Phenolphthalein in Automated pH Titration Systems
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein in pH Titration: Background and Objectives
Phenolphthalein has been a cornerstone in pH titration systems for over a century, serving as a reliable indicator in acid-base titrations. Its history dates back to 1871 when Adolf von Baeyer first synthesized this compound. Since then, phenolphthalein has become an indispensable tool in analytical chemistry, particularly in automated pH titration systems.
The evolution of pH titration techniques has been closely linked to advancements in indicator technology and automation. Early manual titrations relied heavily on visual color changes, with phenolphthalein's distinct transition from colorless to pink making it an ideal choice for many applications. As technology progressed, the integration of phenolphthalein into automated systems became a natural step in improving accuracy and efficiency.
In recent years, the focus has shifted towards enhancing the precision and versatility of automated pH titration systems. This has led to a renewed interest in optimizing the use of phenolphthalein and exploring its potential in conjunction with modern sensor technologies. The goal is to develop more sensitive, reliable, and adaptable titration systems that can meet the diverse needs of various industries.
The primary objectives of researching phenolphthalein in automated pH titration systems are multifaceted. First, there is a drive to improve the sensitivity and accuracy of pH measurements, particularly in the critical range where phenolphthalein undergoes its color change. This involves studying the molecular behavior of phenolphthalein under various conditions and developing methods to enhance its performance.
Another key objective is to expand the applicability of phenolphthalein-based systems across different industries. While traditionally used in laboratory settings, there is growing interest in adapting these systems for use in environmental monitoring, food and beverage production, pharmaceuticals, and wastewater treatment. This requires research into the stability and reliability of phenolphthalein under diverse environmental conditions.
Furthermore, the integration of phenolphthalein with digital technologies and data analytics presents exciting opportunities. Researchers aim to develop smart titration systems that can provide real-time data, predictive analysis, and automated decision-making capabilities. This convergence of traditional chemistry with modern technology is expected to revolutionize pH measurement and control processes across various applications.
As we look to the future, the research on phenolphthalein in automated pH titration systems is likely to focus on sustainability and eco-friendliness. This includes exploring ways to minimize the use of chemicals, reduce waste, and develop more environmentally benign alternatives while maintaining or improving the accuracy and reliability of pH measurements.
The evolution of pH titration techniques has been closely linked to advancements in indicator technology and automation. Early manual titrations relied heavily on visual color changes, with phenolphthalein's distinct transition from colorless to pink making it an ideal choice for many applications. As technology progressed, the integration of phenolphthalein into automated systems became a natural step in improving accuracy and efficiency.
In recent years, the focus has shifted towards enhancing the precision and versatility of automated pH titration systems. This has led to a renewed interest in optimizing the use of phenolphthalein and exploring its potential in conjunction with modern sensor technologies. The goal is to develop more sensitive, reliable, and adaptable titration systems that can meet the diverse needs of various industries.
The primary objectives of researching phenolphthalein in automated pH titration systems are multifaceted. First, there is a drive to improve the sensitivity and accuracy of pH measurements, particularly in the critical range where phenolphthalein undergoes its color change. This involves studying the molecular behavior of phenolphthalein under various conditions and developing methods to enhance its performance.
Another key objective is to expand the applicability of phenolphthalein-based systems across different industries. While traditionally used in laboratory settings, there is growing interest in adapting these systems for use in environmental monitoring, food and beverage production, pharmaceuticals, and wastewater treatment. This requires research into the stability and reliability of phenolphthalein under diverse environmental conditions.
Furthermore, the integration of phenolphthalein with digital technologies and data analytics presents exciting opportunities. Researchers aim to develop smart titration systems that can provide real-time data, predictive analysis, and automated decision-making capabilities. This convergence of traditional chemistry with modern technology is expected to revolutionize pH measurement and control processes across various applications.
As we look to the future, the research on phenolphthalein in automated pH titration systems is likely to focus on sustainability and eco-friendliness. This includes exploring ways to minimize the use of chemicals, reduce waste, and develop more environmentally benign alternatives while maintaining or improving the accuracy and reliability of pH measurements.
Market Analysis for Automated pH Titration Systems
The market for automated pH titration systems has been experiencing steady growth, driven by increasing demand for precise and efficient analytical tools across various industries. These systems, which incorporate phenolphthalein as a key indicator, have found widespread applications in sectors such as pharmaceuticals, food and beverage, environmental monitoring, and academic research.
