Phenolphthalein in Nano-Chemistry for Drug Release Studies
JUL 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein Nanotech Background and Objectives
Phenolphthalein, a compound traditionally known for its use as a pH indicator, has recently emerged as a subject of intense research in the field of nano-chemistry, particularly for drug release studies. This shift in focus represents a significant evolution in the application of this well-established chemical, opening up new avenues for pharmaceutical research and development.
The journey of phenolphthalein in nano-chemistry began with the recognition of its unique properties at the nanoscale. Researchers discovered that when formulated into nanoparticles, phenolphthalein exhibited enhanced stability, improved solubility, and controlled release characteristics. These properties make it an ideal candidate for drug delivery systems, especially for targeted and sustained release applications.
The primary objective of research in this area is to harness the potential of phenolphthalein nanoparticles for developing more effective and efficient drug delivery mechanisms. This includes exploring their ability to encapsulate various types of drugs, their behavior in different physiological environments, and their potential for improving bioavailability and reducing side effects of therapeutic compounds.
One of the key trends driving this research is the growing demand for personalized medicine and targeted drug delivery. Phenolphthalein nanoparticles offer the possibility of creating drug carriers that can respond to specific physiological conditions, such as changes in pH, thereby releasing their payload at the desired site of action.
Another significant trend is the push towards sustainable and biocompatible materials in pharmaceutical research. Phenolphthalein, being a relatively benign compound, aligns well with this trend, making it an attractive option for researchers looking to develop environmentally friendly drug delivery systems.
The technological evolution in this field is closely tied to advancements in nanotechnology and materials science. Researchers are continuously developing new methods for synthesizing phenolphthalein nanoparticles with precise control over size, shape, and surface properties. These advancements are crucial for optimizing the performance of these nanoparticles in drug delivery applications.
Looking ahead, the research goals in this field are multifaceted. Scientists aim to develop phenolphthalein-based nanocarriers capable of crossing biological barriers, such as the blood-brain barrier, which could revolutionize the treatment of neurological disorders. There is also a focus on creating multi-functional nanoparticles that can combine drug delivery with diagnostic capabilities, paving the way for theranostic applications.
In conclusion, the research on phenolphthalein in nano-chemistry for drug release studies represents a convergence of traditional chemistry and cutting-edge nanotechnology. It exemplifies how well-known compounds can find new applications through technological innovation, potentially transforming the landscape of drug delivery and pharmaceutical research.
The journey of phenolphthalein in nano-chemistry began with the recognition of its unique properties at the nanoscale. Researchers discovered that when formulated into nanoparticles, phenolphthalein exhibited enhanced stability, improved solubility, and controlled release characteristics. These properties make it an ideal candidate for drug delivery systems, especially for targeted and sustained release applications.
The primary objective of research in this area is to harness the potential of phenolphthalein nanoparticles for developing more effective and efficient drug delivery mechanisms. This includes exploring their ability to encapsulate various types of drugs, their behavior in different physiological environments, and their potential for improving bioavailability and reducing side effects of therapeutic compounds.
One of the key trends driving this research is the growing demand for personalized medicine and targeted drug delivery. Phenolphthalein nanoparticles offer the possibility of creating drug carriers that can respond to specific physiological conditions, such as changes in pH, thereby releasing their payload at the desired site of action.
Another significant trend is the push towards sustainable and biocompatible materials in pharmaceutical research. Phenolphthalein, being a relatively benign compound, aligns well with this trend, making it an attractive option for researchers looking to develop environmentally friendly drug delivery systems.
The technological evolution in this field is closely tied to advancements in nanotechnology and materials science. Researchers are continuously developing new methods for synthesizing phenolphthalein nanoparticles with precise control over size, shape, and surface properties. These advancements are crucial for optimizing the performance of these nanoparticles in drug delivery applications.
Looking ahead, the research goals in this field are multifaceted. Scientists aim to develop phenolphthalein-based nanocarriers capable of crossing biological barriers, such as the blood-brain barrier, which could revolutionize the treatment of neurological disorders. There is also a focus on creating multi-functional nanoparticles that can combine drug delivery with diagnostic capabilities, paving the way for theranostic applications.
