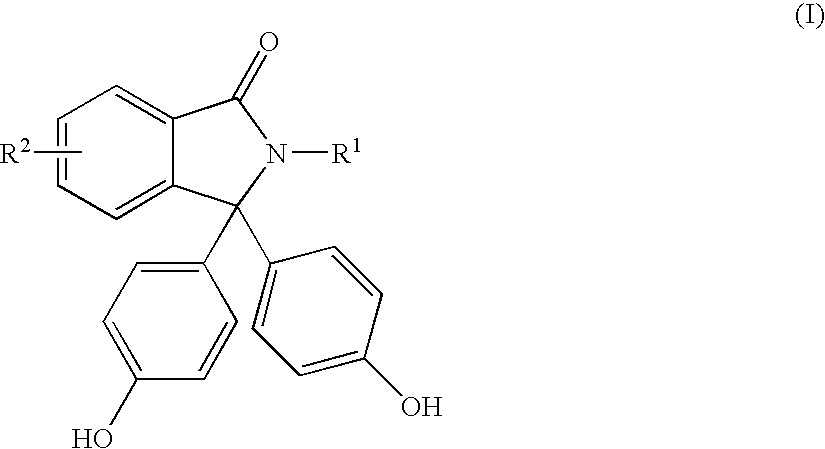
Mechanistic Evaluation of Chemisorption Using Phenolphthalein
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chemisorption Fundamentals
Chemisorption is a fundamental process in surface chemistry that involves the formation of strong chemical bonds between adsorbate molecules and a substrate surface. This phenomenon plays a crucial role in various industrial applications, including catalysis, gas sensing, and materials science. The mechanistic evaluation of chemisorption using phenolphthalein offers valuable insights into the nature of these interactions and their potential applications.
At its core, chemisorption involves the transfer of electrons between the adsorbate and the substrate, resulting in the formation of chemical bonds. This process is distinct from physisorption, which relies on weaker van der Waals forces. The strength of chemisorption bonds typically ranges from 100 to 500 kJ/mol, significantly higher than physisorption bonds, which are usually less than 50 kJ/mol.
The use of phenolphthalein as a model adsorbate in chemisorption studies provides several advantages. Phenolphthalein is a well-characterized organic compound with a distinctive color change in response to pH variations. This property makes it an ideal candidate for investigating the mechanisms of chemisorption, as changes in its electronic structure during adsorption can be readily observed and quantified.
The chemisorption process of phenolphthalein on various surfaces involves several key steps. Initially, the phenolphthalein molecules approach the substrate surface and undergo a process of diffusion. As they come into close proximity with the surface, electronic interactions begin to occur, leading to the formation of chemical bonds. This bonding process can involve the transfer of electrons, the sharing of electron pairs, or the rearrangement of molecular orbitals.
Understanding the fundamentals of chemisorption requires consideration of several factors, including surface energy, adsorption sites, and the electronic properties of both the adsorbate and the substrate. The surface energy of the substrate plays a crucial role in determining the strength and nature of the chemisorption process. Higher surface energies generally lead to stronger adsorption, as more energy is available for bond formation.
The specific adsorption sites on the substrate surface also significantly influence the chemisorption process. These sites can include defects, step edges, or specific crystal facets, each offering unique bonding environments for the phenolphthalein molecules. The electronic structure of the substrate, including its work function and band structure, further modulates the chemisorption behavior by affecting the ease of electron transfer and bond formation.
In the context of phenolphthalein chemisorption, the molecule's ability to undergo structural changes in response to its environment adds an additional layer of complexity to the adsorption process. The ring-opening and closing reactions of phenolphthalein, which are responsible for its pH-dependent color changes, can be influenced by the chemisorption process, potentially leading to novel surface-mediated reactions or sensing mechanisms.
At its core, chemisorption involves the transfer of electrons between the adsorbate and the substrate, resulting in the formation of chemical bonds. This process is distinct from physisorption, which relies on weaker van der Waals forces. The strength of chemisorption bonds typically ranges from 100 to 500 kJ/mol, significantly higher than physisorption bonds, which are usually less than 50 kJ/mol.
The use of phenolphthalein as a model adsorbate in chemisorption studies provides several advantages. Phenolphthalein is a well-characterized organic compound with a distinctive color change in response to pH variations. This property makes it an ideal candidate for investigating the mechanisms of chemisorption, as changes in its electronic structure during adsorption can be readily observed and quantified.