In the pharmaceutical industry, automated pH titration systems are essential for quality control processes, drug formulation, and stability testing. The stringent regulatory requirements in this sector have led to a higher adoption rate of automated systems, as they offer improved accuracy and reproducibility compared to manual methods. This trend is expected to continue as pharmaceutical companies strive to enhance their production efficiency and maintain compliance with evolving standards.
The food and beverage industry represents another significant market for automated pH titration systems. These systems are crucial for ensuring product quality, safety, and consistency. With consumers becoming increasingly health-conscious and demanding transparency in food production, manufacturers are investing in advanced analytical tools to meet these expectations. The ability of automated systems to provide rapid and reliable pH measurements is particularly valuable in this fast-paced industry.
Environmental monitoring agencies and research institutions are also key contributors to the market growth. As concerns about water quality and pollution continue to rise, there is an increasing need for efficient pH measurement tools in environmental analysis. Automated pH titration systems offer the advantage of high-throughput testing, making them ideal for large-scale environmental monitoring programs.
The academic and research sector presents a stable market for these systems, with universities and research laboratories continually upgrading their equipment to stay at the forefront of scientific discovery. The versatility of automated pH titration systems in various research applications, from material science to biochemistry, ensures a consistent demand from this sector.
Geographically, North America and Europe currently dominate the market for automated pH titration systems, owing to their well-established pharmaceutical and food industries, as well as stringent regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing research and development activities, and growing awareness about the importance of quality control in manufacturing processes.
The market is characterized by the presence of several key players, including established analytical instrument manufacturers and specialized titration system providers. These companies are focusing on product innovation, such as the integration of advanced software for data analysis and the development of more user-friendly interfaces, to maintain their competitive edge.
In the pharmaceutical industry, automated pH titration systems are essential for quality control processes, drug formulation, and stability testing. The stringent regulatory requirements in this sector have led to a higher adoption rate of automated systems, as they offer improved accuracy and reproducibility compared to manual methods. This trend is expected to continue as pharmaceutical companies strive to enhance their production efficiency and maintain compliance with evolving standards.
The food and beverage industry represents another significant market for automated pH titration systems. These systems are crucial for ensuring product quality, safety, and consistency. With consumers becoming increasingly health-conscious and demanding transparency in food production, manufacturers are investing in advanced analytical tools to meet these expectations. The ability of automated systems to provide rapid and reliable pH measurements is particularly valuable in this fast-paced industry.
Environmental monitoring agencies and research institutions are also key contributors to the market growth. As concerns about water quality and pollution continue to rise, there is an increasing need for efficient pH measurement tools in environmental analysis. Automated pH titration systems offer the advantage of high-throughput testing, making them ideal for large-scale environmental monitoring programs.
The academic and research sector presents a stable market for these systems, with universities and research laboratories continually upgrading their equipment to stay at the forefront of scientific discovery. The versatility of automated pH titration systems in various research applications, from material science to biochemistry, ensures a consistent demand from this sector.
Geographically, North America and Europe currently dominate the market for automated pH titration systems, owing to their well-established pharmaceutical and food industries, as well as stringent regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing research and development activities, and growing awareness about the importance of quality control in manufacturing processes.
The market is characterized by the presence of several key players, including established analytical instrument manufacturers and specialized titration system providers. These companies are focusing on product innovation, such as the integration of advanced software for data analysis and the development of more user-friendly interfaces, to maintain their competitive edge.
Current Challenges in Phenolphthalein-based pH Detection
Despite the widespread use of phenolphthalein in automated pH titration systems, several challenges persist in its application for accurate and reliable pH detection. One of the primary issues is the narrow pH range in which phenolphthalein exhibits its color change. The indicator transitions from colorless to pink between pH 8.2 and 10.0, limiting its effectiveness in detecting pH changes outside this range.
The stability of phenolphthalein solutions poses another significant challenge. Over time, these solutions can degrade due to exposure to light, heat, and certain chemical environments. This degradation can lead to inconsistent results in automated titration systems, potentially compromising the accuracy of pH measurements in long-term or high-volume applications.
Interference from other ions present in the sample is a persistent issue in phenolphthalein-based pH detection. Certain metal ions, particularly those with high valency, can form complexes with phenolphthalein, altering its color change characteristics and potentially leading to erroneous pH readings. This interference can be especially problematic in complex sample matrices commonly encountered in industrial and environmental applications.
