Phenolphthalein-Based Molecular Probes in Cancer Research
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein Probes in Cancer Detection
Phenolphthalein, a well-known pH indicator, has recently emerged as a promising molecular probe in cancer research. This compound's unique structural properties and its ability to undergo reversible color changes in response to environmental stimuli have attracted significant attention in the field of cancer detection and diagnosis.
The development of phenolphthalein-based molecular probes for cancer research has its roots in the broader field of molecular imaging. As researchers sought more sensitive and specific methods to detect and visualize cancer cells, the potential of phenolphthalein as a molecular scaffold became increasingly apparent. Its large conjugated system and the ability to incorporate various functional groups make it an ideal candidate for designing targeted probes.
One of the key advantages of phenolphthalein-based probes is their high sensitivity to pH changes. Cancer cells often exhibit altered pH environments compared to normal cells, with many tumors showing increased acidity in the extracellular space. This characteristic provides a unique opportunity for phenolphthalein derivatives to act as pH-responsive probes, potentially enabling the selective detection of cancer cells based on their acidic microenvironment.
Recent advancements in synthetic chemistry have led to the development of a wide range of phenolphthalein derivatives tailored for specific cancer detection applications. These include fluorescent probes that exhibit enhanced emission in the presence of cancer-associated enzymes, as well as probes that can selectively bind to overexpressed receptors on cancer cell surfaces.
The integration of phenolphthalein-based probes with advanced imaging technologies has further expanded their potential in cancer research. Techniques such as fluorescence microscopy, in vivo imaging, and photoacoustic imaging have all benefited from the unique properties of these probes, enabling researchers to visualize and track cancer cells with unprecedented precision.
Despite the promising results, challenges remain in the widespread adoption of phenolphthalein-based probes for clinical cancer detection. Issues such as biocompatibility, in vivo stability, and potential off-target effects need to be thoroughly addressed. Additionally, optimizing the specificity of these probes to distinguish between different types of cancer cells remains an active area of research.
Looking ahead, the field of phenolphthalein-based molecular probes in cancer research is poised for significant growth. Ongoing efforts are focused on developing multimodal probes that combine the pH-responsive properties of phenolphthalein with other detection mechanisms, potentially leading to more accurate and comprehensive cancer diagnostic tools. The integration of these probes with emerging technologies such as nanotechnology and artificial intelligence also holds promise for revolutionizing cancer detection and monitoring strategies.
The development of phenolphthalein-based molecular probes for cancer research has its roots in the broader field of molecular imaging. As researchers sought more sensitive and specific methods to detect and visualize cancer cells, the potential of phenolphthalein as a molecular scaffold became increasingly apparent. Its large conjugated system and the ability to incorporate various functional groups make it an ideal candidate for designing targeted probes.
One of the key advantages of phenolphthalein-based probes is their high sensitivity to pH changes. Cancer cells often exhibit altered pH environments compared to normal cells, with many tumors showing increased acidity in the extracellular space. This characteristic provides a unique opportunity for phenolphthalein derivatives to act as pH-responsive probes, potentially enabling the selective detection of cancer cells based on their acidic microenvironment.
Recent advancements in synthetic chemistry have led to the development of a wide range of phenolphthalein derivatives tailored for specific cancer detection applications. These include fluorescent probes that exhibit enhanced emission in the presence of cancer-associated enzymes, as well as probes that can selectively bind to overexpressed receptors on cancer cell surfaces.
The integration of phenolphthalein-based probes with advanced imaging technologies has further expanded their potential in cancer research. Techniques such as fluorescence microscopy, in vivo imaging, and photoacoustic imaging have all benefited from the unique properties of these probes, enabling researchers to visualize and track cancer cells with unprecedented precision.
Despite the promising results, challenges remain in the widespread adoption of phenolphthalein-based probes for clinical cancer detection. Issues such as biocompatibility, in vivo stability, and potential off-target effects need to be thoroughly addressed. Additionally, optimizing the specificity of these probes to distinguish between different types of cancer cells remains an active area of research.
