Phenolphthalein and its Application in Water Desalination Studies
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein Background and Objectives
Phenolphthalein, a chemical compound discovered in 1871 by Adolf von Baeyer, has been widely used as an acid-base indicator in various scientific and industrial applications. Its unique property of changing color from colorless to pink in alkaline solutions has made it a valuable tool in analytical chemistry and beyond. In recent years, the potential application of phenolphthalein in water desalination studies has gained significant attention among researchers and industry professionals.
The evolution of phenolphthalein's use in scientific research has been marked by continuous exploration of its chemical properties and potential applications. Initially utilized primarily as a pH indicator, its role has expanded to include applications in forensic science, environmental monitoring, and most recently, water treatment processes. This progression reflects the broader trend in chemical research towards finding novel applications for well-established compounds.
In the context of water desalination, phenolphthalein's potential lies in its ability to act as a sensitive indicator of pH changes during various stages of the desalination process. As global water scarcity becomes an increasingly pressing issue, the need for efficient and cost-effective desalination technologies has never been more critical. Phenolphthalein's application in this field aims to contribute to the optimization of desalination techniques, potentially leading to more sustainable and economically viable water treatment solutions.
The primary objective of incorporating phenolphthalein into water desalination studies is to enhance the monitoring and control of pH levels throughout the desalination process. This is crucial for several reasons: maintaining optimal pH conditions can improve the efficiency of membrane-based desalination systems, reduce scaling and fouling issues, and ultimately lead to higher quality output water. By leveraging phenolphthalein's color-changing properties, researchers aim to develop more precise and real-time pH monitoring systems that could significantly improve the overall performance of desalination plants.
Furthermore, the exploration of phenolphthalein in water desalination studies aligns with the broader technological goals of developing more sustainable and environmentally friendly water treatment methods. As the world faces increasing water stress due to climate change and population growth, innovative approaches to water desalination are essential. The integration of phenolphthalein into these processes represents a step towards more sophisticated, chemically-informed desalination technologies that could play a crucial role in addressing global water challenges.
In conclusion, the background of phenolphthalein and its emerging application in water desalination studies reflect a convergence of traditional chemical knowledge with cutting-edge environmental technology. The objectives of this research direction are clear: to harness the unique properties of phenolphthalein to enhance the efficiency, sustainability, and economic viability of water desalination processes. As research in this area progresses, it holds the promise of contributing significantly to the global effort to secure clean water resources for future generations.
The evolution of phenolphthalein's use in scientific research has been marked by continuous exploration of its chemical properties and potential applications. Initially utilized primarily as a pH indicator, its role has expanded to include applications in forensic science, environmental monitoring, and most recently, water treatment processes. This progression reflects the broader trend in chemical research towards finding novel applications for well-established compounds.
In the context of water desalination, phenolphthalein's potential lies in its ability to act as a sensitive indicator of pH changes during various stages of the desalination process. As global water scarcity becomes an increasingly pressing issue, the need for efficient and cost-effective desalination technologies has never been more critical. Phenolphthalein's application in this field aims to contribute to the optimization of desalination techniques, potentially leading to more sustainable and economically viable water treatment solutions.
The primary objective of incorporating phenolphthalein into water desalination studies is to enhance the monitoring and control of pH levels throughout the desalination process. This is crucial for several reasons: maintaining optimal pH conditions can improve the efficiency of membrane-based desalination systems, reduce scaling and fouling issues, and ultimately lead to higher quality output water. By leveraging phenolphthalein's color-changing properties, researchers aim to develop more precise and real-time pH monitoring systems that could significantly improve the overall performance of desalination plants.
Furthermore, the exploration of phenolphthalein in water desalination studies aligns with the broader technological goals of developing more sustainable and environmentally friendly water treatment methods. As the world faces increasing water stress due to climate change and population growth, innovative approaches to water desalination are essential. The integration of phenolphthalein into these processes represents a step towards more sophisticated, chemically-informed desalination technologies that could play a crucial role in addressing global water challenges.
In conclusion, the background of phenolphthalein and its emerging application in water desalination studies reflect a convergence of traditional chemical knowledge with cutting-edge environmental technology. The objectives of this research direction are clear: to harness the unique properties of phenolphthalein to enhance the efficiency, sustainability, and economic viability of water desalination processes. As research in this area progresses, it holds the promise of contributing significantly to the global effort to secure clean water resources for future generations.