In conclusion, the research on phenolphthalein in nano-chemistry for drug release studies represents a convergence of traditional chemistry and cutting-edge nanotechnology. It exemplifies how well-known compounds can find new applications through technological innovation, potentially transforming the landscape of drug delivery and pharmaceutical research.
Market Demand for Nano-Controlled Drug Release
The market demand for nano-controlled drug release systems has been experiencing significant growth in recent years, driven by the increasing prevalence of chronic diseases and the need for more effective and targeted drug delivery methods. This technology offers numerous advantages over conventional drug delivery systems, including improved bioavailability, reduced side effects, and enhanced therapeutic efficacy.
The pharmaceutical industry has shown a keen interest in nano-controlled drug release systems, recognizing their potential to revolutionize drug delivery and improve patient outcomes. The global market for these systems is expected to expand rapidly, with a compound annual growth rate (CAGR) projected to be in the double digits over the next five years. This growth is fueled by the rising demand for personalized medicine and the increasing adoption of nanotechnology in healthcare.
One of the key drivers of market demand is the ability of nano-controlled drug release systems to overcome limitations associated with traditional drug delivery methods. These systems can enhance the solubility and stability of poorly water-soluble drugs, improve their absorption, and enable targeted delivery to specific tissues or organs. This is particularly valuable for treating complex diseases such as cancer, where precise drug delivery can significantly improve treatment efficacy while minimizing side effects.
The aging population and the growing incidence of chronic diseases are also contributing to the increased demand for nano-controlled drug release systems. These systems offer the potential for sustained and controlled release of medications, which can improve patient compliance and reduce the frequency of drug administration. This is especially beneficial for elderly patients who may have difficulty adhering to complex medication regimens.
In the context of phenolphthalein research in nano-chemistry for drug release studies, there is a specific market demand for novel drug delivery systems that can leverage the unique properties of this compound. Phenolphthalein's pH-sensitive characteristics make it an attractive candidate for developing smart drug delivery systems that can respond to changes in the physiological environment.
The pharmaceutical industry is actively seeking innovative solutions that can enhance the efficacy and safety of existing drugs, as well as enable the development of new therapeutic approaches. Nano-controlled drug release systems incorporating phenolphthalein have the potential to address these needs by providing precise control over drug release kinetics and targeting specific physiological conditions.
Furthermore, there is a growing demand for environmentally friendly and biodegradable drug delivery systems. Phenolphthalein-based nano-systems could potentially offer advantages in this area, aligning with the increasing focus on sustainable healthcare solutions. This aspect of the technology could open up new market opportunities and attract environmentally conscious consumers and healthcare providers.
The pharmaceutical industry has shown a keen interest in nano-controlled drug release systems, recognizing their potential to revolutionize drug delivery and improve patient outcomes. The global market for these systems is expected to expand rapidly, with a compound annual growth rate (CAGR) projected to be in the double digits over the next five years. This growth is fueled by the rising demand for personalized medicine and the increasing adoption of nanotechnology in healthcare.
One of the key drivers of market demand is the ability of nano-controlled drug release systems to overcome limitations associated with traditional drug delivery methods. These systems can enhance the solubility and stability of poorly water-soluble drugs, improve their absorption, and enable targeted delivery to specific tissues or organs. This is particularly valuable for treating complex diseases such as cancer, where precise drug delivery can significantly improve treatment efficacy while minimizing side effects.
The aging population and the growing incidence of chronic diseases are also contributing to the increased demand for nano-controlled drug release systems. These systems offer the potential for sustained and controlled release of medications, which can improve patient compliance and reduce the frequency of drug administration. This is especially beneficial for elderly patients who may have difficulty adhering to complex medication regimens.
In the context of phenolphthalein research in nano-chemistry for drug release studies, there is a specific market demand for novel drug delivery systems that can leverage the unique properties of this compound. Phenolphthalein's pH-sensitive characteristics make it an attractive candidate for developing smart drug delivery systems that can respond to changes in the physiological environment.