The response time of phenolphthalein to pH changes is another area of concern in automated systems. While generally considered rapid, the indicator's color transition may not be instantaneous enough for high-speed titration processes, potentially leading to overshooting the endpoint and reducing the overall accuracy of the titration.
Temperature sensitivity of phenolphthalein solutions presents an additional challenge. The pH at which the color change occurs can shift slightly with temperature variations, necessitating careful temperature control or compensation in automated titration systems to maintain accuracy across different operating conditions.
The subjective nature of color interpretation, even in automated systems, can introduce variability in results. While spectrophotometric methods have improved objectivity, differences in light sources, detectors, and optical pathways between instruments can still lead to inconsistencies in pH endpoint determination across different automated titration setups.
Lastly, the environmental and health concerns associated with phenolphthalein usage pose challenges in certain applications. Its classification as a potential carcinogen has led to increased scrutiny and regulatory restrictions, prompting the need for alternative indicators in some contexts and complicating waste management procedures in laboratories and industrial settings using automated pH titration systems.
The stability of phenolphthalein solutions poses another significant challenge. Over time, these solutions can degrade due to exposure to light, heat, and certain chemical environments. This degradation can lead to inconsistent results in automated titration systems, potentially compromising the accuracy of pH measurements in long-term or high-volume applications.
Interference from other ions present in the sample is a persistent issue in phenolphthalein-based pH detection. Certain metal ions, particularly those with high valency, can form complexes with phenolphthalein, altering its color change characteristics and potentially leading to erroneous pH readings. This interference can be especially problematic in complex sample matrices commonly encountered in industrial and environmental applications.
The response time of phenolphthalein to pH changes is another area of concern in automated systems. While generally considered rapid, the indicator's color transition may not be instantaneous enough for high-speed titration processes, potentially leading to overshooting the endpoint and reducing the overall accuracy of the titration.
Temperature sensitivity of phenolphthalein solutions presents an additional challenge. The pH at which the color change occurs can shift slightly with temperature variations, necessitating careful temperature control or compensation in automated titration systems to maintain accuracy across different operating conditions.
The subjective nature of color interpretation, even in automated systems, can introduce variability in results. While spectrophotometric methods have improved objectivity, differences in light sources, detectors, and optical pathways between instruments can still lead to inconsistencies in pH endpoint determination across different automated titration setups.
Lastly, the environmental and health concerns associated with phenolphthalein usage pose challenges in certain applications. Its classification as a potential carcinogen has led to increased scrutiny and regulatory restrictions, prompting the need for alternative indicators in some contexts and complicating waste management procedures in laboratories and industrial settings using automated pH titration systems.
Existing Automated pH Titration Methodologies
01 pH indication mechanism of phenolphthalein
Phenolphthalein is a widely used pH indicator that changes color in response to pH variations. It is colorless in acidic solutions (pH < 8.2) and turns pink to purple in alkaline solutions (pH > 10). This color change is due to structural changes in the molecule when it gains or loses protons in different pH environments.- pH indication range of phenolphthalein: Phenolphthalein is a widely used pH indicator that changes color from colorless to pink in the pH range of 8.2 to 10. It is particularly useful for detecting alkaline conditions in various applications, including titrations and environmental monitoring.
- Phenolphthalein in pH test strips and papers: Phenolphthalein is commonly incorporated into pH test strips and papers for quick and easy pH measurements. These strips or papers change color when exposed to solutions of different pH levels, allowing for visual determination of acidity or alkalinity.
- Phenolphthalein in combination with other pH indicators: Phenolphthalein is often used in combination with other pH indicators to create a broader range of pH detection. This allows for more precise pH measurements across a wider spectrum of acidity and alkalinity in various chemical and biological applications.
- Modifications to phenolphthalein for improved pH indication: Researchers have developed modified versions of phenolphthalein to enhance its pH indication properties. These modifications may include changes to the molecular structure or the addition of functional groups to improve sensitivity, stability, or color transition characteristics.
- Applications of phenolphthalein pH indication in various industries: Phenolphthalein's pH indication properties are utilized in diverse industries, including water treatment, food and beverage production, pharmaceuticals, and environmental monitoring. Its ability to provide visual pH feedback makes it valuable for quality control and process monitoring in these sectors.