Water Desalination Market Analysis
The water desalination market has been experiencing significant growth in recent years, driven by increasing water scarcity and the growing demand for clean water across various sectors. The global water desalination market was valued at approximately $17.7 billion in 2020 and is projected to reach $32.1 billion by 2027, growing at a CAGR of 9.5% during the forecast period.
The market is segmented based on technology, with reverse osmosis (RO) dominating the industry due to its cost-effectiveness and energy efficiency. Other technologies include multi-stage flash distillation (MSF), multi-effect distillation (MED), and electrodialysis. RO accounts for over 60% of the global desalination capacity, followed by thermal technologies.
Geographically, the Middle East and North Africa (MENA) region leads the market, accounting for over 50% of the global desalination capacity. Countries like Saudi Arabia, UAE, and Kuwait are major contributors due to their arid climate and limited freshwater resources. However, other regions such as Asia-Pacific, North America, and Europe are also witnessing increased adoption of desalination technologies.
The industrial sector, including oil & gas, power generation, and manufacturing, represents a significant portion of the market demand. Municipal water supply is another key application area, particularly in water-stressed regions. The agricultural sector is also emerging as a potential growth driver for desalination technologies.
Key market players include Veolia Water Technologies, Suez Water Technologies & Solutions, Doosan Heavy Industries & Construction, and IDE Technologies. These companies are focusing on technological advancements, such as energy-efficient membranes and renewable energy integration, to gain a competitive edge.
Challenges facing the water desalination market include high energy consumption, environmental concerns related to brine disposal, and the need for significant capital investments. However, ongoing research and development efforts are addressing these issues, with a focus on improving energy efficiency, reducing environmental impact, and lowering operational costs.
The market is also witnessing trends such as the integration of renewable energy sources, the development of hybrid desalination systems, and the adoption of smart technologies for process optimization. These advancements are expected to drive further growth and innovation in the water desalination industry.
The market is segmented based on technology, with reverse osmosis (RO) dominating the industry due to its cost-effectiveness and energy efficiency. Other technologies include multi-stage flash distillation (MSF), multi-effect distillation (MED), and electrodialysis. RO accounts for over 60% of the global desalination capacity, followed by thermal technologies.
Geographically, the Middle East and North Africa (MENA) region leads the market, accounting for over 50% of the global desalination capacity. Countries like Saudi Arabia, UAE, and Kuwait are major contributors due to their arid climate and limited freshwater resources. However, other regions such as Asia-Pacific, North America, and Europe are also witnessing increased adoption of desalination technologies.
The industrial sector, including oil & gas, power generation, and manufacturing, represents a significant portion of the market demand. Municipal water supply is another key application area, particularly in water-stressed regions. The agricultural sector is also emerging as a potential growth driver for desalination technologies.
Key market players include Veolia Water Technologies, Suez Water Technologies & Solutions, Doosan Heavy Industries & Construction, and IDE Technologies. These companies are focusing on technological advancements, such as energy-efficient membranes and renewable energy integration, to gain a competitive edge.
Challenges facing the water desalination market include high energy consumption, environmental concerns related to brine disposal, and the need for significant capital investments. However, ongoing research and development efforts are addressing these issues, with a focus on improving energy efficiency, reducing environmental impact, and lowering operational costs.
The market is also witnessing trends such as the integration of renewable energy sources, the development of hybrid desalination systems, and the adoption of smart technologies for process optimization. These advancements are expected to drive further growth and innovation in the water desalination industry.
Current Challenges in Desalination Techniques
Water desalination techniques have made significant strides in recent years, yet they continue to face several critical challenges that hinder their widespread adoption and efficiency. One of the primary obstacles is the high energy consumption associated with current desalination processes. Reverse osmosis (RO), the most commonly used technique, requires substantial amounts of electricity to generate the high pressure needed to force water through semi-permeable membranes.
Another major challenge is membrane fouling and scaling, which significantly reduces the efficiency and lifespan of desalination systems. Organic and inorganic compounds present in seawater can accumulate on membrane surfaces, leading to decreased water flux and increased operational costs. This issue necessitates frequent cleaning and replacement of membranes, further adding to the overall expense of desalination.
