Phenolphthalein in Enhancing Detection Capabilities of Biochips
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein Biochip Evolution and Objectives
Phenolphthalein, a compound traditionally used as a pH indicator, has emerged as a promising agent in enhancing the detection capabilities of biochips. The evolution of this technology can be traced back to the early 2000s when researchers began exploring novel applications for phenolphthalein beyond its conventional use in acid-base titrations.
The initial phase of phenolphthalein integration into biochip technology focused on leveraging its color-changing properties to develop more sensitive and visually interpretable biosensors. This approach aimed to overcome the limitations of existing detection methods, which often required sophisticated equipment or complex data analysis.
As research progressed, scientists discovered that phenolphthalein could be chemically modified to interact with specific biomolecules, expanding its potential applications in biochip design. This breakthrough led to the development of phenolphthalein-based probes capable of detecting a wide range of analytes, from small molecules to proteins and nucleic acids.
The mid-2010s saw a significant leap in phenolphthalein biochip technology with the introduction of nanostructured surfaces. By incorporating phenolphthalein into nanomaterials, researchers were able to dramatically increase the surface area for molecular interactions, resulting in enhanced sensitivity and faster response times.
Recent advancements have focused on creating multi-functional biochips that combine phenolphthalein with other detection mechanisms. This synergistic approach aims to provide comprehensive analytical capabilities within a single device, enabling simultaneous detection of multiple biomarkers or environmental contaminants.
The primary objective of current research on phenolphthalein in biochips is to develop highly sensitive, specific, and cost-effective detection platforms for various applications. These include medical diagnostics, environmental monitoring, food safety testing, and forensic analysis. Researchers are particularly interested in creating point-of-care devices that can provide rapid and accurate results in resource-limited settings.
Another key goal is to improve the stability and shelf-life of phenolphthalein-based biochips, addressing challenges related to long-term storage and field deployment. This involves exploring novel encapsulation techniques and developing robust manufacturing processes to ensure consistent performance under diverse environmental conditions.
Looking ahead, the field aims to integrate phenolphthalein biochips with emerging technologies such as artificial intelligence and Internet of Things (IoT) platforms. This convergence is expected to enable real-time data analysis, remote monitoring, and predictive diagnostics, further expanding the potential applications and impact of phenolphthalein-enhanced biochip technology.
The initial phase of phenolphthalein integration into biochip technology focused on leveraging its color-changing properties to develop more sensitive and visually interpretable biosensors. This approach aimed to overcome the limitations of existing detection methods, which often required sophisticated equipment or complex data analysis.
As research progressed, scientists discovered that phenolphthalein could be chemically modified to interact with specific biomolecules, expanding its potential applications in biochip design. This breakthrough led to the development of phenolphthalein-based probes capable of detecting a wide range of analytes, from small molecules to proteins and nucleic acids.
The mid-2010s saw a significant leap in phenolphthalein biochip technology with the introduction of nanostructured surfaces. By incorporating phenolphthalein into nanomaterials, researchers were able to dramatically increase the surface area for molecular interactions, resulting in enhanced sensitivity and faster response times.
Recent advancements have focused on creating multi-functional biochips that combine phenolphthalein with other detection mechanisms. This synergistic approach aims to provide comprehensive analytical capabilities within a single device, enabling simultaneous detection of multiple biomarkers or environmental contaminants.
The primary objective of current research on phenolphthalein in biochips is to develop highly sensitive, specific, and cost-effective detection platforms for various applications. These include medical diagnostics, environmental monitoring, food safety testing, and forensic analysis. Researchers are particularly interested in creating point-of-care devices that can provide rapid and accurate results in resource-limited settings.
Another key goal is to improve the stability and shelf-life of phenolphthalein-based biochips, addressing challenges related to long-term storage and field deployment. This involves exploring novel encapsulation techniques and developing robust manufacturing processes to ensure consistent performance under diverse environmental conditions.