The chemisorption process of phenolphthalein on various surfaces involves several key steps. Initially, the phenolphthalein molecules approach the substrate surface and undergo a process of diffusion. As they come into close proximity with the surface, electronic interactions begin to occur, leading to the formation of chemical bonds. This bonding process can involve the transfer of electrons, the sharing of electron pairs, or the rearrangement of molecular orbitals.
Understanding the fundamentals of chemisorption requires consideration of several factors, including surface energy, adsorption sites, and the electronic properties of both the adsorbate and the substrate. The surface energy of the substrate plays a crucial role in determining the strength and nature of the chemisorption process. Higher surface energies generally lead to stronger adsorption, as more energy is available for bond formation.
The specific adsorption sites on the substrate surface also significantly influence the chemisorption process. These sites can include defects, step edges, or specific crystal facets, each offering unique bonding environments for the phenolphthalein molecules. The electronic structure of the substrate, including its work function and band structure, further modulates the chemisorption behavior by affecting the ease of electron transfer and bond formation.
In the context of phenolphthalein chemisorption, the molecule's ability to undergo structural changes in response to its environment adds an additional layer of complexity to the adsorption process. The ring-opening and closing reactions of phenolphthalein, which are responsible for its pH-dependent color changes, can be influenced by the chemisorption process, potentially leading to novel surface-mediated reactions or sensing mechanisms.
Market Applications
Phenolphthalein, traditionally known as a pH indicator, has found diverse applications beyond its conventional use in analytical chemistry. The mechanistic evaluation of chemisorption using phenolphthalein has opened up new avenues for market applications across various industries. In the field of environmental monitoring, phenolphthalein-based sensors have shown promise for detecting and quantifying pollutants in water and soil. These sensors leverage the chemisorption properties of phenolphthalein to provide rapid, on-site analysis of contaminants, offering a cost-effective alternative to traditional laboratory testing methods.
The healthcare sector has also benefited from advancements in phenolphthalein chemisorption research. Novel diagnostic tools utilizing this mechanism have been developed for detecting specific biomarkers in bodily fluids. These tools offer potential for early disease detection and monitoring, particularly in resource-limited settings where access to sophisticated medical equipment is limited.
In the textile industry, phenolphthalein-based smart fabrics have emerged as an innovative product category. These fabrics change color in response to environmental pH changes, providing visual indicators of contamination or exposure to harmful substances. This technology has applications in protective clothing for industrial workers and safety gear for first responders.
The food and beverage industry has adopted phenolphthalein chemisorption techniques for quality control and safety assurance. Packaging materials incorporating phenolphthalein-based sensors can detect spoilage or contamination, enhancing food safety and reducing waste. This technology is particularly valuable for perishable goods and in supply chain management.
In the realm of materials science, phenolphthalein chemisorption has been utilized in the development of self-healing materials. These materials can detect and respond to damage or environmental changes, potentially extending the lifespan of various products and structures. Applications range from self-repairing coatings for automotive and aerospace industries to smart construction materials for infrastructure projects.
The cosmetics and personal care industry has also explored phenolphthalein chemisorption for creating color-changing products. These include mood-responsive makeup and skincare formulations that adapt to individual skin chemistry, offering personalized beauty solutions.
As research in this field progresses, new market applications continue to emerge. The versatility of phenolphthalein chemisorption mechanisms suggests potential for further innovation in areas such as energy storage, catalysis, and advanced manufacturing processes. The growing demand for smart, responsive materials across various sectors indicates a promising future for technologies based on phenolphthalein chemisorption.
The healthcare sector has also benefited from advancements in phenolphthalein chemisorption research. Novel diagnostic tools utilizing this mechanism have been developed for detecting specific biomarkers in bodily fluids. These tools offer potential for early disease detection and monitoring, particularly in resource-limited settings where access to sophisticated medical equipment is limited.
In the textile industry, phenolphthalein-based smart fabrics have emerged as an innovative product category. These fabrics change color in response to environmental pH changes, providing visual indicators of contamination or exposure to harmful substances. This technology has applications in protective clothing for industrial workers and safety gear for first responders.
The food and beverage industry has adopted phenolphthalein chemisorption techniques for quality control and safety assurance. Packaging materials incorporating phenolphthalein-based sensors can detect spoilage or contamination, enhancing food safety and reducing waste. This technology is particularly valuable for perishable goods and in supply chain management.