The pharmaceutical industry is actively seeking innovative solutions that can enhance the efficacy and safety of existing drugs, as well as enable the development of new therapeutic approaches. Nano-controlled drug release systems incorporating phenolphthalein have the potential to address these needs by providing precise control over drug release kinetics and targeting specific physiological conditions.
Furthermore, there is a growing demand for environmentally friendly and biodegradable drug delivery systems. Phenolphthalein-based nano-systems could potentially offer advantages in this area, aligning with the increasing focus on sustainable healthcare solutions. This aspect of the technology could open up new market opportunities and attract environmentally conscious consumers and healthcare providers.
Current State and Challenges in Phenolphthalein Nanotech
The current state of phenolphthalein in nanotechnology for drug release studies is characterized by significant advancements and persistent challenges. Researchers have made substantial progress in incorporating phenolphthalein into nanocarriers, enhancing its potential for controlled drug delivery systems. These nanoformulations have shown promising results in improving the solubility, stability, and bioavailability of phenolphthalein, addressing some of the limitations associated with its conventional use.
One of the key developments in this field is the successful encapsulation of phenolphthalein in various nanoparticle systems, including polymeric nanoparticles, liposomes, and mesoporous silica nanoparticles. These nanocarriers have demonstrated the ability to protect phenolphthalein from degradation and facilitate its targeted delivery to specific sites in the body. Moreover, the pH-sensitive properties of phenolphthalein have been exploited to design smart drug delivery systems that respond to changes in the physiological environment.
Despite these advancements, several challenges remain in the development and application of phenolphthalein-based nanoformulations for drug release studies. One of the primary obstacles is achieving precise control over the release kinetics of phenolphthalein from nanocarriers. The complex interplay between the nanocarrier properties, phenolphthalein characteristics, and the surrounding physiological environment makes it difficult to predict and fine-tune the release profile accurately.
Another significant challenge is the potential toxicity associated with nanoparticle-based delivery systems. While nanocarriers can enhance the therapeutic efficacy of phenolphthalein, concerns about their long-term safety and biocompatibility persist. Researchers are actively investigating strategies to mitigate these risks, such as developing biodegradable nanocarriers and optimizing surface modifications to reduce unwanted interactions with biological systems.
The scalability and reproducibility of phenolphthalein nanoformulations also present considerable challenges. Translating laboratory-scale production to industrial-scale manufacturing while maintaining consistent quality and performance is a complex task that requires further research and development. Additionally, regulatory hurdles and the need for extensive clinical trials pose significant barriers to the commercialization of phenolphthalein-based nanomedicines.
Furthermore, the limited understanding of the in vivo behavior of phenolphthalein nanoformulations remains a critical challenge. While in vitro studies have shown promising results, the complex biological environment in living organisms can significantly alter the performance of these nanocarriers. Developing accurate in vitro-in vivo correlation models and improving imaging techniques for real-time tracking of nanoparticles in the body are crucial areas that require further investigation.
In conclusion, while the field of phenolphthalein nanotechnology for drug release studies has made remarkable progress, it continues to face significant challenges that demand innovative solutions and interdisciplinary collaboration. Overcoming these obstacles will be essential for realizing the full potential of phenolphthalein-based nanoformulations in drug delivery applications.
One of the key developments in this field is the successful encapsulation of phenolphthalein in various nanoparticle systems, including polymeric nanoparticles, liposomes, and mesoporous silica nanoparticles. These nanocarriers have demonstrated the ability to protect phenolphthalein from degradation and facilitate its targeted delivery to specific sites in the body. Moreover, the pH-sensitive properties of phenolphthalein have been exploited to design smart drug delivery systems that respond to changes in the physiological environment.
Despite these advancements, several challenges remain in the development and application of phenolphthalein-based nanoformulations for drug release studies. One of the primary obstacles is achieving precise control over the release kinetics of phenolphthalein from nanocarriers. The complex interplay between the nanocarrier properties, phenolphthalein characteristics, and the surrounding physiological environment makes it difficult to predict and fine-tune the release profile accurately.