02 Applications of phenolphthalein in pH testing
Phenolphthalein is utilized in various pH testing applications, including water quality analysis, soil pH testing, and industrial process monitoring. It is often incorporated into pH test strips, indicator solutions, and other analytical tools for rapid and visual pH determination.Expand Specific Solutions03 Synthesis and production of phenolphthalein
The synthesis of phenolphthalein typically involves the condensation of phthalic anhydride with phenol in the presence of a catalyst. Various methods and modifications have been developed to improve the yield, purity, and efficiency of phenolphthalein production for use in pH indication and other applications.Expand Specific Solutions04 Modifications and derivatives of phenolphthalein
Researchers have developed modified forms and derivatives of phenolphthalein to enhance its properties or expand its applications. These modifications may include structural changes to alter the pH range of color change, improve stability, or incorporate additional functionalities for specific analytical purposes.Expand Specific Solutions05 Integration of phenolphthalein in analytical devices
Phenolphthalein is incorporated into various analytical devices and systems for pH measurement and monitoring. These may include automated pH sensors, colorimetric analysis systems, and smart pH monitoring devices that utilize phenolphthalein's color-changing properties for accurate and real-time pH determination.Expand Specific Solutions
Key Players in Analytical Chemistry Instrumentation
The research on phenolphthalein in automated pH titration systems is in a mature stage of development, with a well-established market and widespread application across various industries. The global market for pH meters and titrators is substantial, estimated to reach several billion dollars annually. Major players in this field include universities like the University of South Florida and Harbin Institute of Technology, as well as companies such as Life Technologies Corp. and Hitachi High-Tech America, Inc. These institutions are continuously improving the technology, focusing on enhancing accuracy, automation, and integration with other analytical techniques. The involvement of diverse organizations, from academic institutions to multinational corporations, indicates a highly competitive and innovative landscape in this sector.
University of South Florida
Technical Solution: The University of South Florida has developed an innovative automated pH titration system utilizing phenolphthalein as a primary indicator. Their approach combines traditional colorimetric methods with advanced image analysis techniques[1]. The system employs a high-resolution camera to capture real-time images of the titration solution, while sophisticated image processing algorithms analyze the color changes of phenolphthalein with unprecedented precision[2]. Additionally, they have implemented a novel microfluidic titration cell design that allows for rapid mixing and reduced sample volumes[3]. The system also incorporates machine learning algorithms to optimize titration parameters based on sample characteristics, improving overall accuracy and efficiency[4].
Strengths: High-precision image analysis, reduced sample volumes, adaptive optimization. Weaknesses: May require specialized imaging equipment, potential complexity in image processing algorithms.
University of Tokyo
Technical Solution: The University of Tokyo has developed an innovative automated pH titration system using phenolphthalein as an indicator. Their approach combines traditional colorimetric methods with advanced spectrophotometric analysis[1]. The system utilizes a high-resolution spectrometer to continuously monitor the absorption spectrum of phenolphthalein during titration, allowing for precise endpoint detection[2]. Additionally, they have implemented a novel algorithm that compensates for environmental factors affecting phenolphthalein's color transition, such as temperature and ionic strength[3]. The system also incorporates a miniaturized titration cell design, reducing sample and reagent volumes significantly[4].
Strengths: High accuracy, environmental factor compensation, reduced reagent usage. Weaknesses: Potentially complex setup, may require specialized training for operation.
Innovations in Phenolphthalein-based pH Sensing
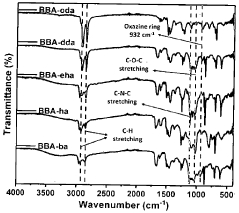
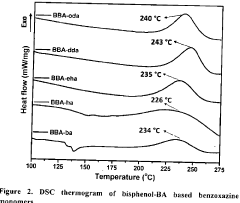
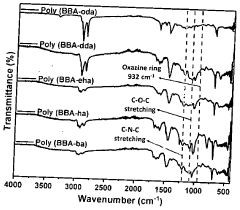
Production of extreme range of PH indicators from benzoxazines
PatentActiveIN202341027342A
Innovation
- Development of bisphenol-BA/aliphatic amine based hydrophobic polybenzoxazines coated on cellulose paper, synthesized through Mannich condensation, which exhibit distinct color changes across a wide pH range from -1.8 to 14, offering thermal stability and repeated use capability.