In the realm of materials science, phenolphthalein chemisorption has been utilized in the development of self-healing materials. These materials can detect and respond to damage or environmental changes, potentially extending the lifespan of various products and structures. Applications range from self-repairing coatings for automotive and aerospace industries to smart construction materials for infrastructure projects.
The cosmetics and personal care industry has also explored phenolphthalein chemisorption for creating color-changing products. These include mood-responsive makeup and skincare formulations that adapt to individual skin chemistry, offering personalized beauty solutions.
As research in this field progresses, new market applications continue to emerge. The versatility of phenolphthalein chemisorption mechanisms suggests potential for further innovation in areas such as energy storage, catalysis, and advanced manufacturing processes. The growing demand for smart, responsive materials across various sectors indicates a promising future for technologies based on phenolphthalein chemisorption.
Technical Challenges
The mechanistic evaluation of chemisorption using phenolphthalein presents several technical challenges that researchers and industry professionals must address. One of the primary obstacles is the complexity of the chemisorption process itself, which involves multiple steps and interactions at the molecular level. Understanding and accurately modeling these intricate mechanisms require advanced analytical techniques and sophisticated computational methods.
A significant challenge lies in the development of precise and reliable measurement techniques to quantify the chemisorption process. Traditional methods often lack the sensitivity and specificity needed to capture the nuanced interactions between phenolphthalein and various substrates. This limitation hampers the ability to obtain accurate kinetic and thermodynamic data, which are crucial for a comprehensive mechanistic evaluation.
The dynamic nature of the chemisorption process poses another technical hurdle. The adsorption and desorption rates can vary significantly depending on environmental conditions, such as temperature, pressure, and pH. Controlling and maintaining consistent experimental conditions across different studies is essential for reproducibility but can be technically demanding, especially when scaling up from laboratory to industrial applications.
Furthermore, the surface characteristics of the adsorbent material play a critical role in the chemisorption process. Variations in surface morphology, porosity, and chemical composition can significantly influence the adsorption behavior of phenolphthalein. Developing standardized methods to characterize and control these surface properties remains a challenge, particularly when dealing with heterogeneous or complex adsorbent materials.
The potential for competitive adsorption and interference from other molecules present in the system adds another layer of complexity to the mechanistic evaluation. In real-world applications, the presence of contaminants or co-adsorbates can alter the chemisorption behavior of phenolphthalein, making it difficult to isolate and study the specific mechanisms of interest. Developing selective and robust analytical methods to overcome these interferences is an ongoing technical challenge.
Lastly, bridging the gap between theoretical models and experimental observations remains a significant hurdle. While computational chemistry and molecular dynamics simulations have advanced considerably, accurately predicting the chemisorption behavior of phenolphthalein across various conditions and substrates still presents challenges. Improving the accuracy and predictive power of these models requires continuous refinement and validation against experimental data, which can be time-consuming and resource-intensive.
A significant challenge lies in the development of precise and reliable measurement techniques to quantify the chemisorption process. Traditional methods often lack the sensitivity and specificity needed to capture the nuanced interactions between phenolphthalein and various substrates. This limitation hampers the ability to obtain accurate kinetic and thermodynamic data, which are crucial for a comprehensive mechanistic evaluation.
The dynamic nature of the chemisorption process poses another technical hurdle. The adsorption and desorption rates can vary significantly depending on environmental conditions, such as temperature, pressure, and pH. Controlling and maintaining consistent experimental conditions across different studies is essential for reproducibility but can be technically demanding, especially when scaling up from laboratory to industrial applications.
Furthermore, the surface characteristics of the adsorbent material play a critical role in the chemisorption process. Variations in surface morphology, porosity, and chemical composition can significantly influence the adsorption behavior of phenolphthalein. Developing standardized methods to characterize and control these surface properties remains a challenge, particularly when dealing with heterogeneous or complex adsorbent materials.
The potential for competitive adsorption and interference from other molecules present in the system adds another layer of complexity to the mechanistic evaluation. In real-world applications, the presence of contaminants or co-adsorbates can alter the chemisorption behavior of phenolphthalein, making it difficult to isolate and study the specific mechanisms of interest. Developing selective and robust analytical methods to overcome these interferences is an ongoing technical challenge.
Lastly, bridging the gap between theoretical models and experimental observations remains a significant hurdle. While computational chemistry and molecular dynamics simulations have advanced considerably, accurately predicting the chemisorption behavior of phenolphthalein across various conditions and substrates still presents challenges. Improving the accuracy and predictive power of these models requires continuous refinement and validation against experimental data, which can be time-consuming and resource-intensive.