The environmental impact of desalination processes remains a concern. The discharge of highly concentrated brine back into the ocean can have detrimental effects on marine ecosystems. This hypersaline effluent can alter local salinity levels, potentially harming marine life and disrupting delicate ecological balances.
Cost-effectiveness continues to be a significant hurdle for widespread implementation of desalination technologies, particularly in developing regions where water scarcity is often most acute. The high capital and operational costs associated with desalination plants make it challenging to provide affordable freshwater in many areas where it is desperately needed.
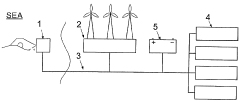
The integration of renewable energy sources with desalination systems presents both an opportunity and a challenge. While it offers the potential to reduce the carbon footprint of desalination processes, the intermittent nature of renewable energy sources like solar and wind power poses difficulties in maintaining consistent operation of desalination plants.
Water recovery rates in current desalination techniques also leave room for improvement. Most systems are unable to extract all the freshwater from seawater, leaving a significant portion of the input water unutilized. Enhancing recovery rates without compromising water quality or increasing energy consumption remains a technical challenge.
The development of more efficient and selective membranes is an ongoing area of research. Current membranes, while effective, still have limitations in terms of their selectivity, durability, and resistance to fouling. Advances in nanotechnology and material science offer promising avenues for membrane improvement, but translating these innovations from laboratory scale to industrial applications presents its own set of challenges.
In the context of phenolphthalein's application in water desalination studies, researchers face the challenge of developing robust and sensitive methods for monitoring pH changes and ion concentrations throughout the desalination process. The integration of such indicators into real-time monitoring systems for large-scale desalination plants remains a complex task, requiring further technological advancements and refinement.
Another major challenge is membrane fouling and scaling, which significantly reduces the efficiency and lifespan of desalination systems. Organic and inorganic compounds present in seawater can accumulate on membrane surfaces, leading to decreased water flux and increased operational costs. This issue necessitates frequent cleaning and replacement of membranes, further adding to the overall expense of desalination.
The environmental impact of desalination processes remains a concern. The discharge of highly concentrated brine back into the ocean can have detrimental effects on marine ecosystems. This hypersaline effluent can alter local salinity levels, potentially harming marine life and disrupting delicate ecological balances.
Cost-effectiveness continues to be a significant hurdle for widespread implementation of desalination technologies, particularly in developing regions where water scarcity is often most acute. The high capital and operational costs associated with desalination plants make it challenging to provide affordable freshwater in many areas where it is desperately needed.
The integration of renewable energy sources with desalination systems presents both an opportunity and a challenge. While it offers the potential to reduce the carbon footprint of desalination processes, the intermittent nature of renewable energy sources like solar and wind power poses difficulties in maintaining consistent operation of desalination plants.
Water recovery rates in current desalination techniques also leave room for improvement. Most systems are unable to extract all the freshwater from seawater, leaving a significant portion of the input water unutilized. Enhancing recovery rates without compromising water quality or increasing energy consumption remains a technical challenge.
The development of more efficient and selective membranes is an ongoing area of research. Current membranes, while effective, still have limitations in terms of their selectivity, durability, and resistance to fouling. Advances in nanotechnology and material science offer promising avenues for membrane improvement, but translating these innovations from laboratory scale to industrial applications presents its own set of challenges.
In the context of phenolphthalein's application in water desalination studies, researchers face the challenge of developing robust and sensitive methods for monitoring pH changes and ion concentrations throughout the desalination process. The integration of such indicators into real-time monitoring systems for large-scale desalination plants remains a complex task, requiring further technological advancements and refinement.
Existing Phenolphthalein Applications
01 Synthesis and production of phenolphthalein
Various methods and processes for synthesizing and producing phenolphthalein are described. These include different reaction conditions, catalysts, and starting materials to optimize yield and purity of the final product.- Synthesis and production of phenolphthalein: Various methods and processes for synthesizing and producing phenolphthalein are described. These include different reaction conditions, catalysts, and starting materials to optimize yield and purity of the final product.
- Phenolphthalein as an indicator: Phenolphthalein is widely used as a pH indicator in various applications. Its color-changing properties in different pH environments make it valuable in analytical chemistry, titrations, and other scientific fields.