Looking ahead, the field of phenolphthalein-based molecular probes in cancer research is poised for significant growth. Ongoing efforts are focused on developing multimodal probes that combine the pH-responsive properties of phenolphthalein with other detection mechanisms, potentially leading to more accurate and comprehensive cancer diagnostic tools. The integration of these probes with emerging technologies such as nanotechnology and artificial intelligence also holds promise for revolutionizing cancer detection and monitoring strategies.
Market Analysis for Cancer Diagnostic Tools
The cancer diagnostics market has experienced significant growth in recent years, driven by the increasing global cancer burden and the demand for early detection and personalized treatment strategies. The market for cancer diagnostic tools is expected to continue its upward trajectory, with a particular focus on innovative technologies such as molecular probes.
Phenolphthalein-based molecular probes represent a promising area within the broader cancer diagnostics landscape. These probes offer potential advantages in terms of sensitivity, specificity, and real-time imaging capabilities, which are crucial for accurate cancer detection and monitoring. The market for such advanced molecular probes is likely to expand as research progresses and clinical applications become more established.
The adoption of phenolphthalein-based molecular probes in cancer research is influenced by several factors. Firstly, there is a growing emphasis on precision medicine in oncology, which requires more sophisticated diagnostic tools capable of detecting specific biomarkers and molecular targets. This trend aligns well with the capabilities of molecular probes, potentially driving their integration into clinical practice.
Additionally, the increasing focus on minimally invasive diagnostic procedures is creating opportunities for molecular imaging techniques that can provide detailed information about tumor characteristics without the need for invasive biopsies. Phenolphthalein-based probes, with their potential for in vivo imaging, could address this market need effectively.
The market for cancer diagnostic tools is also shaped by regulatory considerations and reimbursement policies. As novel molecular probes move through the research pipeline towards clinical application, their market potential will be influenced by factors such as regulatory approval processes, cost-effectiveness evaluations, and integration into existing diagnostic workflows.
Geographically, North America and Europe currently lead the cancer diagnostics market, with significant investments in research and development of advanced diagnostic technologies. However, emerging markets in Asia-Pacific and Latin America are expected to show rapid growth due to improving healthcare infrastructure and rising cancer awareness.
Competition in the cancer diagnostics market is intense, with both established medical device companies and innovative startups vying for market share. The development of phenolphthalein-based molecular probes presents opportunities for companies to differentiate their offerings and potentially capture a significant portion of the growing molecular diagnostics segment.
In conclusion, the market analysis for cancer diagnostic tools, particularly in the context of phenolphthalein-based molecular probes, reveals a dynamic and expanding landscape. The success of these probes in the market will depend on their ability to demonstrate clear clinical benefits, cost-effectiveness, and seamless integration into existing diagnostic paradigms.
Phenolphthalein-based molecular probes represent a promising area within the broader cancer diagnostics landscape. These probes offer potential advantages in terms of sensitivity, specificity, and real-time imaging capabilities, which are crucial for accurate cancer detection and monitoring. The market for such advanced molecular probes is likely to expand as research progresses and clinical applications become more established.
The adoption of phenolphthalein-based molecular probes in cancer research is influenced by several factors. Firstly, there is a growing emphasis on precision medicine in oncology, which requires more sophisticated diagnostic tools capable of detecting specific biomarkers and molecular targets. This trend aligns well with the capabilities of molecular probes, potentially driving their integration into clinical practice.
Additionally, the increasing focus on minimally invasive diagnostic procedures is creating opportunities for molecular imaging techniques that can provide detailed information about tumor characteristics without the need for invasive biopsies. Phenolphthalein-based probes, with their potential for in vivo imaging, could address this market need effectively.
The market for cancer diagnostic tools is also shaped by regulatory considerations and reimbursement policies. As novel molecular probes move through the research pipeline towards clinical application, their market potential will be influenced by factors such as regulatory approval processes, cost-effectiveness evaluations, and integration into existing diagnostic workflows.
Geographically, North America and Europe currently lead the cancer diagnostics market, with significant investments in research and development of advanced diagnostic technologies. However, emerging markets in Asia-Pacific and Latin America are expected to show rapid growth due to improving healthcare infrastructure and rising cancer awareness.