Looking ahead, the field aims to integrate phenolphthalein biochips with emerging technologies such as artificial intelligence and Internet of Things (IoT) platforms. This convergence is expected to enable real-time data analysis, remote monitoring, and predictive diagnostics, further expanding the potential applications and impact of phenolphthalein-enhanced biochip technology.
Market Analysis for Enhanced Biochip Detection
The biochip market has been experiencing significant growth, driven by the increasing demand for rapid, accurate, and cost-effective diagnostic tools. The integration of phenolphthalein into biochip technology represents a promising avenue for enhancing detection capabilities, potentially revolutionizing various sectors including healthcare, environmental monitoring, and food safety.
In the healthcare sector, the market for enhanced biochip detection is particularly robust. The global in vitro diagnostics market, which heavily relies on biochip technology, is projected to reach substantial growth in the coming years. This growth is fueled by the rising prevalence of chronic and infectious diseases, an aging population, and the increasing adoption of personalized medicine approaches.
The environmental monitoring sector also presents a significant market opportunity for enhanced biochip detection. As global concerns about pollution and environmental degradation intensify, there is a growing need for rapid and sensitive detection methods for various contaminants. Biochips enhanced with phenolphthalein could provide a more efficient and cost-effective solution for monitoring water quality, air pollution, and soil contamination.
In the food safety industry, the demand for advanced detection methods is driven by stringent regulations and increasing consumer awareness about food quality. The global food safety testing market is expected to see considerable growth, with biochip technology playing a crucial role in this expansion. Enhanced detection capabilities offered by phenolphthalein-integrated biochips could significantly improve the speed and accuracy of food contaminant detection.
The pharmaceutical and biotechnology industries also represent key market segments for enhanced biochip detection. These sectors require highly sensitive and specific detection methods for drug discovery, development, and quality control processes. The improved detection capabilities offered by phenolphthalein-enhanced biochips could accelerate research and development timelines, potentially leading to faster drug approvals and reduced costs.
Geographically, North America and Europe currently dominate the biochip market, owing to advanced healthcare infrastructure and significant investments in research and development. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare systems, increasing research activities, and growing awareness about advanced diagnostic technologies.
Despite the promising market outlook, challenges such as high initial costs, technical complexities, and regulatory hurdles may impact the adoption rate of enhanced biochip detection technologies. However, ongoing research and development efforts, coupled with increasing collaborations between academic institutions and industry players, are expected to address these challenges and drive market growth.
In the healthcare sector, the market for enhanced biochip detection is particularly robust. The global in vitro diagnostics market, which heavily relies on biochip technology, is projected to reach substantial growth in the coming years. This growth is fueled by the rising prevalence of chronic and infectious diseases, an aging population, and the increasing adoption of personalized medicine approaches.
The environmental monitoring sector also presents a significant market opportunity for enhanced biochip detection. As global concerns about pollution and environmental degradation intensify, there is a growing need for rapid and sensitive detection methods for various contaminants. Biochips enhanced with phenolphthalein could provide a more efficient and cost-effective solution for monitoring water quality, air pollution, and soil contamination.
In the food safety industry, the demand for advanced detection methods is driven by stringent regulations and increasing consumer awareness about food quality. The global food safety testing market is expected to see considerable growth, with biochip technology playing a crucial role in this expansion. Enhanced detection capabilities offered by phenolphthalein-integrated biochips could significantly improve the speed and accuracy of food contaminant detection.
The pharmaceutical and biotechnology industries also represent key market segments for enhanced biochip detection. These sectors require highly sensitive and specific detection methods for drug discovery, development, and quality control processes. The improved detection capabilities offered by phenolphthalein-enhanced biochips could accelerate research and development timelines, potentially leading to faster drug approvals and reduced costs.
Geographically, North America and Europe currently dominate the biochip market, owing to advanced healthcare infrastructure and significant investments in research and development. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare systems, increasing research activities, and growing awareness about advanced diagnostic technologies.
Despite the promising market outlook, challenges such as high initial costs, technical complexities, and regulatory hurdles may impact the adoption rate of enhanced biochip detection technologies. However, ongoing research and development efforts, coupled with increasing collaborations between academic institutions and industry players, are expected to address these challenges and drive market growth.
