Phenolphthalein for Detection of pH-Dependent Kinetic Changes
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein pH Detection Background
Phenolphthalein, a widely used pH indicator, has been a cornerstone in analytical chemistry for over a century. Discovered in 1871 by Adolf von Baeyer, this compound has found extensive applications in various fields, particularly in the detection of pH-dependent kinetic changes. Its unique color-changing properties in response to pH variations have made it an invaluable tool in both research and industrial settings.
The fundamental principle behind phenolphthalein's pH detection capability lies in its molecular structure. In acidic and neutral solutions, phenolphthalein exists in a colorless lactone form. However, as the pH increases above 8.2, the molecule undergoes a structural change, opening its lactone ring and forming a quinoid structure. This transformation results in the characteristic pink color associated with phenolphthalein in basic solutions.
The sensitivity of phenolphthalein to pH changes has led to its widespread use in titrations, particularly acid-base titrations. Its sharp color transition from colorless to pink occurs over a narrow pH range, typically between 8.2 and 10.0, making it an excellent endpoint indicator for many analytical procedures. This property has made phenolphthalein indispensable in educational laboratories, environmental monitoring, and quality control processes in various industries.
In recent years, the application of phenolphthalein has expanded beyond simple pH indication. Researchers have begun exploring its potential in detecting pH-dependent kinetic changes in complex biological and chemical systems. This new avenue of research aims to leverage the compound's rapid response to pH fluctuations to monitor dynamic processes in real-time.
The growing interest in phenolphthalein for kinetic studies stems from its ability to provide visual and quantifiable data on pH changes occurring during chemical reactions or biological processes. This capability is particularly valuable in studying enzyme kinetics, where pH often plays a crucial role in reaction rates and mechanisms. By incorporating phenolphthalein into these systems, researchers can gain insights into the temporal aspects of pH-dependent reactions, offering a new dimension to traditional kinetic studies.
Moreover, the development of advanced spectroscopic techniques has enhanced the precision with which phenolphthalein-based pH changes can be measured. These advancements have opened up possibilities for more sophisticated applications, such as in microfluidic devices and high-throughput screening assays, where rapid and accurate pH detection is critical.
As research in this field progresses, there is a growing focus on understanding the molecular dynamics of phenolphthalein's color change mechanism. This deeper understanding could lead to the development of modified phenolphthalein derivatives with enhanced properties, such as broader pH ranges or increased sensitivity, further expanding its utility in both research and industrial applications.
The fundamental principle behind phenolphthalein's pH detection capability lies in its molecular structure. In acidic and neutral solutions, phenolphthalein exists in a colorless lactone form. However, as the pH increases above 8.2, the molecule undergoes a structural change, opening its lactone ring and forming a quinoid structure. This transformation results in the characteristic pink color associated with phenolphthalein in basic solutions.
The sensitivity of phenolphthalein to pH changes has led to its widespread use in titrations, particularly acid-base titrations. Its sharp color transition from colorless to pink occurs over a narrow pH range, typically between 8.2 and 10.0, making it an excellent endpoint indicator for many analytical procedures. This property has made phenolphthalein indispensable in educational laboratories, environmental monitoring, and quality control processes in various industries.
In recent years, the application of phenolphthalein has expanded beyond simple pH indication. Researchers have begun exploring its potential in detecting pH-dependent kinetic changes in complex biological and chemical systems. This new avenue of research aims to leverage the compound's rapid response to pH fluctuations to monitor dynamic processes in real-time.
The growing interest in phenolphthalein for kinetic studies stems from its ability to provide visual and quantifiable data on pH changes occurring during chemical reactions or biological processes. This capability is particularly valuable in studying enzyme kinetics, where pH often plays a crucial role in reaction rates and mechanisms. By incorporating phenolphthalein into these systems, researchers can gain insights into the temporal aspects of pH-dependent reactions, offering a new dimension to traditional kinetic studies.
Moreover, the development of advanced spectroscopic techniques has enhanced the precision with which phenolphthalein-based pH changes can be measured. These advancements have opened up possibilities for more sophisticated applications, such as in microfluidic devices and high-throughput screening assays, where rapid and accurate pH detection is critical.
As research in this field progresses, there is a growing focus on understanding the molecular dynamics of phenolphthalein's color change mechanism. This deeper understanding could lead to the development of modified phenolphthalein derivatives with enhanced properties, such as broader pH ranges or increased sensitivity, further expanding its utility in both research and industrial applications.