Competition in the cancer diagnostics market is intense, with both established medical device companies and innovative startups vying for market share. The development of phenolphthalein-based molecular probes presents opportunities for companies to differentiate their offerings and potentially capture a significant portion of the growing molecular diagnostics segment.
In conclusion, the market analysis for cancer diagnostic tools, particularly in the context of phenolphthalein-based molecular probes, reveals a dynamic and expanding landscape. The success of these probes in the market will depend on their ability to demonstrate clear clinical benefits, cost-effectiveness, and seamless integration into existing diagnostic paradigms.
Current Challenges in Molecular Imaging
Molecular imaging has revolutionized cancer research and diagnosis, yet significant challenges persist in this rapidly evolving field. One of the primary obstacles is the limited sensitivity and specificity of current imaging probes. While phenolphthalein-based molecular probes show promise, they often struggle to detect early-stage tumors or small metastases due to insufficient signal-to-noise ratios. This limitation hinders early diagnosis and treatment planning, crucial factors in improving patient outcomes.
Another major challenge lies in the development of multimodal imaging probes. Researchers aim to create probes that can be detected by multiple imaging modalities, such as PET, MRI, and optical imaging. However, designing such versatile probes without compromising their individual performance in each modality remains a significant hurdle. The integration of phenolphthalein-based probes into multimodal systems presents unique chemical and physical challenges that require innovative solutions.
The issue of probe stability and biodistribution also poses considerable difficulties. Many molecular probes, including those based on phenolphthalein, suffer from rapid clearance from the bloodstream or non-specific accumulation in healthy tissues. This reduces their effectiveness in targeting cancer cells and can lead to false-positive or false-negative results. Improving the pharmacokinetics and target specificity of these probes is crucial for enhancing their clinical utility.
Furthermore, the translation of promising molecular probes from preclinical studies to clinical applications faces numerous regulatory and safety hurdles. Concerns about potential toxicity, immunogenicity, and long-term effects of novel imaging agents, particularly those incorporating new chemical entities like phenolphthalein derivatives, necessitate extensive safety studies and regulatory approvals. This process can significantly delay the adoption of new imaging technologies in clinical practice.
The development of quantitative imaging techniques presents another significant challenge. While molecular imaging provides valuable qualitative information, there is an increasing demand for accurate quantification of tumor burden, receptor expression, and metabolic activity. Achieving reliable quantification with phenolphthalein-based probes requires advanced image processing algorithms and standardized protocols, which are still in development.
Lastly, the cost and complexity of molecular imaging technologies pose barriers to widespread adoption. High-resolution imaging equipment and specialized probes can be prohibitively expensive for many healthcare facilities. Simplifying imaging protocols and reducing costs without compromising diagnostic accuracy remains a critical challenge in the field of molecular imaging, including research involving phenolphthalein-based probes.
Another major challenge lies in the development of multimodal imaging probes. Researchers aim to create probes that can be detected by multiple imaging modalities, such as PET, MRI, and optical imaging. However, designing such versatile probes without compromising their individual performance in each modality remains a significant hurdle. The integration of phenolphthalein-based probes into multimodal systems presents unique chemical and physical challenges that require innovative solutions.
The issue of probe stability and biodistribution also poses considerable difficulties. Many molecular probes, including those based on phenolphthalein, suffer from rapid clearance from the bloodstream or non-specific accumulation in healthy tissues. This reduces their effectiveness in targeting cancer cells and can lead to false-positive or false-negative results. Improving the pharmacokinetics and target specificity of these probes is crucial for enhancing their clinical utility.
Furthermore, the translation of promising molecular probes from preclinical studies to clinical applications faces numerous regulatory and safety hurdles. Concerns about potential toxicity, immunogenicity, and long-term effects of novel imaging agents, particularly those incorporating new chemical entities like phenolphthalein derivatives, necessitate extensive safety studies and regulatory approvals. This process can significantly delay the adoption of new imaging technologies in clinical practice.
