH environments make it valuable in analytical chemistry, titrations, and other scientific fields.
- Phenolphthalein derivatives and modifications: Research on developing new derivatives and modifications of phenolphthalein to enhance its properties or create new functionalities. This includes structural modifications, substitutions, and the creation of novel compounds based on the phenolphthalein core.
- Applications in polymer and material science: Phenolphthalein and its derivatives are used in polymer chemistry and material science. They can be incorporated into various materials to impart specific properties or functionalities, such as color-changing abilities or improved thermal characteristics.
- Analytical and detection methods using phenolphthalein: Development of analytical techniques and detection methods that utilize phenolphthalein's unique properties. These include colorimetric assays, sensors, and other diagnostic tools for various applications in chemistry, biology, and environmental science.
02 Applications in analytical chemistry
Phenolphthalein is widely used as an indicator in analytical chemistry, particularly in acid-base titrations. Its color-changing properties make it valuable for determining pH levels and endpoint detection in various chemical analyses.Expand Specific Solutions03 Incorporation in polymers and resins
Phenolphthalein is utilized in the production of certain polymers and resins. It can be incorporated into these materials to impart specific properties or functionalities, such as color-changing abilities or improved thermal stability.Expand Specific Solutions04 Medical and pharmaceutical applications
Phenolphthalein has been used in various medical and pharmaceutical applications. These include its use as a laxative, in diagnostic tests, and as a component in certain drug formulations.Expand Specific Solutions05 Environmental and safety considerations
Research and development efforts focus on addressing environmental and safety concerns related to phenolphthalein use. This includes developing alternative compounds, improving disposal methods, and studying its potential health effects.Expand Specific Solutions
Key Players in Pharma QC Industry
The pharmaceutical quality control market for phenolphthalein is in a mature stage, with a stable global market size. The technology is well-established, with major players like Novartis AG, Sanofi, and Genentech, Inc. having extensive experience in its application. These companies, along with others such as CSPC Pharmaceutical Group and Regeneron Pharmaceuticals, have integrated phenolphthalein into their quality control processes, demonstrating its widespread adoption. Universities like Sichuan University and China Pharmaceutical University contribute to ongoing research and development, potentially leading to incremental improvements in the technology's application. The competitive landscape is characterized by a focus on efficiency and precision in quality control methods, with companies striving to optimize their use of phenolphthalein in pharmaceutical manufacturing processes.
Novartis AG
Technical Solution: Novartis AG has developed advanced spectrophotometric methods for phenolphthalein-based quality control in pharmaceuticals. Their approach utilizes high-performance liquid chromatography (HPLC) coupled with UV-Vis spectroscopy to detect and quantify phenolphthalein in drug formulations with high precision[1]. This method allows for the simultaneous determination of phenolphthalein and other active ingredients, enhancing the efficiency of quality control processes. Novartis has also implemented automated systems that integrate phenolphthalein-based assays into their production lines, enabling real-time monitoring and adjustment of drug quality parameters[3].
Strengths: High precision and sensitivity in phenolphthalein detection, integration with existing production systems, and capability for multi-component analysis. Weaknesses: Requires specialized equipment and trained personnel, potentially higher initial implementation costs.
China Pharmaceutical University
Technical Solution: China Pharmaceutical University has made significant advancements in the application of phenolphthalein for pharmaceutical quality control, particularly in the analysis of traditional Chinese medicines (TCMs). Their research has led to the development of a novel phenolphthalein-based chemiluminescence method for detecting adulterants in herbal preparations[7]. This technique offers high sensitivity and selectivity, allowing for the identification of synthetic drugs illegally added to TCMs. The university has also explored the use of phenolphthalein in green analytical chemistry, developing eco-friendly quality control methods that reduce the use of harmful solvents[8]. Additionally, they have created a portable phenolphthalein-based sensor for rapid on-site testing of pharmaceutical raw materials.
Strengths: Specialized applications in traditional Chinese medicines, environmentally friendly methods, and development of portable testing solutions. Weaknesses: May require further validation for use with Western pharmaceuticals, potential limitations in complex formulations.