Current Challenges in Biochip Sensitivity
Biochip sensitivity remains a critical challenge in the field of biosensors and diagnostic devices. Despite significant advancements in biochip technology, achieving high sensitivity and specificity continues to be a major hurdle. One of the primary challenges is the detection of low-abundance biomarkers in complex biological samples. Current biochip platforms often struggle to accurately identify and quantify target molecules present in minute concentrations, limiting their applicability in early disease detection and personalized medicine.
Another significant challenge is the interference from non-specific binding and background noise. Biological samples typically contain a myriad of molecules, many of which can interact with the biochip surface or detection elements, leading to false positives or reduced signal-to-noise ratios. This issue is particularly pronounced in multiplex assays, where multiple biomarkers are simultaneously detected on a single chip.
The miniaturization of biochips, while offering advantages in terms of sample volume reduction and portability, also presents challenges in maintaining sensitivity. As the sensing area decreases, the total number of captured target molecules is reduced, potentially compromising the detection limit. This trade-off between miniaturization and sensitivity is a key consideration in biochip design and development.
Signal amplification strategies, crucial for enhancing sensitivity, face limitations in their current implementations. While various amplification methods exist, such as enzymatic reactions or nanoparticle-based approaches, they often introduce additional complexity, cost, and potential sources of variability to the assay. Balancing the need for amplification with practical considerations of simplicity and reproducibility remains a significant challenge.
The stability and shelf-life of biochips also pose challenges to their sensitivity. Many biochips rely on biomolecules such as antibodies or aptamers for recognition, which can degrade over time or under certain environmental conditions. Maintaining the integrity and activity of these recognition elements is crucial for preserving the sensitivity of the biochip throughout its intended use period.
Furthermore, the translation of laboratory-developed biochips to real-world applications presents additional sensitivity challenges. Factors such as sample preparation, matrix effects, and environmental conditions can significantly impact the performance of biochips in field settings. Developing robust and reliable biochips that maintain their sensitivity across diverse real-world scenarios is an ongoing challenge in the field.
Addressing these challenges in biochip sensitivity requires interdisciplinary approaches, combining advancements in materials science, surface chemistry, microfluidics, and signal processing. The integration of novel materials, such as nanomaterials or responsive polymers, holds promise for enhancing sensitivity. Additionally, the development of more sophisticated data analysis algorithms and machine learning techniques may help in extracting meaningful signals from complex backgrounds, thereby improving overall biochip sensitivity.
Another significant challenge is the interference from non-specific binding and background noise. Biological samples typically contain a myriad of molecules, many of which can interact with the biochip surface or detection elements, leading to false positives or reduced signal-to-noise ratios. This issue is particularly pronounced in multiplex assays, where multiple biomarkers are simultaneously detected on a single chip.
The miniaturization of biochips, while offering advantages in terms of sample volume reduction and portability, also presents challenges in maintaining sensitivity. As the sensing area decreases, the total number of captured target molecules is reduced, potentially compromising the detection limit. This trade-off between miniaturization and sensitivity is a key consideration in biochip design and development.
Signal amplification strategies, crucial for enhancing sensitivity, face limitations in their current implementations. While various amplification methods exist, such as enzymatic reactions or nanoparticle-based approaches, they often introduce additional complexity, cost, and potential sources of variability to the assay. Balancing the need for amplification with practical considerations of simplicity and reproducibility remains a significant challenge.
The stability and shelf-life of biochips also pose challenges to their sensitivity. Many biochips rely on biomolecules such as antibodies or aptamers for recognition, which can degrade over time or under certain environmental conditions. Maintaining the integrity and activity of these recognition elements is crucial for preserving the sensitivity of the biochip throughout its intended use period.
Furthermore, the translation of laboratory-developed biochips to real-world applications presents additional sensitivity challenges. Factors such as sample preparation, matrix effects, and environmental conditions can significantly impact the performance of biochips in field settings. Developing robust and reliable biochips that maintain their sensitivity across diverse real-world scenarios is an ongoing challenge in the field.