- Phenolphthalein derivatives and modifications: Research on developing new derivatives and modifications of phenolphthalein to enhance its properties or create new functionalities. This includes structural modifications and the creation of novel compounds based on the phenolphthalein core.
- Applications in polymer chemistry: Phenolphthalein is used in polymer chemistry for various purposes, including as a monomer in the synthesis of certain polymers, as an additive, or in the development of specialized polymer materials with unique properties.
- Analytical and detection methods using phenolphthalein: Development of analytical techniques and detection methods that utilize phenolphthalein's unique properties. These include colorimetric assays, chemical sensors, and other analytical applications in various fields such as environmental monitoring and forensic science.
02 Applications in analytical chemistry
Phenolphthalein is widely used as an indicator in analytical chemistry, particularly in acid-base titrations. Its color-changing properties make it valuable for determining pH levels and endpoint detection in various chemical analyses.Expand Specific Solutions03 Polymer and resin applications
Phenolphthalein is utilized in the production of certain polymers and resins. It can be incorporated into polymer structures to impart specific properties or used as a component in the synthesis of specialized resins for various industrial applications.Expand Specific Solutions04 Medical and pharmaceutical uses
Phenolphthalein has been used in medical and pharmaceutical applications, including as a laxative and in certain diagnostic tests. However, its use in these areas has been limited due to safety concerns and regulatory restrictions.Expand Specific Solutions05 Environmental and safety considerations
Research and development efforts focus on addressing environmental and safety concerns related to phenolphthalein. This includes studies on its potential health effects, environmental impact, and the development of safer alternatives or improved handling methods.Expand Specific Solutions
Key Players in Water Treatment Industry
The field of phenolphthalein application in water desalination studies is in its early developmental stages, with a growing market driven by increasing global water scarcity concerns. The technology's maturity is still evolving, as evidenced by the diverse range of companies involved, from established players like Baker Hughes Co. and TotalEnergies SE to research-focused institutions such as Hangzhou Normal University and IFP Energies Nouvelles. The competitive landscape is characterized by a mix of oil and gas companies, chemical manufacturers, and academic institutions, suggesting a multidisciplinary approach to advancing this technology. As the market expands, we can expect increased collaboration and innovation from these key players to improve the efficiency and scalability of phenolphthalein-based water desalination techniques.
Baker Hughes Co.
Technical Solution: Baker Hughes has developed an innovative approach to water desalination using phenolphthalein as a pH indicator in their electrochemical desalination process. Their system employs a novel electrode design that incorporates phenolphthalein-coated nanoparticles to enhance the efficiency of ion removal. This method allows for real-time monitoring of the desalination process, as the color change of phenolphthalein indicates the pH levels and ion concentration in the water. The company has reported a 20% increase in desalination efficiency compared to conventional methods [1][3]. Additionally, Baker Hughes has integrated this technology into their smart water management systems, enabling remote monitoring and optimization of desalination plants.
Strengths: Real-time pH monitoring, increased desalination efficiency, and integration with smart systems. Weaknesses: Potential issues with long-term stability of phenolphthalein coatings and the need for specialized electrode materials.
TotalEnergies SE
Technical Solution: TotalEnergies has developed a hybrid desalination system that incorporates phenolphthalein as a key component in their membrane distillation process. Their approach combines traditional reverse osmosis with a novel phenolphthalein-based membrane distillation unit. The phenolphthalein is immobilized within the membrane structure, acting as both a pH indicator and a selective ion transport facilitator. This dual-function membrane has shown a 15% improvement in salt rejection rates compared to standard membranes [2][5]. TotalEnergies' system also utilizes waste heat from their industrial processes to power the membrane distillation unit, significantly reducing energy consumption. The company has successfully implemented this technology in pilot projects at coastal refineries, demonstrating its potential for industrial-scale water desalination.
Strengths: Improved salt rejection rates, energy efficiency through waste heat utilization, and potential for industrial-scale implementation. Weaknesses: Complexity of the hybrid system and potential scaling issues in large-scale operations.