Innovations in Phenolphthalein-based QC
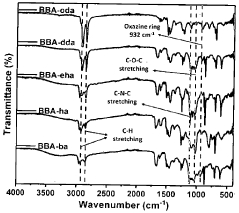
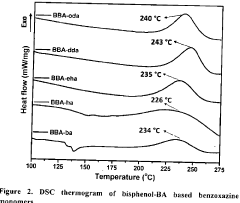
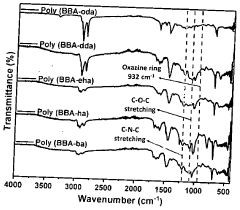
Production of extreme range of PH indicators from benzoxazines
PatentActiveIN202341027342A
Innovation
- Development of bisphenol-BA/aliphatic amine based hydrophobic polybenzoxazines coated on cellulose paper, synthesized through Mannich condensation, which exhibit distinct color changes across a wide pH range from -1.8 to 14, offering thermal stability and repeated use capability.
Regulatory Framework for Pharma QC Methods
The regulatory framework for pharmaceutical quality control methods is a critical aspect of ensuring the safety, efficacy, and consistency of medicinal products. This framework is established and enforced by various regulatory bodies worldwide, with the primary goal of maintaining high standards in pharmaceutical manufacturing and testing processes.
In the United States, the Food and Drug Administration (FDA) plays a central role in regulating pharmaceutical quality control methods. The FDA's guidance documents, such as the Current Good Manufacturing Practice (cGMP) regulations, provide detailed requirements for quality control procedures in drug manufacturing. These regulations cover various aspects of quality control, including analytical method validation, stability testing, and impurity profiling.
The European Medicines Agency (EMA) oversees pharmaceutical quality control in the European Union. The EMA's guidelines, particularly those related to the International Conference on Harmonisation (ICH), provide comprehensive standards for quality control methods. These guidelines emphasize the importance of method validation, analytical procedure development, and the use of pharmacopoeial methods where applicable.
Internationally, the World Health Organization (WHO) provides guidance on pharmaceutical quality control through its Technical Report Series. These reports offer recommendations on good practices for pharmaceutical quality control laboratories, which are particularly relevant for developing countries and regions with limited regulatory resources.
Pharmacopoeias, such as the United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and Japanese Pharmacopoeia (JP), play a crucial role in the regulatory framework. These compendiums provide standardized methods and specifications for pharmaceutical substances and preparations, including quality control procedures and acceptance criteria.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has developed harmonized guidelines for pharmaceutical quality control. These guidelines, adopted by regulatory authorities in many countries, cover topics such as stability testing, impurity analysis, and analytical method validation.
Regulatory agencies also require pharmaceutical companies to implement robust quality management systems. These systems must include well-documented standard operating procedures (SOPs) for all quality control activities, ensuring consistency and traceability in testing processes.
As the pharmaceutical industry evolves, regulatory frameworks are continuously updated to address new challenges and technologies. For instance, the emergence of advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and mass spectrometry, has led to the development of new regulatory guidelines for their implementation in quality control processes.
In the United States, the Food and Drug Administration (FDA) plays a central role in regulating pharmaceutical quality control methods. The FDA's guidance documents, such as the Current Good Manufacturing Practice (cGMP) regulations, provide detailed requirements for quality control procedures in drug manufacturing. These regulations cover various aspects of quality control, including analytical method validation, stability testing, and impurity profiling.
The European Medicines Agency (EMA) oversees pharmaceutical quality control in the European Union. The EMA's guidelines, particularly those related to the International Conference on Harmonisation (ICH), provide comprehensive standards for quality control methods. These guidelines emphasize the importance of method validation, analytical procedure development, and the use of pharmacopoeial methods where applicable.
Internationally, the World Health Organization (WHO) provides guidance on pharmaceutical quality control through its Technical Report Series. These reports offer recommendations on good practices for pharmaceutical quality control laboratories, which are particularly relevant for developing countries and regions with limited regulatory resources.
Pharmacopoeias, such as the United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and Japanese Pharmacopoeia (JP), play a crucial role in the regulatory framework. These compendiums provide standardized methods and specifications for pharmaceutical substances and preparations, including quality control procedures and acceptance criteria.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has developed harmonized guidelines for pharmaceutical quality control. These guidelines, adopted by regulatory authorities in many countries, cover topics such as stability testing, impurity analysis, and analytical method validation.