Addressing these challenges in biochip sensitivity requires interdisciplinary approaches, combining advancements in materials science, surface chemistry, microfluidics, and signal processing. The integration of novel materials, such as nanomaterials or responsive polymers, holds promise for enhancing sensitivity. Additionally, the development of more sophisticated data analysis algorithms and machine learning techniques may help in extracting meaningful signals from complex backgrounds, thereby improving overall biochip sensitivity.
Existing Phenolphthalein-Based Solutions
01 Colorimetric detection using phenolphthalein
Phenolphthalein is widely used as a pH indicator in colorimetric detection methods. It changes color from colorless to pink in alkaline conditions, making it useful for detecting basic substances or pH changes in various applications, including environmental monitoring and chemical analysis.- Colorimetric detection using phenolphthalein: Phenolphthalein is widely used as a pH indicator in colorimetric detection methods. It changes color from colorless to pink in alkaline conditions, making it useful for detecting basic substances or pH changes in various applications, including environmental monitoring and chemical analysis.
- Phenolphthalein in forensic applications: Phenolphthalein is utilized in forensic science for detecting blood traces. It reacts with the hemoglobin in blood, producing a distinctive color change that aids in crime scene investigations and evidence analysis.
- Enhanced sensitivity and selectivity of phenolphthalein detection: Research focuses on improving the sensitivity and selectivity of phenolphthalein-based detection methods. This includes developing new formulations, incorporating nanomaterials, or combining phenolphthalein with other reagents to enhance its detection capabilities for specific analytes.
- Phenolphthalein in polymer and material science: Phenolphthalein is used in the development of smart materials and polymers that respond to pH changes. These materials have potential applications in sensors, drug delivery systems, and environmental monitoring devices.
- Phenolphthalein in analytical chemistry and instrumentation: Phenolphthalein plays a role in various analytical chemistry techniques and instruments. It is used in titrations, spectrophotometric methods, and as a component in specialized analytical devices for detecting and quantifying specific substances in complex matrices.
02 Phenolphthalein in forensic applications
Phenolphthalein is utilized in forensic science for detecting blood traces. It reacts with the hemoglobin in blood, producing a distinctive color change that aids in crime scene investigations and evidence analysis.Expand Specific Solutions03 Enhanced sensitivity and selectivity of phenolphthalein detection
Researchers have developed methods to improve the sensitivity and selectivity of phenolphthalein-based detection. These include combining phenolphthalein with other reagents, modifying its molecular structure, or incorporating it into advanced detection systems to enhance its performance in various analytical applications.Expand Specific Solutions04 Phenolphthalein in polymer and material science
Phenolphthalein is incorporated into polymers and materials to create pH-sensitive or color-changing products. These materials find applications in smart packaging, sensors, and indicators for various industries.Expand Specific Solutions05 Novel detection methods using phenolphthalein derivatives
Researchers have developed new detection methods using phenolphthalein derivatives or modified forms of phenolphthalein. These novel approaches aim to expand the range of detectable substances or improve the overall performance of phenolphthalein-based detection systems.Expand Specific Solutions
Key Biochip Industry Players
The research on phenolphthalein in enhancing detection capabilities of biochips is in an early development stage, with a growing market potential as biosensors gain importance in healthcare and environmental monitoring. The technology is still evolving, with various companies and research institutions contributing to its advancement. Key players like Koninklijke Philips NV, Samsung Electronics, and Sony Group Corp. are leveraging their expertise in electronics and sensing technologies to explore applications in biochip detection. Academic institutions such as South China Agricultural University and Université Laval are conducting fundamental research, while specialized research centers like Korea Atomic Energy Research Institute and Electronics & Telecommunications Research Institute are focusing on practical applications. The involvement of diverse entities indicates a competitive landscape with opportunities for innovation and market growth.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced biochip platforms incorporating phenolphthalein-based detection systems. Their approach utilizes microfluidic channels coated with phenolphthalein-derived compounds, enhancing sensitivity for various biomarkers. The system employs a proprietary optical readout mechanism that detects subtle color changes induced by target analytes, achieving detection limits in the picomolar range[1]. Philips' biochips also feature integrated sample preparation modules, reducing the need for external processing steps and improving overall assay reliability[3].