Market Analysis for pH Indicators
The market for pH indicators, including phenolphthalein, has been experiencing steady growth due to increasing applications in various industries. The global pH indicators market is primarily driven by the rising demand in water treatment, environmental monitoring, and healthcare sectors. In the water treatment industry, pH indicators play a crucial role in ensuring water quality and safety, both for industrial processes and drinking water. Environmental monitoring agencies utilize pH indicators for assessing soil and water quality, contributing to the market's expansion.
The healthcare sector represents a significant portion of the pH indicators market, with applications in clinical diagnostics, pharmaceutical research, and biotechnology. Phenolphthalein, in particular, finds extensive use in medical laboratories for urinalysis and other diagnostic tests. The growing emphasis on personalized medicine and point-of-care diagnostics is expected to further boost the demand for pH indicators in the healthcare industry.
In the food and beverage industry, pH indicators are essential for quality control and safety assurance. With increasing consumer awareness about food safety and stringent regulations, the demand for pH indicators in this sector is projected to grow. Additionally, the cosmetics and personal care industry utilizes pH indicators for product development and quality control, contributing to market growth.
The Asia-Pacific region is anticipated to witness the highest growth rate in the pH indicators market due to rapid industrialization, increasing environmental concerns, and growing healthcare expenditure. North America and Europe continue to hold significant market shares, driven by advanced healthcare systems and stringent environmental regulations.
Key market players in the pH indicators industry include Merck KGaA, Thermo Fisher Scientific, and Sigma-Aldrich (now part of Merck). These companies are focusing on product innovation and strategic partnerships to maintain their market positions. The development of advanced pH-sensitive materials and the integration of digital technologies for real-time pH monitoring are emerging trends in the market.
Challenges in the pH indicators market include the need for improved accuracy and sensitivity, especially in complex biological systems. Research on phenolphthalein for detecting pH-dependent kinetic changes addresses this challenge by exploring novel applications and enhancing the indicator's performance. This research direction aligns with the market's demand for more precise and versatile pH measurement tools across various industries.
The healthcare sector represents a significant portion of the pH indicators market, with applications in clinical diagnostics, pharmaceutical research, and biotechnology. Phenolphthalein, in particular, finds extensive use in medical laboratories for urinalysis and other diagnostic tests. The growing emphasis on personalized medicine and point-of-care diagnostics is expected to further boost the demand for pH indicators in the healthcare industry.
In the food and beverage industry, pH indicators are essential for quality control and safety assurance. With increasing consumer awareness about food safety and stringent regulations, the demand for pH indicators in this sector is projected to grow. Additionally, the cosmetics and personal care industry utilizes pH indicators for product development and quality control, contributing to market growth.
The Asia-Pacific region is anticipated to witness the highest growth rate in the pH indicators market due to rapid industrialization, increasing environmental concerns, and growing healthcare expenditure. North America and Europe continue to hold significant market shares, driven by advanced healthcare systems and stringent environmental regulations.
Key market players in the pH indicators industry include Merck KGaA, Thermo Fisher Scientific, and Sigma-Aldrich (now part of Merck). These companies are focusing on product innovation and strategic partnerships to maintain their market positions. The development of advanced pH-sensitive materials and the integration of digital technologies for real-time pH monitoring are emerging trends in the market.
Challenges in the pH indicators market include the need for improved accuracy and sensitivity, especially in complex biological systems. Research on phenolphthalein for detecting pH-dependent kinetic changes addresses this challenge by exploring novel applications and enhancing the indicator's performance. This research direction aligns with the market's demand for more precise and versatile pH measurement tools across various industries.
Current Challenges in pH-Dependent Kinetics
The field of pH-dependent kinetics faces several significant challenges that hinder progress in understanding and utilizing phenolphthalein for the detection of pH-dependent kinetic changes. One of the primary obstacles is the complexity of the molecular interactions involved in pH-dependent reactions. The intricate interplay between protons, buffer components, and the target molecules creates a multifaceted system that is difficult to model accurately.
Another challenge lies in the precise control and measurement of pH changes in real-time. While phenolphthalein is a well-known pH indicator, its color change is not instantaneous, which can lead to delays in detecting rapid kinetic changes. This temporal resolution limitation can obscure fast reaction dynamics, particularly in systems where pH fluctuations occur rapidly.
The sensitivity of phenolphthalein to environmental factors poses additional complications. Temperature, ionic strength, and the presence of interfering substances can all affect the indicator's performance, potentially leading to misinterpretation of results. Researchers must carefully account for these variables to ensure the reliability of their measurements.