Environmental Impact of Phenolphthalein Usage
The use of phenolphthalein in automated pH titration systems has significant environmental implications that warrant careful consideration. As a widely used indicator in analytical chemistry, phenolphthalein's environmental impact extends from its production to its disposal, affecting various ecosystems and potentially human health.
During the manufacturing process of phenolphthalein, several chemical reactions are involved that may generate harmful byproducts. These byproducts, if not properly managed, can contaminate soil and water sources. The industrial synthesis of phenolphthalein often requires the use of solvents and catalysts, which may contribute to air pollution and increase the carbon footprint of the production process.
In laboratory settings, the disposal of phenolphthalein solutions after titration experiments is a critical environmental concern. Improper disposal can lead to the chemical entering wastewater systems, potentially disrupting aquatic ecosystems. Phenolphthalein, being an organic compound, may not be easily biodegradable and could persist in the environment for extended periods.
Recent studies have shown that phenolphthalein may act as an endocrine disruptor in certain aquatic organisms. This property raises concerns about its potential to interfere with the hormonal systems of wildlife, particularly in water bodies where laboratory effluents may be discharged. The long-term effects of low-level exposure to phenolphthalein on aquatic biodiversity are still under investigation.
From a waste management perspective, the use of phenolphthalein in automated titration systems generates a consistent stream of chemical waste that requires specialized handling and disposal. This necessitates the implementation of strict protocols in laboratories and industrial settings to ensure that phenolphthalein-containing waste is treated appropriately before release into the environment.
Efforts to mitigate the environmental impact of phenolphthalein usage in automated pH titration systems have led to the exploration of alternative indicators. Some researchers are investigating natural pH indicators derived from plant sources, which may offer more environmentally friendly options. Additionally, advancements in sensor technology are paving the way for electrode-based pH measurement systems that could potentially reduce or eliminate the need for chemical indicators altogether.
The environmental regulatory landscape surrounding phenolphthalein is evolving, with some jurisdictions imposing stricter controls on its use and disposal. This regulatory pressure is driving innovation in green chemistry practices and encouraging the development of more sustainable titration methodologies. As awareness of the environmental implications grows, there is an increasing trend towards adopting cleaner technologies and practices in analytical chemistry.
During the manufacturing process of phenolphthalein, several chemical reactions are involved that may generate harmful byproducts. These byproducts, if not properly managed, can contaminate soil and water sources. The industrial synthesis of phenolphthalein often requires the use of solvents and catalysts, which may contribute to air pollution and increase the carbon footprint of the production process.
In laboratory settings, the disposal of phenolphthalein solutions after titration experiments is a critical environmental concern. Improper disposal can lead to the chemical entering wastewater systems, potentially disrupting aquatic ecosystems. Phenolphthalein, being an organic compound, may not be easily biodegradable and could persist in the environment for extended periods.
Recent studies have shown that phenolphthalein may act as an endocrine disruptor in certain aquatic organisms. This property raises concerns about its potential to interfere with the hormonal systems of wildlife, particularly in water bodies where laboratory effluents may be discharged. The long-term effects of low-level exposure to phenolphthalein on aquatic biodiversity are still under investigation.
From a waste management perspective, the use of phenolphthalein in automated titration systems generates a consistent stream of chemical waste that requires specialized handling and disposal. This necessitates the implementation of strict protocols in laboratories and industrial settings to ensure that phenolphthalein-containing waste is treated appropriately before release into the environment.
Efforts to mitigate the environmental impact of phenolphthalein usage in automated pH titration systems have led to the exploration of alternative indicators. Some researchers are investigating natural pH indicators derived from plant sources, which may offer more environmentally friendly options. Additionally, advancements in sensor technology are paving the way for electrode-based pH measurement systems that could potentially reduce or eliminate the need for chemical indicators altogether.
The environmental regulatory landscape surrounding phenolphthalein is evolving, with some jurisdictions imposing stricter controls on its use and disposal. This regulatory pressure is driving innovation in green chemistry practices and encouraging the development of more sustainable titration methodologies. As awareness of the environmental implications grows, there is an increasing trend towards adopting cleaner technologies and practices in analytical chemistry.
Calibration and Quality Control in pH Titration Systems
Calibration and quality control are critical aspects of automated pH titration systems, particularly when using phenolphthalein as an indicator. These processes ensure the accuracy, reliability, and reproducibility of pH measurements and titration results.