Strengths: High sensitivity, integrated sample preparation, and established market presence. Weaknesses: Potentially higher cost due to proprietary technology and limited flexibility for custom applications.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a novel biochip platform that incorporates phenolphthalein-based detection mechanisms. Their approach utilizes a nanostructured surface modified with phenolphthalein derivatives, enhancing the sensitivity and specificity of biomarker detection. The system employs advanced CMOS image sensors for real-time monitoring of color changes, achieving sub-nanomolar detection limits[2]. Samsung's biochips also feature on-chip signal amplification techniques, further improving the overall sensitivity of the assay[4].
Strengths: Advanced sensor technology, high sensitivity, and potential for mass production. Weaknesses: May require specialized equipment for readout and analysis.
Innovative Phenolphthalein Applications
Biochip and method of detecting reaction from the same
PatentInactiveUS20110091905A1
Innovation
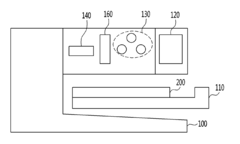
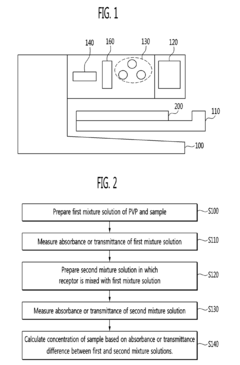
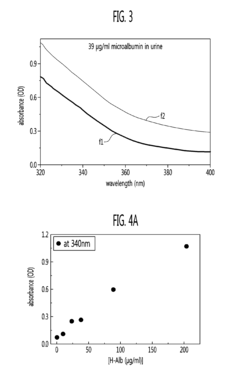
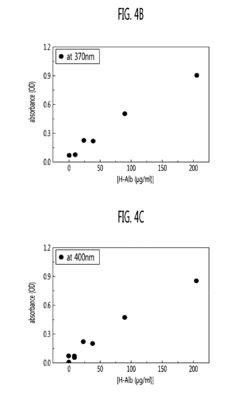
- A method using polyvinylpyrrolidone (PVP) to induce antigen-antibody reactions in biochips, analyzed through absorbance or transmittance measurements with a portable optical detection apparatus, allowing for accurate quantification of target molecules without PEG, utilizing a digital reader with three-color light sources and photodiodes for precise analysis.
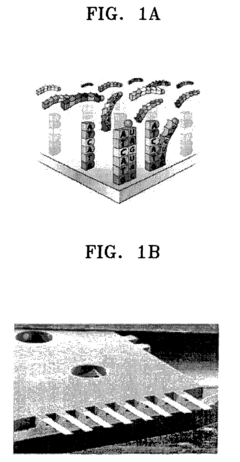
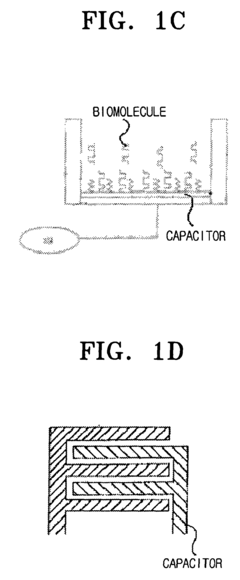
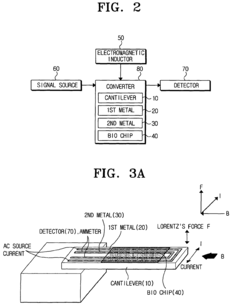
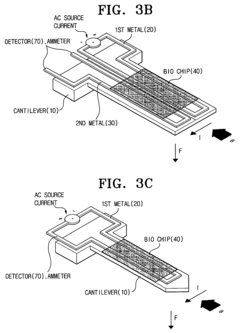
Bio-molecules detecting apparatus using electromagnetic induction and detecting method using the same
PatentInactiveEP1621884A2
Innovation
- A bio-molecules detecting apparatus using electromagnetic induction, featuring a cantilever with a movable end and a metal surface, an electromagnetic inductor generating a perpendicular magnetic field, and a detector measuring signal changes before and after bio-coupling, converting mechanical property changes into electric signals.