Furthermore, the narrow pH range in which phenolphthalein exhibits its color change (typically between pH 8.2 and 10.0) restricts its applicability in studying a broader spectrum of pH-dependent reactions. This limitation necessitates the use of multiple indicators or alternative methods for comprehensive pH-dependent kinetic studies across wider pH ranges.
The quantification of pH-dependent kinetic changes using phenolphthalein also presents challenges in terms of data analysis and interpretation. The non-linear relationship between color intensity and pH requires sophisticated calibration techniques and data processing algorithms to extract meaningful kinetic parameters.
Additionally, the potential for photobleaching and chemical degradation of phenolphthalein during extended experiments can introduce errors in long-term kinetic studies. This instability may lead to drift in measurements over time, complicating the analysis of slow reactions or equilibrium processes.
Lastly, the integration of phenolphthalein-based detection systems with modern high-throughput screening technologies and microfluidic devices presents technical hurdles. Miniaturization and automation of pH-dependent kinetic assays require innovative approaches to maintain sensitivity and accuracy while scaling down reaction volumes and increasing throughput.
Another challenge lies in the precise control and measurement of pH changes in real-time. While phenolphthalein is a well-known pH indicator, its color change is not instantaneous, which can lead to delays in detecting rapid kinetic changes. This temporal resolution limitation can obscure fast reaction dynamics, particularly in systems where pH fluctuations occur rapidly.
The sensitivity of phenolphthalein to environmental factors poses additional complications. Temperature, ionic strength, and the presence of interfering substances can all affect the indicator's performance, potentially leading to misinterpretation of results. Researchers must carefully account for these variables to ensure the reliability of their measurements.
Furthermore, the narrow pH range in which phenolphthalein exhibits its color change (typically between pH 8.2 and 10.0) restricts its applicability in studying a broader spectrum of pH-dependent reactions. This limitation necessitates the use of multiple indicators or alternative methods for comprehensive pH-dependent kinetic studies across wider pH ranges.
The quantification of pH-dependent kinetic changes using phenolphthalein also presents challenges in terms of data analysis and interpretation. The non-linear relationship between color intensity and pH requires sophisticated calibration techniques and data processing algorithms to extract meaningful kinetic parameters.
Additionally, the potential for photobleaching and chemical degradation of phenolphthalein during extended experiments can introduce errors in long-term kinetic studies. This instability may lead to drift in measurements over time, complicating the analysis of slow reactions or equilibrium processes.
Lastly, the integration of phenolphthalein-based detection systems with modern high-throughput screening technologies and microfluidic devices presents technical hurdles. Miniaturization and automation of pH-dependent kinetic assays require innovative approaches to maintain sensitivity and accuracy while scaling down reaction volumes and increasing throughput.
Existing Phenolphthalein-based Solutions
01 pH-dependent color change mechanism of phenolphthalein
Phenolphthalein exhibits a pH-dependent color change due to its structural transformation between colorless and colored forms. This mechanism is based on the protonation and deprotonation of the molecule, which affects its electronic configuration and light absorption properties. The kinetics of this color change can be studied to understand the rate of pH-induced structural changes in phenolphthalein.- pH-dependent color change mechanism of phenolphthalein: Phenolphthalein exhibits a pH-dependent color change due to its structural transformation between colorless and colored forms. This mechanism is based on the protonation and deprotonation of the molecule, which alters its electronic configuration and absorption spectrum. The kinetics of this color change can be studied to understand the rate of pH-induced structural changes in phenolphthalein.
- Applications in pH-sensitive materials and indicators: The pH-dependent kinetic changes of phenolphthalein are utilized in various applications, including pH-sensitive materials and indicators. These applications range from simple pH test strips to more complex systems such as smart polymers and drug delivery systems that respond to changes in pH. The rate of color change can be tailored for specific applications by modifying the chemical environment or incorporating phenolphthalein into different matrices.
- Influence of environmental factors on phenolphthalein kinetics: Environmental factors such as temperature, ionic strength, and the presence of other chemical species can significantly affect the kinetics of phenolphthalein's pH-dependent changes. These factors can alter the rate of protonation/deprotonation, influence the stability of different forms of phenolphthalein, or affect its interaction with the surrounding medium. Understanding these influences is crucial for optimizing phenolphthalein-based systems and interpreting their responses accurately.