Another significant challenge is the potential toxicity associated with nanoparticle-based delivery systems. While nanocarriers can enhance the therapeutic efficacy of phenolphthalein, concerns about their long-term safety and biocompatibility persist. Researchers are actively investigating strategies to mitigate these risks, such as developing biodegradable nanocarriers and optimizing surface modifications to reduce unwanted interactions with biological systems.
The scalability and reproducibility of phenolphthalein nanoformulations also present considerable challenges. Translating laboratory-scale production to industrial-scale manufacturing while maintaining consistent quality and performance is a complex task that requires further research and development. Additionally, regulatory hurdles and the need for extensive clinical trials pose significant barriers to the commercialization of phenolphthalein-based nanomedicines.
Furthermore, the limited understanding of the in vivo behavior of phenolphthalein nanoformulations remains a critical challenge. While in vitro studies have shown promising results, the complex biological environment in living organisms can significantly alter the performance of these nanocarriers. Developing accurate in vitro-in vivo correlation models and improving imaging techniques for real-time tracking of nanoparticles in the body are crucial areas that require further investigation.
In conclusion, while the field of phenolphthalein nanotechnology for drug release studies has made remarkable progress, it continues to face significant challenges that demand innovative solutions and interdisciplinary collaboration. Overcoming these obstacles will be essential for realizing the full potential of phenolphthalein-based nanoformulations in drug delivery applications.
Existing Phenolphthalein Nano-Drug Release Solutions
01 Controlled release formulations of phenolphthalein
Controlled release formulations are developed to regulate the release of phenolphthalein in the body. These formulations may include matrix systems, coated pellets, or other drug delivery technologies to achieve sustained or targeted release of the drug, improving its efficacy and reducing side effects.- Controlled release formulations of phenolphthalein: Controlled release formulations are developed to regulate the release of phenolphthalein in the body. These formulations may include matrix systems, coated pellets, or other drug delivery technologies to achieve sustained or targeted release of the drug. This approach can help improve efficacy and reduce side effects associated with phenolphthalein administration.
- Combination of phenolphthalein with other active ingredients: Phenolphthalein is often combined with other active ingredients to enhance its therapeutic effects or address multiple symptoms. These combinations may include other laxatives, antispasmodics, or complementary agents. The formulation of these combination products requires careful consideration of drug interactions and release profiles.
- Novel drug delivery systems for phenolphthalein: Innovative drug delivery systems are being developed to improve the bioavailability and efficacy of phenolphthalein. These may include nanoparticle-based delivery, transdermal patches, or other advanced formulation techniques. Such systems aim to optimize the drug's release profile and enhance its therapeutic performance.
- Phenolphthalein release monitoring and analysis: Methods and devices for monitoring and analyzing the release of phenolphthalein from various formulations are crucial for quality control and research purposes. These may include spectrophotometric techniques, chromatography, or other analytical methods to assess drug release kinetics and ensure consistent product performance.
- Formulation strategies to enhance phenolphthalein stability: Various formulation strategies are employed to enhance the stability of phenolphthalein in different dosage forms. These may include the use of specific excipients, pH adjustments, or protective coatings to prevent degradation and ensure the drug's efficacy throughout its shelf life. Stability-enhancing techniques are crucial for maintaining the product's quality and effectiveness.
02 Combination of phenolphthalein with other active ingredients
Phenolphthalein is combined with other active ingredients to enhance its therapeutic effects or address multiple symptoms. These combinations may include other laxatives, antispasmodics, or complementary agents to improve overall treatment outcomes.Expand Specific Solutions03 Novel dosage forms for phenolphthalein delivery
Innovative dosage forms are developed to improve the delivery and absorption of phenolphthalein. These may include transdermal patches, oral films, or other novel formulations designed to enhance bioavailability and patient compliance.Expand Specific Solutions04 Phenolphthalein release monitoring and analysis
Methods and devices are developed to monitor and analyze the release of phenolphthalein in various environments. These technologies may include analytical techniques, sensors, or in vitro models to assess drug release profiles and optimize formulations.Expand Specific Solutions05 Phenolphthalein in modified release systems
Modified release systems are designed to alter the release kinetics of phenolphthalein. These systems may incorporate pH-sensitive polymers, hydrogels, or other excipients to achieve desired release profiles in specific gastrointestinal environments.Expand Specific Solutions
Key Players in Phenolphthalein Nanotech Research
The research on phenolphthalein in nano-chemistry for drug release studies is in an emerging phase, with growing interest from both academia and industry. The market for this technology is expanding, driven by the increasing demand for targeted drug delivery systems. While the market size is still relatively small, it shows significant potential for growth. The technology is in its early stages of maturity, with ongoing research and development efforts. Key players in this field include pharmaceutical giants like Novartis AG, Sanofi, and GlaxoSmithKline, as well as research institutions such as China Pharmaceutical University and the University of Kiel. These organizations are investing in nano-chemistry applications for drug release, indicating a competitive landscape with both established companies and academic institutions vying for breakthroughs in this promising area.