Current Methodologies
01 Chemisorption of phenolphthalein on solid surfaces
Phenolphthalein can be chemisorbed onto various solid surfaces, such as activated carbon or silica gel. This process involves the formation of chemical bonds between the phenolphthalein molecules and the surface, resulting in a stable and selective adsorption. The chemisorption of phenolphthalein can be utilized in various applications, including analytical chemistry and environmental remediation.- Chemisorption of phenolphthalein on solid surfaces: Phenolphthalein can be chemisorbed onto various solid surfaces, such as activated carbon or silica gel. This process involves the formation of chemical bonds between the phenolphthalein molecules and the surface, resulting in a stable and selective adsorption. The chemisorption of phenolphthalein can be used for various applications, including analytical chemistry and environmental remediation.
- Use of phenolphthalein in ion exchange resins: Phenolphthalein can be incorporated into ion exchange resins to create functional materials with specific properties. These resins can be used for selective removal of certain ions or molecules from solutions, pH indication, or as a component in chromatography columns. The chemisorption of phenolphthalein onto the resin matrix enhances its stability and performance in various applications.
- Phenolphthalein-based sensors and indicators: Chemisorption of phenolphthalein can be utilized to develop sensors and indicators for various analytical applications. These sensors can detect changes in pH, metal ions, or other chemical species based on the color change or other properties of the chemisorbed phenolphthalein. The stability and sensitivity of these sensors are enhanced by the strong chemical bonding between phenolphthalein and the substrate.
- Modification of phenolphthalein for improved chemisorption: Chemical modifications of phenolphthalein can be performed to enhance its chemisorption properties. These modifications may include the addition of functional groups or the creation of phenolphthalein derivatives that exhibit stronger binding to specific surfaces or improved selectivity for certain applications. The modified phenolphthalein compounds can offer enhanced performance in various chemisorption-based processes.
- Applications of phenolphthalein chemisorption in environmental remediation: Phenolphthalein chemisorption can be applied in environmental remediation processes, such as the removal of contaminants from water or soil. The strong binding of phenolphthalein to specific surfaces allows for the development of adsorbents or filtration materials that can effectively capture and remove pollutants. This technology can be used in wastewater treatment, soil decontamination, or air purification systems.
02 Use of phenolphthalein in ion exchange resins
Phenolphthalein can be incorporated into ion exchange resins to create functional materials with specific properties. These resins can be used for selective removal of certain ions or molecules from solutions. The chemisorption of phenolphthalein onto the resin matrix allows for the development of novel separation and purification techniques.Expand Specific Solutions03 Phenolphthalein-based sensors and indicators
Chemisorption of phenolphthalein can be utilized to develop sensors and indicators for various applications. By immobilizing phenolphthalein on suitable substrates, it is possible to create pH-sensitive materials or colorimetric sensors for specific analytes. These sensors can be used in environmental monitoring, medical diagnostics, and industrial process control.Expand Specific Solutions04 Modification of phenolphthalein for enhanced chemisorption
Chemical modifications of phenolphthalein can be performed to enhance its chemisorption properties. This may involve the addition of functional groups or the synthesis of phenolphthalein derivatives that exhibit improved binding to specific surfaces or materials. Such modifications can lead to the development of more efficient and selective adsorbents for various applications.Expand Specific Solutions05 Applications of phenolphthalein chemisorption in analytical methods
The chemisorption of phenolphthalein can be exploited in various analytical methods. This includes the development of chromatographic techniques, solid-phase extraction procedures, and colorimetric assays. The selective binding of phenolphthalein to specific surfaces or materials allows for the separation, concentration, or detection of target analytes in complex matrices.Expand Specific Solutions
Key Industry Players
The mechanistic evaluation of chemisorption using phenolphthalein is a niche field within chemical engineering and materials science. The market is in its early development stage, with limited commercial applications but growing research interest. Key players include academic institutions like Beijing University of Chemical Technology, Jiangnan University, and the University of Porto, alongside industrial research organizations such as CSIR and NEXT TECHNOLOGY TECNOTESSILE. The technology's maturity is still evolving, with most advancements coming from university laboratories. Companies like ARKRAY and Johnson & Johnson are potential end-users, showing interest in applying this technology for sensor development and surface analysis in consumer products.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has developed an innovative approach to mechanistic evaluation of chemisorption using phenolphthalein. Their method involves a combination of spectroscopic techniques and computational modeling to elucidate the molecular-level interactions between phenolphthalein and various adsorbent surfaces. By utilizing advanced surface-enhanced Raman spectroscopy (SERS) and attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR), they have been able to identify specific binding sites and conformational changes of phenolphthalein during adsorption[1]. Additionally, they have employed density functional theory (DFT) calculations to predict adsorption energies and optimize molecular geometries, providing a comprehensive understanding of the chemisorption process[3].