- Quantitative analysis using phenolphthalein kinetics: The kinetics of phenolphthalein's pH-dependent changes can be used for quantitative analysis in various fields. By monitoring the rate of color change or the time required for complete color transition, it is possible to determine pH values, buffer capacities, or concentrations of specific ions. This approach has applications in analytical chemistry, environmental monitoring, and quality control processes.
- Modification of phenolphthalein for enhanced pH sensitivity: Research efforts have focused on modifying the phenolphthalein molecule or incorporating it into composite materials to enhance its pH sensitivity and kinetic properties. These modifications aim to improve the response time, expand the working pH range, or introduce additional functionalities. Such enhancements can lead to more sensitive and versatile pH-responsive systems with applications in areas like biosensors, smart packaging, and controlled release technologies.
02 Applications in pH-sensitive materials and indicators
The pH-dependent kinetic changes of phenolphthalein are utilized in various applications, including pH-sensitive materials and indicators. These applications range from simple pH test strips to more complex systems such as smart polymers and drug delivery systems that respond to changes in environmental pH. The rate of color change can be tailored for specific applications by modifying the molecular structure or incorporating it into different matrices.Expand Specific Solutions03 Influence of environmental factors on phenolphthalein kinetics
Environmental factors such as temperature, ionic strength, and solvent composition can significantly affect the kinetics of phenolphthalein's pH-dependent changes. These factors influence the rate of protonation/deprotonation reactions and the stability of different molecular forms. Understanding these influences is crucial for optimizing phenolphthalein-based systems and ensuring reliable performance across various conditions.Expand Specific Solutions04 Quantitative analysis using phenolphthalein kinetics
The kinetics of phenolphthalein's pH-dependent changes can be used for quantitative analysis in various fields. By monitoring the rate of color change or the time required to reach a specific color intensity, it is possible to determine pH values, buffer capacities, or concentrations of specific analytes. This approach offers advantages in terms of sensitivity and dynamic range compared to traditional equilibrium-based measurements.Expand Specific Solutions05 Molecular modifications to tune phenolphthalein kinetics
Structural modifications of the phenolphthalein molecule can be used to tune its pH-dependent kinetic properties. These modifications can alter the pKa values, change the color transition range, or modify the rate of structural changes. Such tailored phenolphthalein derivatives find applications in areas requiring specific pH sensitivity or response times, such as in biomedical sensors or industrial process monitoring.Expand Specific Solutions
Key Players in pH Detection Industry
The research on phenolphthalein for detecting pH-dependent kinetic changes is in a mature stage, with established applications in various industries. The market for this technology is moderate but stable, driven by its widespread use in analytical chemistry and biochemistry. Companies like Life Technologies Corp. and Promega Corp. are key players, offering advanced reagents and instruments for pH-sensitive applications. Academic institutions such as MIT and the National University of Singapore contribute significantly to ongoing research and development in this field. The technology's maturity is evident in its integration into standard laboratory practices, with continuous refinements focusing on improving sensitivity and expanding applications in biomedical and environmental monitoring.
Promega Corp.
Technical Solution: Promega has developed a bioluminescent pH sensor system that incorporates phenolphthalein principles for intracellular pH measurement. Their technology uses genetically encoded pH-sensitive luciferase variants that change their light emission properties based on pH, similar to the color change mechanism of phenolphthalein. This allows for non-invasive, real-time monitoring of intracellular pH changes in living cells[6]. Promega has also created assay kits that combine this technology with their established luminescence detection platforms for high-throughput screening of compounds affecting cellular pH homeostasis[8].
Strengths: Non-invasive intracellular measurements, compatibility with existing luminescence detection systems. Weaknesses: May require genetic modification of target cells, potentially limiting its use in some applications.
Endress+Hauser Conducta GmbH+Co. KG
Technical Solution: Endress+Hauser has developed advanced pH measurement systems that incorporate phenolphthalein-based optical sensors for industrial process control. Their technology uses a modified form of phenolphthalein immobilized on optical fibers, allowing for continuous, real-time pH monitoring in harsh industrial environments. The system is designed to withstand high temperatures, pressures, and chemical exposure, making it suitable for applications in chemical manufacturing, wastewater treatment, and food processing[9]. Additionally, they have integrated this technology with their digital communication platforms for remote monitoring and predictive maintenance of pH-critical processes[10].
Strengths: Robust design for industrial applications, integration with digital platforms for process control. Weaknesses: May be overspecialized for general research applications, potentially high cost for non-industrial users.