Regulatory agencies also require pharmaceutical companies to implement robust quality management systems. These systems must include well-documented standard operating procedures (SOPs) for all quality control activities, ensuring consistency and traceability in testing processes.
As the pharmaceutical industry evolves, regulatory frameworks are continuously updated to address new challenges and technologies. For instance, the emergence of advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and mass spectrometry, has led to the development of new regulatory guidelines for their implementation in quality control processes.
Environmental Impact of Phenolphthalein Use
The use of phenolphthalein in pharmaceutical quality control has raised concerns about its potential environmental impact. As a widely used indicator in acid-base titrations and quality assurance processes, the disposal of phenolphthalein-containing solutions and materials can have significant implications for ecosystems and water resources.
One of the primary environmental concerns is the persistence of phenolphthalein in aquatic environments. When improperly disposed of, this compound can enter water systems, potentially affecting aquatic life and water quality. Studies have shown that phenolphthalein can remain stable in water for extended periods, leading to long-term exposure risks for various organisms.
The bioaccumulation potential of phenolphthalein in aquatic organisms is another critical aspect of its environmental impact. Some research suggests that certain aquatic species may accumulate this compound in their tissues, potentially leading to biomagnification up the food chain. This process could result in higher concentrations of phenolphthalein in predatory species, including those consumed by humans.
Soil contamination is also a concern when phenolphthalein-containing waste is improperly managed. The compound can adsorb to soil particles, potentially affecting soil microorganisms and plant growth. This contamination may persist in the environment, leading to long-term ecological effects and potential groundwater contamination.
The degradation products of phenolphthalein in the environment are another area of concern. While the compound itself may break down over time, its degradation products could potentially have their own environmental impacts. Research into these breakdown products and their ecological effects is ongoing, but preliminary studies suggest that some may have estrogenic properties, potentially disrupting endocrine systems in wildlife.
To mitigate these environmental risks, pharmaceutical companies and laboratories using phenolphthalein are increasingly adopting strict waste management protocols. These include proper segregation of phenolphthalein-containing waste, use of specialized treatment methods before disposal, and exploration of alternative, more environmentally friendly indicators for quality control processes.
Regulatory bodies worldwide are also taking notice of the potential environmental impacts of phenolphthalein. Some countries have implemented stricter guidelines for its use and disposal, while others are funding research into more sustainable alternatives. These efforts aim to balance the compound's utility in pharmaceutical quality control with the need to protect environmental integrity.
One of the primary environmental concerns is the persistence of phenolphthalein in aquatic environments. When improperly disposed of, this compound can enter water systems, potentially affecting aquatic life and water quality. Studies have shown that phenolphthalein can remain stable in water for extended periods, leading to long-term exposure risks for various organisms.
The bioaccumulation potential of phenolphthalein in aquatic organisms is another critical aspect of its environmental impact. Some research suggests that certain aquatic species may accumulate this compound in their tissues, potentially leading to biomagnification up the food chain. This process could result in higher concentrations of phenolphthalein in predatory species, including those consumed by humans.
Soil contamination is also a concern when phenolphthalein-containing waste is improperly managed. The compound can adsorb to soil particles, potentially affecting soil microorganisms and plant growth. This contamination may persist in the environment, leading to long-term ecological effects and potential groundwater contamination.
The degradation products of phenolphthalein in the environment are another area of concern. While the compound itself may break down over time, its degradation products could potentially have their own environmental impacts. Research into these breakdown products and their ecological effects is ongoing, but preliminary studies suggest that some may have estrogenic properties, potentially disrupting endocrine systems in wildlife.
To mitigate these environmental risks, pharmaceutical companies and laboratories using phenolphthalein are increasingly adopting strict waste management protocols. These include proper segregation of phenolphthalein-containing waste, use of specialized treatment methods before disposal, and exploration of alternative, more environmentally friendly indicators for quality control processes.
Regulatory bodies worldwide are also taking notice of the potential environmental impacts of phenolphthalein. Some countries have implemented stricter guidelines for its use and disposal, while others are funding research into more sustainable alternatives. These efforts aim to balance the compound's utility in pharmaceutical quality control with the need to protect environmental integrity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!