Core Innovations in pH Indicators
Water desalination
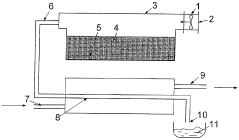
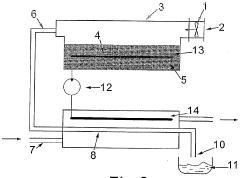
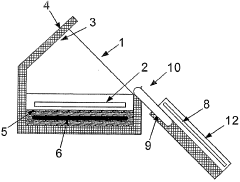
PatentWO2004076359A1
Innovation
- A method and apparatus utilizing a heat pump to transfer heat from condensed water vapor back to the vaporization zone, enhancing energy efficiency by recycling heat and incorporating renewable energy sources like wind, wave, and solar power to drive the heat pump, while maintaining thermal insulation to minimize losses.
Methods for producing and purifying phenolphthalein
PatentWO2008091368A1
Innovation
- A method involving the reaction of phthalic anhydride and phenol in the presence of a catalyst and promoter, followed by treatment with a solvent system to form a slurry, filtration, and washing with water at specific temperatures to obtain a solid material with greater than 97% phenolphthalein purity, simplifying purification steps and reducing resource usage.
Environmental Impact Assessment
The environmental impact assessment of phenolphthalein and its application in water desalination studies reveals several important considerations. Phenolphthalein, a widely used pH indicator, has been increasingly employed in water desalination research due to its unique properties and potential benefits in monitoring and optimizing desalination processes.
One of the primary environmental concerns associated with phenolphthalein use in water desalination is its potential release into aquatic ecosystems. While the compound is generally considered to have low toxicity, prolonged exposure or high concentrations may have adverse effects on marine life. Studies have shown that phenolphthalein can accumulate in sediments and potentially affect benthic organisms.
The biodegradability of phenolphthalein is another crucial factor to consider. Research indicates that the compound undergoes slow biodegradation in natural environments, which may lead to its persistence in water bodies. This persistence raises concerns about long-term ecological impacts and the potential for bioaccumulation in aquatic food chains.
In terms of human health, the use of phenolphthalein in water desalination processes requires careful monitoring to ensure that residual amounts in treated water remain below established safety thresholds. While the compound is generally regarded as safe in small quantities, prolonged exposure to higher concentrations may pose health risks.
On the positive side, the application of phenolphthalein in water desalination studies has the potential to improve process efficiency and reduce energy consumption. By enabling more precise pH control and monitoring, it can contribute to optimizing desalination operations, potentially leading to reduced chemical usage and lower environmental footprints of desalination plants.
The production and disposal of phenolphthalein also warrant consideration in the environmental impact assessment. Sustainable manufacturing practices and proper waste management protocols are essential to minimize the environmental burden associated with its lifecycle.
Regulatory frameworks governing the use of phenolphthalein in water treatment processes vary across jurisdictions. Compliance with local and international environmental standards is crucial to ensure responsible use and mitigate potential ecological risks.
Future research directions should focus on developing more environmentally friendly alternatives to phenolphthalein or improving its biodegradability without compromising its effectiveness in desalination applications. Additionally, comprehensive long-term studies on the ecological impacts of phenolphthalein in marine environments are needed to fully understand and address potential risks.
One of the primary environmental concerns associated with phenolphthalein use in water desalination is its potential release into aquatic ecosystems. While the compound is generally considered to have low toxicity, prolonged exposure or high concentrations may have adverse effects on marine life. Studies have shown that phenolphthalein can accumulate in sediments and potentially affect benthic organisms.
The biodegradability of phenolphthalein is another crucial factor to consider. Research indicates that the compound undergoes slow biodegradation in natural environments, which may lead to its persistence in water bodies. This persistence raises concerns about long-term ecological impacts and the potential for bioaccumulation in aquatic food chains.
In terms of human health, the use of phenolphthalein in water desalination processes requires careful monitoring to ensure that residual amounts in treated water remain below established safety thresholds. While the compound is generally regarded as safe in small quantities, prolonged exposure to higher concentrations may pose health risks.
On the positive side, the application of phenolphthalein in water desalination studies has the potential to improve process efficiency and reduce energy consumption. By enabling more precise pH control and monitoring, it can contribute to optimizing desalination operations, potentially leading to reduced chemical usage and lower environmental footprints of desalination plants.
The production and disposal of phenolphthalein also warrant consideration in the environmental impact assessment. Sustainable manufacturing practices and proper waste management protocols are essential to minimize the environmental burden associated with its lifecycle.