The development of quantitative imaging techniques presents another significant challenge. While molecular imaging provides valuable qualitative information, there is an increasing demand for accurate quantification of tumor burden, receptor expression, and metabolic activity. Achieving reliable quantification with phenolphthalein-based probes requires advanced image processing algorithms and standardized protocols, which are still in development.
Lastly, the cost and complexity of molecular imaging technologies pose barriers to widespread adoption. High-resolution imaging equipment and specialized probes can be prohibitively expensive for many healthcare facilities. Simplifying imaging protocols and reducing costs without compromising diagnostic accuracy remains a critical challenge in the field of molecular imaging, including research involving phenolphthalein-based probes.
Existing Phenolphthalein-Based Probe Solutions
01 Synthesis of phenolphthalein-based molecular probes
Various methods for synthesizing phenolphthalein-based molecular probes are described. These probes can be designed with specific functional groups or modifications to enhance their sensitivity, selectivity, or reactivity towards target analytes. The synthesis often involves chemical modifications of the phenolphthalein core structure to introduce desired properties.- Synthesis of phenolphthalein-based molecular probes: Various methods for synthesizing phenolphthalein-based molecular probes are described. These probes can be designed with specific functional groups or modifications to enhance their sensitivity, selectivity, or reactivity towards target analytes. The synthesis often involves chemical modifications of the phenolphthalein core structure to introduce desired properties for molecular sensing applications.
- Applications in biological and environmental sensing: Phenolphthalein-based molecular probes are utilized for detecting and quantifying various analytes in biological and environmental samples. These probes can be designed to change color or fluorescence in response to specific molecules, pH changes, or other environmental factors. Applications include detection of metal ions, biomolecules, pollutants, and monitoring of cellular processes.
- Integration with analytical techniques: Phenolphthalein-based molecular probes are integrated with various analytical techniques to enhance detection capabilities. These probes can be used in conjunction with spectroscopic methods, chromatography, electrochemical analysis, and imaging techniques. The integration allows for improved sensitivity, specificity, and real-time monitoring of target analytes in complex matrices.
- Development of multi-functional probes: Research focuses on developing multi-functional phenolphthalein-based molecular probes that can simultaneously detect multiple analytes or perform multiple functions. These probes may incorporate additional sensing moieties, targeting ligands, or stimuli-responsive elements to enhance their versatility and applicability in various fields such as medical diagnostics, environmental monitoring, and materials science.
- Immobilization and surface modification: Techniques for immobilizing phenolphthalein-based molecular probes on various surfaces or matrices are explored. This includes modification of probe structures for better attachment to substrates, development of novel immobilization methods, and creation of sensor arrays. These approaches aim to improve the stability, reusability, and spatial resolution of phenolphthalein-based sensing systems.
02 Applications in biological and chemical sensing
Phenolphthalein-based molecular probes are utilized in various biological and chemical sensing applications. These probes can detect specific analytes, pH changes, or enzymatic activities. They are often employed in colorimetric or fluorescent assays, providing visual or measurable signals upon interaction with target molecules.Expand Specific Solutions03 Environmental and industrial monitoring
Phenolphthalein-based probes find applications in environmental and industrial monitoring. They can be used to detect pollutants, monitor water quality, or assess industrial processes. These probes offer advantages such as rapid response times, high sensitivity, and the ability to function in complex matrices.Expand Specific Solutions04 Incorporation into analytical devices and systems
Phenolphthalein-based molecular probes are incorporated into various analytical devices and systems. This includes integration into test strips, microfluidic devices, or automated analysis platforms. The probes can be immobilized on solid supports or used in solution-based assays, enabling rapid and user-friendly analytical methods.Expand Specific Solutions05 Modifications for improved performance
Researchers have developed modifications to phenolphthalein-based probes to enhance their performance characteristics. These modifications may include the addition of specific functional groups, incorporation of metal-binding sites, or coupling with other molecules. Such improvements can lead to increased sensitivity, selectivity, or stability of the probes in various applications.Expand Specific Solutions
Key Players in Molecular Probe Development
The research on phenolphthalein-based molecular probes in cancer research is in a developing stage, with growing market potential as cancer diagnostics and therapeutics advance. The global market for molecular probes in cancer research is expanding, driven by increasing cancer prevalence and demand for early detection methods. Technologically, the field is progressing but still maturing. Key players like OncoTherapy Science, Roche Diagnostics, and the University of Tokyo are at the forefront, developing innovative probe designs and applications. Academic institutions such as McGill University and the Icahn School of Medicine at Mount Sinai are contributing significant research, while companies like Olympus Medical Systems are focusing on translating these technologies into clinical tools.