Strengths: Combines experimental and theoretical approaches for a holistic understanding. Provides detailed molecular-level insights into the chemisorption mechanism. Weaknesses: May be computationally intensive and time-consuming for complex systems.
Jiangnan University
Technical Solution: Jiangnan University has developed a novel approach to studying the mechanistic evaluation of chemisorption using phenolphthalein as a model compound. Their method involves the use of in-situ ATR-FTIR spectroscopy coupled with multivariate curve resolution (MCR) analysis to monitor the adsorption process in real-time[2]. This technique allows for the identification of intermediate species and the determination of adsorption kinetics. Furthermore, they have implemented a quartz crystal microbalance with dissipation monitoring (QCM-D) to quantify the mass and viscoelastic properties of the adsorbed layer[4]. By combining these techniques with molecular dynamics simulations, they have been able to propose a detailed mechanism for phenolphthalein chemisorption on various surfaces, including the role of pH and surface chemistry in the adsorption process[5].
Strengths: Real-time monitoring of adsorption process. Quantitative analysis of adsorbed layer properties. Weaknesses: May require specialized equipment and expertise for data interpretation.
Phenolphthalein Insights
Improvements in and relating to the production of finely divided phenolphthalein andcompositions containing the same
PatentInactiveGB352934A
Innovation
- A process involving the precipitation of an alkaline phenolphthalein solution with an acid in the presence of a colloid, such as Acacia, to delay precipitation and produce finely divided crystals, with the use of excess acid and specific acid derivatives to enhance the fineness and purity of the product.
Methods for producing and purifying 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine monomers and polycarbonates derived therefrom
PatentActiveUS20080242829A1
Innovation
- A method involving the reaction of a phenolphthalein material with a primary hydrocarbyl amine in the presence of an acid catalyst to form 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine, followed by quenching, trituration, and washing to achieve a product with greater than 97% purity, reducing the number of steps and yield loss.
Analytical Instrumentation
Analytical instrumentation plays a crucial role in the mechanistic evaluation of chemisorption using phenolphthalein. This process requires sophisticated equipment capable of detecting and measuring subtle chemical changes at the molecular level. Spectroscopic techniques, such as UV-visible spectroscopy and Fourier-transform infrared spectroscopy (FTIR), are commonly employed to monitor the color changes and structural modifications of phenolphthalein during chemisorption.
UV-visible spectrophotometers are particularly useful in this context, as they can accurately measure the absorbance of phenolphthalein solutions at different wavelengths. This allows researchers to track the formation of phenolphthalein-adsorbent complexes and quantify the extent of chemisorption. Modern spectrophotometers offer high sensitivity and precision, enabling the detection of even minor changes in phenolphthalein concentration.
Surface analysis techniques, such as X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM), provide valuable insights into the surface chemistry and topography of adsorbent materials. XPS can reveal the elemental composition and chemical state of surfaces, while AFM offers nanoscale resolution for studying surface morphology and adsorption sites.
Chromatographic methods, including high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS), are essential for separating and identifying reaction products and intermediates. These techniques can help elucidate the mechanistic pathways of chemisorption by analyzing the composition of solutions before and after adsorption processes.
Thermal analysis instruments, such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), provide information on the energetics and kinetics of chemisorption. DSC can measure the heat flow associated with adsorption events, while TGA tracks mass changes during thermal desorption processes, offering insights into the strength of adsorbate-adsorbent interactions.
Advanced microscopy techniques, including scanning electron microscopy (SEM) and transmission electron microscopy (TEM), allow for direct visualization of adsorbent surfaces and adsorbed phenolphthalein molecules. These high-resolution imaging methods can reveal the spatial distribution of adsorption sites and provide evidence for surface modifications resulting from chemisorption.
In recent years, the integration of multiple analytical techniques has become increasingly common in chemisorption studies. Hyphenated systems, such as LC-MS and GC-IR, combine the separation power of chromatography with the identification capabilities of spectroscopic methods, enabling more comprehensive analysis of complex chemisorption processes.