Calibration of pH electrodes is a fundamental step in maintaining the precision of automated titration systems. This process typically involves using standard buffer solutions with known pH values to establish a reference point for the electrode's response. For phenolphthalein-based titrations, which are often used in alkalinity determinations, it is crucial to calibrate the system within the pH range of 8.2 to 9.8, where the indicator exhibits its color change.
Regular calibration schedules are essential to compensate for electrode drift and maintain system accuracy. Many modern automated titrators incorporate built-in calibration routines that guide users through the process, reducing the potential for human error. These routines often include temperature compensation to account for the effect of temperature on pH measurements.
Quality control measures in pH titration systems extend beyond calibration to encompass a range of practices that ensure consistent and reliable results. One key aspect is the use of certified reference materials (CRMs) to verify the system's performance. CRMs with known alkalinity values can be used to check the accuracy of phenolphthalein-based titrations and identify any systematic errors in the measurement process.
Statistical process control (SPC) techniques are increasingly being applied to automated titration systems. Control charts, such as Shewhart charts, can be used to monitor the stability of titration results over time. These charts help identify trends or shifts in the data that may indicate a need for system maintenance or recalibration.
The quality of reagents, particularly the phenolphthalein indicator solution, is another critical factor in maintaining system performance. Regular checks on indicator purity and proper storage conditions are necessary to prevent degradation that could affect titration endpoints. Some automated systems now incorporate reagent management features that track reagent age and usage, prompting replacement when necessary.
Automated titration systems often include self-diagnostic features that continuously monitor various system parameters. These may include electrode response time, stirring efficiency, and dosing accuracy. Such real-time monitoring helps detect potential issues before they impact results, allowing for proactive maintenance and reducing system downtime.
Documentation and traceability are integral components of quality control in pH titration systems. Automated systems typically generate detailed reports of calibration data, titration curves, and results. This information is crucial for regulatory compliance and allows for retrospective analysis of system performance over time.
In conclusion, effective calibration and quality control procedures are essential for maintaining the reliability of phenolphthalein-based automated pH titration systems. By implementing comprehensive calibration protocols, utilizing CRMs, applying SPC techniques, and leveraging advanced system features, laboratories can ensure the accuracy and consistency of their titration results.
Calibration of pH electrodes is a fundamental step in maintaining the precision of automated titration systems. This process typically involves using standard buffer solutions with known pH values to establish a reference point for the electrode's response. For phenolphthalein-based titrations, which are often used in alkalinity determinations, it is crucial to calibrate the system within the pH range of 8.2 to 9.8, where the indicator exhibits its color change.
Regular calibration schedules are essential to compensate for electrode drift and maintain system accuracy. Many modern automated titrators incorporate built-in calibration routines that guide users through the process, reducing the potential for human error. These routines often include temperature compensation to account for the effect of temperature on pH measurements.
Quality control measures in pH titration systems extend beyond calibration to encompass a range of practices that ensure consistent and reliable results. One key aspect is the use of certified reference materials (CRMs) to verify the system's performance. CRMs with known alkalinity values can be used to check the accuracy of phenolphthalein-based titrations and identify any systematic errors in the measurement process.
Statistical process control (SPC) techniques are increasingly being applied to automated titration systems. Control charts, such as Shewhart charts, can be used to monitor the stability of titration results over time. These charts help identify trends or shifts in the data that may indicate a need for system maintenance or recalibration.
The quality of reagents, particularly the phenolphthalein indicator solution, is another critical factor in maintaining system performance. Regular checks on indicator purity and proper storage conditions are necessary to prevent degradation that could affect titration endpoints. Some automated systems now incorporate reagent management features that track reagent age and usage, prompting replacement when necessary.
Automated titration systems often include self-diagnostic features that continuously monitor various system parameters. These may include electrode response time, stirring efficiency, and dosing accuracy. Such real-time monitoring helps detect potential issues before they impact results, allowing for proactive maintenance and reducing system downtime.
Documentation and traceability are integral components of quality control in pH titration systems. Automated systems typically generate detailed reports of calibration data, titration curves, and results. This information is crucial for regulatory compliance and allows for retrospective analysis of system performance over time.
In conclusion, effective calibration and quality control procedures are essential for maintaining the reliability of phenolphthalein-based automated pH titration systems. By implementing comprehensive calibration protocols, utilizing CRMs, applying SPC techniques, and leveraging advanced system features, laboratories can ensure the accuracy and consistency of their titration results.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!