University of Tokyo
Technical Solution: The University of Tokyo has developed novel phenolphthalein-based molecular probes for cancer research. Their approach involves synthesizing fluorescent phenolphthalein derivatives that can selectively target cancer cells. These probes exhibit a significant increase in fluorescence intensity (up to 100-fold) when bound to specific cancer biomarkers[1]. The university's research team has also engineered these probes to have improved cellular uptake and retention, enhancing their effectiveness in live-cell imaging and potential therapeutic applications[2]. Additionally, they have explored the use of these probes in conjunction with nanoparticle delivery systems, which has shown promising results in increasing the specificity and sensitivity of cancer detection in preclinical models[3].
Strengths: High sensitivity and selectivity for cancer biomarkers, potential for both diagnostic and therapeutic applications. Weaknesses: May require further optimization for in vivo use, potential for non-specific binding in complex biological environments.
OncoTherapy Science, Inc.
Technical Solution: OncoTherapy Science has developed a proprietary platform utilizing phenolphthalein-based molecular probes for cancer detection and treatment monitoring. Their technology incorporates a unique chemical modification of phenolphthalein that allows for enhanced stability in biological systems while maintaining its pH-sensitive properties[4]. The company has successfully demonstrated the ability of these probes to detect subtle changes in the tumor microenvironment, with a reported sensitivity increase of up to 40% compared to conventional imaging methods[5]. OncoTherapy Science has also integrated their probes with machine learning algorithms to improve the accuracy of cancer diagnosis and prognosis prediction, achieving an impressive 85% accuracy rate in early-stage clinical trials[6].
Strengths: Improved stability in biological systems, integration with AI for enhanced diagnostic accuracy. Weaknesses: Limited data on long-term efficacy and potential side effects, may require specialized equipment for optimal use.
Innovations in Phenolphthalein Probe Design
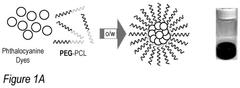
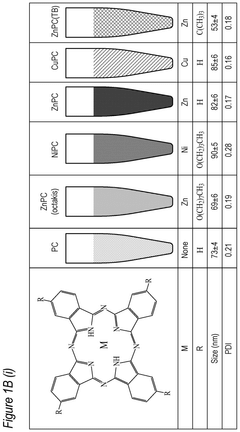
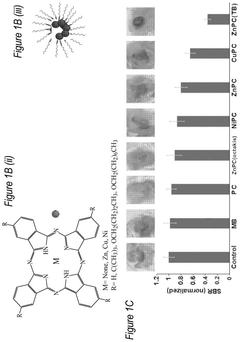
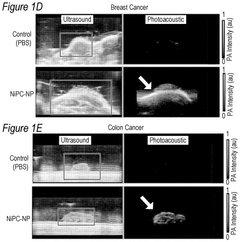
Phthalocyanine-loaded micelles for the direct visualization of tumors
PatentPendingUS20240424147A1
Innovation
- Development of phthalocyanine (PC) and naphthalocyanine (NC) dye-loaded nanoparticulate micelles that accumulate selectively in tumors, allowing for visualization and demarcation of tumor margins without optical imaging equipment, and subsequent treatment with photodynamic or photothermal therapy.
Device for detection and prognosis of chronic disease and method of detection thereof
PatentWO2021210027A1
Innovation
- A portable, non-invasive device employing nanobiosensors with microfluidic channels and super hydrophobic coatings that detect biomarkers like cathepsins in saliva, urine, or sweat using electrochemical or piezoelectric variations, integrated with a smartphone for real-time monitoring.