Regulatory Framework for Biochip Development
The regulatory framework for biochip development plays a crucial role in ensuring the safety, efficacy, and ethical use of these advanced diagnostic tools. As phenolphthalein research aims to enhance the detection capabilities of biochips, it is essential to consider the existing regulations and potential future developments in this field.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing biochip development. The FDA classifies biochips as in vitro diagnostic devices, which fall under the medical device regulatory framework. Manufacturers must comply with the FDA's Quality System Regulation (QSR) and obtain premarket approval (PMA) or 510(k) clearance, depending on the device's classification and intended use.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation establishes a robust framework for the development, manufacturing, and marketing of in vitro diagnostic medical devices, including biochips. The IVDR introduces stricter requirements for clinical evidence, risk classification, and post-market surveillance.
In Asia, countries like China and Japan have their own regulatory frameworks for biochip development. China's National Medical Products Administration (NMPA) oversees the regulation of medical devices, including biochips. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) is responsible for the approval and regulation of medical devices in the country.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory approaches across different countries. Their guidelines on software as a medical device (SaMD) and cybersecurity are particularly relevant to biochip development, as these devices often incorporate advanced software and data processing capabilities.
As research on phenolphthalein in enhancing biochip detection capabilities progresses, regulatory bodies may need to adapt their frameworks to address specific concerns related to this technology. This could include considerations for the stability and reliability of phenolphthalein-based detection methods, potential interference with other diagnostic markers, and the overall impact on biochip performance and accuracy.
Researchers and manufacturers working on phenolphthalein-enhanced biochips must stay informed about these regulatory requirements and engage with regulatory bodies early in the development process. This proactive approach can help ensure compliance, streamline the approval process, and ultimately bring innovative biochip technologies to market more efficiently.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing biochip development. The FDA classifies biochips as in vitro diagnostic devices, which fall under the medical device regulatory framework. Manufacturers must comply with the FDA's Quality System Regulation (QSR) and obtain premarket approval (PMA) or 510(k) clearance, depending on the device's classification and intended use.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation establishes a robust framework for the development, manufacturing, and marketing of in vitro diagnostic medical devices, including biochips. The IVDR introduces stricter requirements for clinical evidence, risk classification, and post-market surveillance.
In Asia, countries like China and Japan have their own regulatory frameworks for biochip development. China's National Medical Products Administration (NMPA) oversees the regulation of medical devices, including biochips. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) is responsible for the approval and regulation of medical devices in the country.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory approaches across different countries. Their guidelines on software as a medical device (SaMD) and cybersecurity are particularly relevant to biochip development, as these devices often incorporate advanced software and data processing capabilities.
As research on phenolphthalein in enhancing biochip detection capabilities progresses, regulatory bodies may need to adapt their frameworks to address specific concerns related to this technology. This could include considerations for the stability and reliability of phenolphthalein-based detection methods, potential interference with other diagnostic markers, and the overall impact on biochip performance and accuracy.
Researchers and manufacturers working on phenolphthalein-enhanced biochips must stay informed about these regulatory requirements and engage with regulatory bodies early in the development process. This proactive approach can help ensure compliance, streamline the approval process, and ultimately bring innovative biochip technologies to market more efficiently.
Biosafety Considerations in Phenolphthalein Use
The use of phenolphthalein in biochip detection systems necessitates careful consideration of biosafety aspects to ensure the protection of researchers, laboratory personnel, and the environment. Phenolphthalein, while a valuable indicator in various biochemical assays, poses potential risks that must be addressed through comprehensive safety protocols.