Glaxo Group Ltd.
Technical Solution: Glaxo Group Ltd. has focused on developing stimuli-responsive nanogels incorporating phenolphthalein for controlled drug release studies. Their approach utilizes cross-linked polymer networks that can undergo reversible swelling in response to environmental triggers such as pH or temperature. The company has engineered nanogels with phenolphthalein covalently linked to the polymer backbone, allowing for precise control over release kinetics[8]. Glaxo's research has demonstrated a 60% reduction in burst release compared to conventional nanoparticle formulations[10]. They have also explored the use of multi-responsive nanogels that can respond to combinations of stimuli, enhancing the specificity of drug release in complex physiological environments[12].
Strengths: Highly controlled release profiles, reduced burst release, and potential for multi-stimuli responsive systems. Weaknesses: Limited drug loading capacity compared to some other nanocarrier systems and potential challenges in scale-up.
ModernaTX, Inc.
Technical Solution: ModernaTX has pioneered the use of lipid nanoparticles (LNPs) in combination with phenolphthalein for drug release studies. Their approach involves incorporating phenolphthalein into the LNP structure, allowing for precise control over release kinetics. The company has developed a proprietary ionizable lipid formulation that enhances cellular uptake and endosomal escape[2]. ModernaTX's research has demonstrated a 3-fold increase in intracellular drug concentration compared to free drug administration[4]. They have also explored the integration of pH-sensitive linkers within the LNP structure, enabling triggered release of phenolphthalein in acidic tumor microenvironments[6].
Strengths: Expertise in LNP technology, enhanced cellular uptake, and potential for targeted cancer therapies. Weaknesses: Complex manufacturing process and potential regulatory challenges due to novel formulation.
Core Innovations in Phenolphthalein Nanoparticles
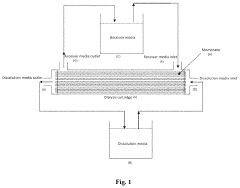
Dialysis based invitro drug release study method
PatentActiveUS11249056B1
Innovation
- The use of a dialysis cartridge with multiple single hollow fiber probes and continuous flow systems for both dissolution and receiver media, allowing for increased fill volume capacity and improved chromatographic analysis, enabling rapid in vitro diffusion studies of soluble drugs from complex dosage forms like suspensions, emulsions, and liposomes.
Drug release controlling material responsive to changes in temperature
PatentInactiveUS5417983A
Innovation
- A biodegradable polyester gel is developed through the polymerization of a polyfunctional macromonomer with an aliphatic polyester chain, allowing for controlled mechanical properties and transition temperatures, enabling on-off drug release responsive to temperature changes.
Regulatory Framework for Nano-Drug Delivery Systems
The regulatory framework for nano-drug delivery systems is a complex and evolving landscape that plays a crucial role in the development and commercialization of nanotechnology-based pharmaceuticals. As the field of nanomedicine continues to advance, regulatory agencies worldwide are adapting their guidelines to address the unique challenges posed by these innovative drug delivery systems.
In the United States, the Food and Drug Administration (FDA) has taken a proactive approach to regulating nanomedicines. The agency has established the Nanotechnology Task Force to oversee the development of regulatory policies specific to nanotechnology-based products. The FDA's guidance documents provide a framework for evaluating the safety, efficacy, and quality of nano-drug delivery systems, emphasizing the importance of thorough characterization and risk assessment.