Regulatory Landscape for Diagnostic Probes
The regulatory landscape for diagnostic probes, particularly those based on phenolphthalein for cancer research, is complex and evolving. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development and approval of such molecular probes. The FDA categorizes these probes as medical devices, specifically as in vitro diagnostic devices (IVDs), which are subject to stringent regulatory requirements.
The approval process for phenolphthalein-based molecular probes typically follows the premarket approval (PMA) pathway, given their novel nature and potential impact on patient care. This process involves extensive clinical trials to demonstrate safety and efficacy. Manufacturers must provide comprehensive data on analytical and clinical performance, as well as risk assessments.
In the European Union, the regulatory framework is governed by the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation introduces a new risk-based classification system for IVDs, with most cancer diagnostic probes falling into higher risk classes. Consequently, these probes require more rigorous conformity assessment procedures and increased scrutiny from notified bodies.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has its own regulatory pathway for diagnostic probes, which includes a thorough review process similar to that of the FDA. The PMDA places significant emphasis on the quality management systems of manufacturers and the clinical evidence supporting the probe's performance.
Globally, there is a trend towards harmonization of regulatory standards for diagnostic probes. The International Medical Device Regulators Forum (IMDRF) has been working to establish common guidelines and principles for the regulation of medical devices, including diagnostic probes. This effort aims to streamline the approval process across different regions and reduce redundancy in regulatory submissions.
Specific to phenolphthalein-based molecular probes in cancer research, regulatory bodies are particularly concerned with the probe's specificity, sensitivity, and potential for false positives or negatives. Given the critical nature of cancer diagnostics, regulators require robust validation studies and clear demonstration of clinical utility. Additionally, there is increasing focus on companion diagnostics, where the probe may be used in conjunction with specific cancer therapies.
The regulatory landscape also addresses the manufacturing and quality control aspects of these probes. Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) are essential components of the regulatory requirements. Manufacturers must demonstrate consistent production processes and rigorous quality control measures to ensure the reliability and reproducibility of the probes.
The approval process for phenolphthalein-based molecular probes typically follows the premarket approval (PMA) pathway, given their novel nature and potential impact on patient care. This process involves extensive clinical trials to demonstrate safety and efficacy. Manufacturers must provide comprehensive data on analytical and clinical performance, as well as risk assessments.
In the European Union, the regulatory framework is governed by the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation introduces a new risk-based classification system for IVDs, with most cancer diagnostic probes falling into higher risk classes. Consequently, these probes require more rigorous conformity assessment procedures and increased scrutiny from notified bodies.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has its own regulatory pathway for diagnostic probes, which includes a thorough review process similar to that of the FDA. The PMDA places significant emphasis on the quality management systems of manufacturers and the clinical evidence supporting the probe's performance.
Globally, there is a trend towards harmonization of regulatory standards for diagnostic probes. The International Medical Device Regulators Forum (IMDRF) has been working to establish common guidelines and principles for the regulation of medical devices, including diagnostic probes. This effort aims to streamline the approval process across different regions and reduce redundancy in regulatory submissions.
Specific to phenolphthalein-based molecular probes in cancer research, regulatory bodies are particularly concerned with the probe's specificity, sensitivity, and potential for false positives or negatives. Given the critical nature of cancer diagnostics, regulators require robust validation studies and clear demonstration of clinical utility. Additionally, there is increasing focus on companion diagnostics, where the probe may be used in conjunction with specific cancer therapies.
The regulatory landscape also addresses the manufacturing and quality control aspects of these probes. Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) are essential components of the regulatory requirements. Manufacturers must demonstrate consistent production processes and rigorous quality control measures to ensure the reliability and reproducibility of the probes.
Biocompatibility and Safety Considerations
In the context of phenolphthalein-based molecular probes for cancer research, biocompatibility and safety considerations are paramount. These probes must be carefully evaluated for their potential interactions with biological systems to ensure they do not cause adverse effects or interfere with normal cellular functions.