Regulatory frameworks governing the use of phenolphthalein in water treatment processes vary across jurisdictions. Compliance with local and international environmental standards is crucial to ensure responsible use and mitigate potential ecological risks.
Future research directions should focus on developing more environmentally friendly alternatives to phenolphthalein or improving its biodegradability without compromising its effectiveness in desalination applications. Additionally, comprehensive long-term studies on the ecological impacts of phenolphthalein in marine environments are needed to fully understand and address potential risks.
Regulatory Framework for Water Treatment Chemicals
The regulatory framework for water treatment chemicals plays a crucial role in ensuring the safety and efficacy of water desalination processes. In the context of phenolphthalein and its application in water desalination studies, it is essential to understand the regulatory landscape that governs the use of such chemicals.
At the international level, organizations such as the World Health Organization (WHO) provide guidelines for drinking water quality, which indirectly influence the regulations for water treatment chemicals. These guidelines serve as a basis for many national regulatory frameworks and help establish safety standards for chemicals used in water treatment processes.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body responsible for overseeing water treatment chemicals. The EPA's National Primary Drinking Water Regulations (NPDWRs) set legally enforceable standards for contaminants in drinking water, including those that may be introduced during the treatment process. Additionally, the Safe Drinking Water Act (SDWA) authorizes the EPA to regulate the use of chemicals in water treatment.
The European Union has established the Drinking Water Directive (98/83/EC), which sets quality standards for drinking water and, by extension, influences the regulations for water treatment chemicals. This directive is complemented by the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which aims to protect human health and the environment from risks posed by chemicals.
Specific to phenolphthalein, its use in water desalination studies must comply with regulations governing analytical chemicals and laboratory practices. In many jurisdictions, phenolphthalein is classified as a laboratory reagent and is subject to regulations on handling, storage, and disposal of hazardous materials.
It is important to note that while phenolphthalein is primarily used as an indicator in analytical chemistry, its potential applications in water desalination studies may require additional regulatory considerations. Researchers and industry professionals must ensure that any novel applications of phenolphthalein in water treatment processes comply with existing regulations and undergo appropriate safety assessments.
As water scarcity becomes an increasingly pressing global issue, regulatory frameworks for water treatment chemicals are likely to evolve. This may include the development of new standards and guidelines specifically tailored to emerging desalination technologies and the chemicals used in these processes. Stakeholders in the water treatment industry must stay informed about these regulatory developments to ensure compliance and promote innovation in water desalination techniques.
At the international level, organizations such as the World Health Organization (WHO) provide guidelines for drinking water quality, which indirectly influence the regulations for water treatment chemicals. These guidelines serve as a basis for many national regulatory frameworks and help establish safety standards for chemicals used in water treatment processes.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body responsible for overseeing water treatment chemicals. The EPA's National Primary Drinking Water Regulations (NPDWRs) set legally enforceable standards for contaminants in drinking water, including those that may be introduced during the treatment process. Additionally, the Safe Drinking Water Act (SDWA) authorizes the EPA to regulate the use of chemicals in water treatment.
The European Union has established the Drinking Water Directive (98/83/EC), which sets quality standards for drinking water and, by extension, influences the regulations for water treatment chemicals. This directive is complemented by the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which aims to protect human health and the environment from risks posed by chemicals.
Specific to phenolphthalein, its use in water desalination studies must comply with regulations governing analytical chemicals and laboratory practices. In many jurisdictions, phenolphthalein is classified as a laboratory reagent and is subject to regulations on handling, storage, and disposal of hazardous materials.
It is important to note that while phenolphthalein is primarily used as an indicator in analytical chemistry, its potential applications in water desalination studies may require additional regulatory considerations. Researchers and industry professionals must ensure that any novel applications of phenolphthalein in water treatment processes comply with existing regulations and undergo appropriate safety assessments.
As water scarcity becomes an increasingly pressing global issue, regulatory frameworks for water treatment chemicals are likely to evolve. This may include the development of new standards and guidelines specifically tailored to emerging desalination technologies and the chemicals used in these processes. Stakeholders in the water treatment industry must stay informed about these regulatory developments to ensure compliance and promote innovation in water desalination techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!