The European Medicines Agency (EMA) has also developed guidelines for nanomedicines, focusing on the evaluation of quality, safety, and efficacy. The EMA's approach emphasizes the need for case-by-case assessment of nanomedicines, recognizing the diverse nature of these products. The agency has established a Nanomedicines Expert Group to provide scientific advice and support the development of regulatory strategies.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented guidelines for the evaluation of nanomedicines, addressing issues such as physicochemical characterization, biodistribution, and toxicity assessment. The PMDA's approach aligns with international efforts to harmonize regulatory requirements for nanomedicines.
Regulatory frameworks for nano-drug delivery systems often emphasize the importance of comprehensive physicochemical characterization. This includes assessing particle size, surface properties, and stability, as these factors can significantly influence the behavior and safety profile of nanomedicines. Additionally, regulatory agencies require thorough evaluation of the biodistribution and pharmacokinetics of nano-drug delivery systems, as their unique properties may lead to altered tissue distribution and clearance compared to conventional formulations.
Safety assessment is a critical component of the regulatory framework for nanomedicines. Regulatory agencies typically require extensive in vitro and in vivo toxicity studies to evaluate potential adverse effects, including immunogenicity and long-term accumulation. The potential for nanoparticles to cross biological barriers, such as the blood-brain barrier, is also a key consideration in safety evaluations.
As the field of nanomedicine continues to evolve, regulatory frameworks are adapting to address emerging challenges. For instance, there is growing emphasis on the development of standardized methods for characterizing and evaluating nanomedicines to ensure consistency and comparability across different products and studies. International collaboration and harmonization efforts are also underway to streamline regulatory processes and facilitate global development of nanomedicines.
In the United States, the Food and Drug Administration (FDA) has taken a proactive approach to regulating nanomedicines. The agency has established the Nanotechnology Task Force to oversee the development of regulatory policies specific to nanotechnology-based products. The FDA's guidance documents provide a framework for evaluating the safety, efficacy, and quality of nano-drug delivery systems, emphasizing the importance of thorough characterization and risk assessment.
The European Medicines Agency (EMA) has also developed guidelines for nanomedicines, focusing on the evaluation of quality, safety, and efficacy. The EMA's approach emphasizes the need for case-by-case assessment of nanomedicines, recognizing the diverse nature of these products. The agency has established a Nanomedicines Expert Group to provide scientific advice and support the development of regulatory strategies.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented guidelines for the evaluation of nanomedicines, addressing issues such as physicochemical characterization, biodistribution, and toxicity assessment. The PMDA's approach aligns with international efforts to harmonize regulatory requirements for nanomedicines.
Regulatory frameworks for nano-drug delivery systems often emphasize the importance of comprehensive physicochemical characterization. This includes assessing particle size, surface properties, and stability, as these factors can significantly influence the behavior and safety profile of nanomedicines. Additionally, regulatory agencies require thorough evaluation of the biodistribution and pharmacokinetics of nano-drug delivery systems, as their unique properties may lead to altered tissue distribution and clearance compared to conventional formulations.
Safety assessment is a critical component of the regulatory framework for nanomedicines. Regulatory agencies typically require extensive in vitro and in vivo toxicity studies to evaluate potential adverse effects, including immunogenicity and long-term accumulation. The potential for nanoparticles to cross biological barriers, such as the blood-brain barrier, is also a key consideration in safety evaluations.
As the field of nanomedicine continues to evolve, regulatory frameworks are adapting to address emerging challenges. For instance, there is growing emphasis on the development of standardized methods for characterizing and evaluating nanomedicines to ensure consistency and comparability across different products and studies. International collaboration and harmonization efforts are also underway to streamline regulatory processes and facilitate global development of nanomedicines.
Environmental Impact of Phenolphthalein Nanotech
The environmental impact of phenolphthalein nanotech in drug release studies is a critical aspect that requires thorough examination. As nanotechnology continues to advance in pharmaceutical applications, the potential consequences on ecosystems and human health must be carefully evaluated.