Core Innovations in pH-Dependent Kinetics
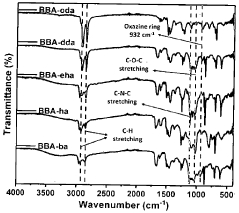
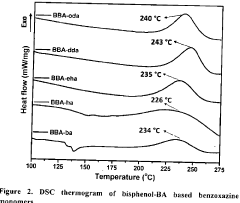
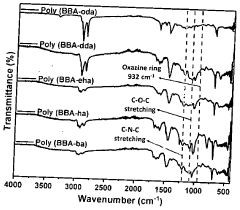
Production of extreme range of PH indicators from benzoxazines
PatentActiveIN202341027342A
Innovation
- Development of bisphenol-BA/aliphatic amine based hydrophobic polybenzoxazines coated on cellulose paper, synthesized through Mannich condensation, which exhibit distinct color changes across a wide pH range from -1.8 to 14, offering thermal stability and repeated use capability.
Environmental Impact of pH Indicators
The use of pH indicators, such as phenolphthalein, in various scientific and industrial applications has raised concerns about their potential environmental impact. As these chemicals are increasingly utilized for detecting pH-dependent kinetic changes, it is crucial to assess their effects on ecosystems and human health.
One of the primary environmental concerns associated with pH indicators is their persistence in aquatic environments. When released into water bodies, these compounds can remain stable for extended periods, potentially affecting aquatic organisms and disrupting natural pH balances. Studies have shown that some pH indicators, including phenolphthalein, may accumulate in sediments and bioaccumulate in aquatic organisms, leading to long-term ecological consequences.
The toxicity of pH indicators to aquatic life is another significant consideration. While many of these compounds are considered relatively safe at low concentrations, prolonged exposure or higher concentrations can have adverse effects on fish, invertebrates, and algae. For instance, phenolphthalein has been found to cause reproductive issues in certain fish species and inhibit algal growth at elevated concentrations.
Soil contamination is an additional concern when pH indicators are improperly disposed of or accidentally released into the environment. These chemicals can alter soil pH, potentially affecting plant growth and soil microbial communities. The mobility of pH indicators in soil can also lead to groundwater contamination, posing risks to drinking water sources and underground ecosystems.
The production and disposal of pH indicators contribute to their environmental footprint. Manufacturing processes often involve the use of hazardous chemicals and energy-intensive procedures, resulting in greenhouse gas emissions and potential chemical waste. Improper disposal of these indicators, particularly in laboratory and industrial settings, can lead to environmental contamination if not managed correctly.
Efforts to mitigate the environmental impact of pH indicators include developing more environmentally friendly alternatives, improving waste management practices, and implementing stricter regulations on their use and disposal. Some researchers are exploring biodegradable pH indicators or those derived from natural sources to reduce environmental persistence and toxicity.
In conclusion, while pH indicators like phenolphthalein play a crucial role in scientific research and industrial processes, their potential environmental impact cannot be overlooked. Balancing the benefits of these compounds with their ecological risks requires ongoing research, responsible use, and the development of sustainable alternatives to ensure the long-term health of our ecosystems.
One of the primary environmental concerns associated with pH indicators is their persistence in aquatic environments. When released into water bodies, these compounds can remain stable for extended periods, potentially affecting aquatic organisms and disrupting natural pH balances. Studies have shown that some pH indicators, including phenolphthalein, may accumulate in sediments and bioaccumulate in aquatic organisms, leading to long-term ecological consequences.
The toxicity of pH indicators to aquatic life is another significant consideration. While many of these compounds are considered relatively safe at low concentrations, prolonged exposure or higher concentrations can have adverse effects on fish, invertebrates, and algae. For instance, phenolphthalein has been found to cause reproductive issues in certain fish species and inhibit algal growth at elevated concentrations.
Soil contamination is an additional concern when pH indicators are improperly disposed of or accidentally released into the environment. These chemicals can alter soil pH, potentially affecting plant growth and soil microbial communities. The mobility of pH indicators in soil can also lead to groundwater contamination, posing risks to drinking water sources and underground ecosystems.
The production and disposal of pH indicators contribute to their environmental footprint. Manufacturing processes often involve the use of hazardous chemicals and energy-intensive procedures, resulting in greenhouse gas emissions and potential chemical waste. Improper disposal of these indicators, particularly in laboratory and industrial settings, can lead to environmental contamination if not managed correctly.