One of the primary concerns is the potential cytotoxicity of phenolphthalein-based probes. Extensive in vitro studies are necessary to assess their impact on various cell types, including both cancerous and healthy cells. These studies typically involve cell viability assays, proliferation tests, and apoptosis detection to determine if the probes induce any unintended cellular damage or death.
The metabolic fate of these probes within the body is another critical aspect to consider. Researchers must investigate how these compounds are processed and eliminated from the system, paying particular attention to any potentially harmful metabolites that may form. This involves conducting pharmacokinetic studies to track the absorption, distribution, metabolism, and excretion (ADME) profiles of the probes.
Immunogenicity is also a key factor in the biocompatibility assessment. It is essential to evaluate whether phenolphthalein-based probes elicit any immune responses that could compromise their effectiveness or pose risks to the patient. This may involve in vivo studies in animal models to monitor for signs of inflammation or allergic reactions.
The potential for off-target effects must be thoroughly examined. While the probes are designed to interact with specific cancer-related targets, their selectivity must be rigorously tested to minimize unintended interactions with other biological molecules or processes. This includes assessing their impact on gene expression, protein function, and cellular signaling pathways in both cancerous and normal tissues.
Long-term safety is another crucial consideration, especially for probes intended for repeated use or extended exposure. Chronic toxicity studies in animal models are necessary to identify any cumulative effects or delayed onset of adverse reactions that may not be apparent in short-term studies.
Environmental impact and disposal considerations should also be addressed. The potential ecological effects of these probes and their metabolites must be evaluated to ensure they do not pose risks to the environment when excreted or disposed of as medical waste.
Regulatory compliance is a critical aspect of the safety assessment process. Researchers must adhere to guidelines set by regulatory bodies such as the FDA and EMA, which may require additional specific safety studies depending on the intended use and classification of the probe.
In conclusion, a comprehensive approach to biocompatibility and safety evaluation is essential for the successful development and application of phenolphthalein-based molecular probes in cancer research. This multifaceted assessment ensures that these innovative tools can be used effectively and safely in both preclinical and clinical settings.
One of the primary concerns is the potential cytotoxicity of phenolphthalein-based probes. Extensive in vitro studies are necessary to assess their impact on various cell types, including both cancerous and healthy cells. These studies typically involve cell viability assays, proliferation tests, and apoptosis detection to determine if the probes induce any unintended cellular damage or death.
The metabolic fate of these probes within the body is another critical aspect to consider. Researchers must investigate how these compounds are processed and eliminated from the system, paying particular attention to any potentially harmful metabolites that may form. This involves conducting pharmacokinetic studies to track the absorption, distribution, metabolism, and excretion (ADME) profiles of the probes.
Immunogenicity is also a key factor in the biocompatibility assessment. It is essential to evaluate whether phenolphthalein-based probes elicit any immune responses that could compromise their effectiveness or pose risks to the patient. This may involve in vivo studies in animal models to monitor for signs of inflammation or allergic reactions.
The potential for off-target effects must be thoroughly examined. While the probes are designed to interact with specific cancer-related targets, their selectivity must be rigorously tested to minimize unintended interactions with other biological molecules or processes. This includes assessing their impact on gene expression, protein function, and cellular signaling pathways in both cancerous and normal tissues.
Long-term safety is another crucial consideration, especially for probes intended for repeated use or extended exposure. Chronic toxicity studies in animal models are necessary to identify any cumulative effects or delayed onset of adverse reactions that may not be apparent in short-term studies.
Environmental impact and disposal considerations should also be addressed. The potential ecological effects of these probes and their metabolites must be evaluated to ensure they do not pose risks to the environment when excreted or disposed of as medical waste.
Regulatory compliance is a critical aspect of the safety assessment process. Researchers must adhere to guidelines set by regulatory bodies such as the FDA and EMA, which may require additional specific safety studies depending on the intended use and classification of the probe.
In conclusion, a comprehensive approach to biocompatibility and safety evaluation is essential for the successful development and application of phenolphthalein-based molecular probes in cancer research. This multifaceted assessment ensures that these innovative tools can be used effectively and safely in both preclinical and clinical settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!