One primary concern is the potential toxicity of phenolphthalein. Long-term exposure or ingestion has been associated with carcinogenic effects in animal studies. Therefore, proper handling procedures and personal protective equipment (PPE) are essential when working with this compound in biochip applications. Researchers should wear appropriate gloves, lab coats, and eye protection to minimize direct contact.
Inhalation risks must also be mitigated, particularly when dealing with powdered forms of phenolphthalein. The use of fume hoods or well-ventilated spaces is crucial to prevent the accumulation of airborne particles. Additionally, proper storage and containment measures should be implemented to prevent accidental spills or releases.
Environmental considerations play a significant role in biosafety protocols for phenolphthalein use. Proper disposal methods must be established to prevent contamination of water systems or soil. This may involve specialized waste management procedures and the use of designated chemical waste containers.
The potential for cross-contamination in biochip experiments is another critical biosafety aspect. Stringent cleaning and decontamination procedures should be implemented to ensure the integrity of experimental results and prevent the spread of phenolphthalein residues to other laboratory areas or equipment.
Researchers must also be aware of the potential for phenolphthalein to interfere with other biological assays or detection methods. This necessitates careful experimental design and validation to avoid false positives or negatives in biochip readings.
Training and education form a cornerstone of biosafety measures. All personnel involved in biochip research using phenolphthalein should receive comprehensive training on proper handling, storage, and disposal procedures. Regular safety audits and updates to protocols should be conducted to ensure compliance with current best practices and regulations.
In the context of biochip manufacturing and quality control, additional biosafety measures may be necessary. This could include the implementation of cleanroom environments, specialized air filtration systems, and rigorous testing protocols to ensure the absence of phenolphthalein contamination in final products intended for diagnostic or research use.
By addressing these biosafety considerations, researchers can harness the benefits of phenolphthalein in enhancing biochip detection capabilities while minimizing potential risks to human health and the environment. This balanced approach ensures the responsible advancement of biochip technology in various applications, from medical diagnostics to environmental monitoring.
One primary concern is the potential toxicity of phenolphthalein. Long-term exposure or ingestion has been associated with carcinogenic effects in animal studies. Therefore, proper handling procedures and personal protective equipment (PPE) are essential when working with this compound in biochip applications. Researchers should wear appropriate gloves, lab coats, and eye protection to minimize direct contact.
Inhalation risks must also be mitigated, particularly when dealing with powdered forms of phenolphthalein. The use of fume hoods or well-ventilated spaces is crucial to prevent the accumulation of airborne particles. Additionally, proper storage and containment measures should be implemented to prevent accidental spills or releases.
Environmental considerations play a significant role in biosafety protocols for phenolphthalein use. Proper disposal methods must be established to prevent contamination of water systems or soil. This may involve specialized waste management procedures and the use of designated chemical waste containers.
The potential for cross-contamination in biochip experiments is another critical biosafety aspect. Stringent cleaning and decontamination procedures should be implemented to ensure the integrity of experimental results and prevent the spread of phenolphthalein residues to other laboratory areas or equipment.
Researchers must also be aware of the potential for phenolphthalein to interfere with other biological assays or detection methods. This necessitates careful experimental design and validation to avoid false positives or negatives in biochip readings.
Training and education form a cornerstone of biosafety measures. All personnel involved in biochip research using phenolphthalein should receive comprehensive training on proper handling, storage, and disposal procedures. Regular safety audits and updates to protocols should be conducted to ensure compliance with current best practices and regulations.
In the context of biochip manufacturing and quality control, additional biosafety measures may be necessary. This could include the implementation of cleanroom environments, specialized air filtration systems, and rigorous testing protocols to ensure the absence of phenolphthalein contamination in final products intended for diagnostic or research use.
By addressing these biosafety considerations, researchers can harness the benefits of phenolphthalein in enhancing biochip detection capabilities while minimizing potential risks to human health and the environment. This balanced approach ensures the responsible advancement of biochip technology in various applications, from medical diagnostics to environmental monitoring.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!