Efforts to mitigate the environmental impact of pH indicators include developing more environmentally friendly alternatives, improving waste management practices, and implementing stricter regulations on their use and disposal. Some researchers are exploring biodegradable pH indicators or those derived from natural sources to reduce environmental persistence and toxicity.
In conclusion, while pH indicators like phenolphthalein play a crucial role in scientific research and industrial processes, their potential environmental impact cannot be overlooked. Balancing the benefits of these compounds with their ecological risks requires ongoing research, responsible use, and the development of sustainable alternatives to ensure the long-term health of our ecosystems.
Standardization of pH Detection Methods
The standardization of pH detection methods is crucial for ensuring consistent and reliable results in research and industrial applications. This process involves establishing uniform protocols, calibration procedures, and quality control measures across different laboratories and industries.
One of the primary considerations in standardizing pH detection methods is the selection of appropriate pH indicators. Phenolphthalein, a widely used indicator, has been extensively studied for its ability to detect pH-dependent kinetic changes. Its color transition from colorless to pink in the pH range of 8.2 to 10.0 makes it particularly useful for alkaline titrations and endpoint detection in various analytical procedures.
To standardize pH detection methods using phenolphthalein, it is essential to establish precise preparation protocols for indicator solutions. This includes specifying the concentration of phenolphthalein, the solvent composition, and storage conditions to maintain stability and consistency. Additionally, guidelines for the volume of indicator to be used in different applications should be clearly defined to ensure reproducibility.
Calibration procedures play a critical role in standardization efforts. This involves the use of certified buffer solutions with known pH values to create calibration curves for pH meters and spectrophotometric methods. Regular calibration checks and the use of quality control samples are necessary to maintain accuracy and precision in pH measurements across different laboratories and over time.
Temperature compensation is another crucial aspect of standardizing pH detection methods. As pH measurements are temperature-dependent, standardized protocols should include guidelines for temperature control during measurements and the use of temperature compensation algorithms in pH meters.
Interlaboratory comparisons and proficiency testing programs are essential components of the standardization process. These initiatives help identify discrepancies between different laboratories and methodologies, leading to the refinement of protocols and the establishment of best practices.
The development of standard operating procedures (SOPs) for pH detection using phenolphthalein is a key outcome of the standardization process. These SOPs should cover all aspects of the measurement process, including sample preparation, indicator addition, measurement techniques, data recording, and interpretation of results.
Lastly, the standardization of pH detection methods must also consider the limitations and potential interferences associated with phenolphthalein. This includes understanding its behavior in the presence of certain ions, organic compounds, and extreme temperatures. By addressing these factors, the standardized methods can provide more reliable and comparable results across different applications and research settings.
One of the primary considerations in standardizing pH detection methods is the selection of appropriate pH indicators. Phenolphthalein, a widely used indicator, has been extensively studied for its ability to detect pH-dependent kinetic changes. Its color transition from colorless to pink in the pH range of 8.2 to 10.0 makes it particularly useful for alkaline titrations and endpoint detection in various analytical procedures.
To standardize pH detection methods using phenolphthalein, it is essential to establish precise preparation protocols for indicator solutions. This includes specifying the concentration of phenolphthalein, the solvent composition, and storage conditions to maintain stability and consistency. Additionally, guidelines for the volume of indicator to be used in different applications should be clearly defined to ensure reproducibility.
Calibration procedures play a critical role in standardization efforts. This involves the use of certified buffer solutions with known pH values to create calibration curves for pH meters and spectrophotometric methods. Regular calibration checks and the use of quality control samples are necessary to maintain accuracy and precision in pH measurements across different laboratories and over time.
Temperature compensation is another crucial aspect of standardizing pH detection methods. As pH measurements are temperature-dependent, standardized protocols should include guidelines for temperature control during measurements and the use of temperature compensation algorithms in pH meters.
Interlaboratory comparisons and proficiency testing programs are essential components of the standardization process. These initiatives help identify discrepancies between different laboratories and methodologies, leading to the refinement of protocols and the establishment of best practices.
The development of standard operating procedures (SOPs) for pH detection using phenolphthalein is a key outcome of the standardization process. These SOPs should cover all aspects of the measurement process, including sample preparation, indicator addition, measurement techniques, data recording, and interpretation of results.
Lastly, the standardization of pH detection methods must also consider the limitations and potential interferences associated with phenolphthalein. This includes understanding its behavior in the presence of certain ions, organic compounds, and extreme temperatures. By addressing these factors, the standardized methods can provide more reliable and comparable results across different applications and research settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!