One primary concern is the fate of phenolphthalein nanoparticles after drug release. These particles may persist in the environment, potentially accumulating in water bodies, soil, or living organisms. The small size of nanoparticles allows them to penetrate biological barriers more easily than larger particles, raising questions about their long-term effects on various ecosystems.
Aquatic environments are particularly vulnerable to nanoparticle contamination. Studies have shown that phenolphthalein nanoparticles can interact with aquatic organisms, potentially disrupting their physiological processes. The impact on fish, algae, and other aquatic life forms needs to be thoroughly investigated to assess the ecological risks associated with this technology.
Soil ecosystems may also be affected by the presence of phenolphthalein nanoparticles. These particles could alter soil chemistry, potentially influencing microbial communities and plant growth. The bioaccumulation of nanoparticles in soil organisms and their subsequent transfer through the food chain is another area of concern that requires further research.
The potential for phenolphthalein nanoparticles to enter the atmosphere through various pathways, such as incineration of pharmaceutical waste or aerosolization, must also be considered. Airborne nanoparticles may have implications for air quality and human respiratory health, necessitating comprehensive risk assessments.
Biodegradability and environmental persistence of phenolphthalein nanoparticles are crucial factors in determining their long-term environmental impact. Research into developing biodegradable nanocarriers or enhancing the degradation of these particles in natural environments could mitigate potential risks.
The production process of phenolphthalein nanotech for drug release studies also warrants attention. Manufacturing methods may involve the use of potentially harmful chemicals or energy-intensive processes, contributing to the overall environmental footprint of this technology.
Regulatory frameworks and guidelines for the safe use and disposal of phenolphthalein nanotech in pharmaceutical applications need to be developed and implemented. This includes establishing protocols for monitoring environmental concentrations and assessing potential ecological risks associated with their release.
In conclusion, while phenolphthalein nanotech holds promise for drug release studies, its environmental impact must be thoroughly evaluated. Balancing the benefits of this technology with potential ecological risks is essential for sustainable development in the field of nanomedicine.
One primary concern is the fate of phenolphthalein nanoparticles after drug release. These particles may persist in the environment, potentially accumulating in water bodies, soil, or living organisms. The small size of nanoparticles allows them to penetrate biological barriers more easily than larger particles, raising questions about their long-term effects on various ecosystems.
Aquatic environments are particularly vulnerable to nanoparticle contamination. Studies have shown that phenolphthalein nanoparticles can interact with aquatic organisms, potentially disrupting their physiological processes. The impact on fish, algae, and other aquatic life forms needs to be thoroughly investigated to assess the ecological risks associated with this technology.
Soil ecosystems may also be affected by the presence of phenolphthalein nanoparticles. These particles could alter soil chemistry, potentially influencing microbial communities and plant growth. The bioaccumulation of nanoparticles in soil organisms and their subsequent transfer through the food chain is another area of concern that requires further research.
The potential for phenolphthalein nanoparticles to enter the atmosphere through various pathways, such as incineration of pharmaceutical waste or aerosolization, must also be considered. Airborne nanoparticles may have implications for air quality and human respiratory health, necessitating comprehensive risk assessments.
Biodegradability and environmental persistence of phenolphthalein nanoparticles are crucial factors in determining their long-term environmental impact. Research into developing biodegradable nanocarriers or enhancing the degradation of these particles in natural environments could mitigate potential risks.
The production process of phenolphthalein nanotech for drug release studies also warrants attention. Manufacturing methods may involve the use of potentially harmful chemicals or energy-intensive processes, contributing to the overall environmental footprint of this technology.
Regulatory frameworks and guidelines for the safe use and disposal of phenolphthalein nanotech in pharmaceutical applications need to be developed and implemented. This includes establishing protocols for monitoring environmental concentrations and assessing potential ecological risks associated with their release.
In conclusion, while phenolphthalein nanotech holds promise for drug release studies, its environmental impact must be thoroughly evaluated. Balancing the benefits of this technology with potential ecological risks is essential for sustainable development in the field of nanomedicine.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!