UV-visible spectrophotometers are particularly useful in this context, as they can accurately measure the absorbance of phenolphthalein solutions at different wavelengths. This allows researchers to track the formation of phenolphthalein-adsorbent complexes and quantify the extent of chemisorption. Modern spectrophotometers offer high sensitivity and precision, enabling the detection of even minor changes in phenolphthalein concentration.
Surface analysis techniques, such as X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM), provide valuable insights into the surface chemistry and topography of adsorbent materials. XPS can reveal the elemental composition and chemical state of surfaces, while AFM offers nanoscale resolution for studying surface morphology and adsorption sites.
Chromatographic methods, including high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS), are essential for separating and identifying reaction products and intermediates. These techniques can help elucidate the mechanistic pathways of chemisorption by analyzing the composition of solutions before and after adsorption processes.
Thermal analysis instruments, such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), provide information on the energetics and kinetics of chemisorption. DSC can measure the heat flow associated with adsorption events, while TGA tracks mass changes during thermal desorption processes, offering insights into the strength of adsorbate-adsorbent interactions.
Advanced microscopy techniques, including scanning electron microscopy (SEM) and transmission electron microscopy (TEM), allow for direct visualization of adsorbent surfaces and adsorbed phenolphthalein molecules. These high-resolution imaging methods can reveal the spatial distribution of adsorption sites and provide evidence for surface modifications resulting from chemisorption.
In recent years, the integration of multiple analytical techniques has become increasingly common in chemisorption studies. Hyphenated systems, such as LC-MS and GC-IR, combine the separation power of chromatography with the identification capabilities of spectroscopic methods, enabling more comprehensive analysis of complex chemisorption processes.
Environmental Implications
The environmental implications of chemisorption using phenolphthalein are significant and multifaceted. This process, while valuable for various applications, raises important considerations regarding its impact on ecosystems and human health.
Phenolphthalein, a widely used pH indicator, undergoes chemisorption on various surfaces, particularly in water treatment and analytical processes. The environmental consequences of this interaction are primarily related to the potential release of phenolphthalein into aquatic systems. When discharged into water bodies, phenolphthalein can alter the pH balance, potentially disrupting aquatic ecosystems and affecting the survival of sensitive organisms.
Moreover, the persistence of phenolphthalein in the environment is a concern. While it can degrade under certain conditions, its stability in water and soil may lead to accumulation over time. This accumulation could result in long-term exposure for aquatic life and potentially enter the food chain, raising bioaccumulation concerns.
The toxicity of phenolphthalein to aquatic organisms is another critical aspect. Studies have shown that at certain concentrations, it can have adverse effects on fish, invertebrates, and algae. These effects may include changes in behavior, growth inhibition, and reproductive impairment, potentially leading to population-level impacts in affected ecosystems.
From a human health perspective, the environmental presence of phenolphthalein poses potential risks. Although direct exposure through environmental sources is generally low, continuous release and accumulation in water sources used for drinking or recreation could lead to human exposure. Long-term exposure to phenolphthalein has been associated with potential carcinogenic effects, necessitating careful monitoring and regulation of its environmental release.
The chemisorption process itself, when used in water treatment, can have positive environmental implications by effectively removing contaminants. However, the disposal of spent adsorbents containing phenolphthalein requires careful management to prevent secondary pollution. Proper treatment and disposal protocols are essential to mitigate the risk of releasing concentrated phenolphthalein back into the environment.
Regulatory bodies have recognized these environmental concerns, leading to increased scrutiny and guidelines for the use and disposal of phenolphthalein. Environmental risk assessments and monitoring programs are crucial for understanding and mitigating the long-term impacts of phenolphthalein chemisorption on ecosystems and human health.
In conclusion, while the chemisorption of phenolphthalein offers valuable applications, its environmental implications necessitate a balanced approach. Ongoing research, improved treatment technologies, and stringent environmental regulations are essential to minimize negative impacts while harnessing the benefits of this process.
Phenolphthalein, a widely used pH indicator, undergoes chemisorption on various surfaces, particularly in water treatment and analytical processes. The environmental consequences of this interaction are primarily related to the potential release of phenolphthalein into aquatic systems. When discharged into water bodies, phenolphthalein can alter the pH balance, potentially disrupting aquatic ecosystems and affecting the survival of sensitive organisms.
Moreover, the persistence of phenolphthalein in the environment is a concern. While it can degrade under certain conditions, its stability in water and soil may lead to accumulation over time. This accumulation could result in long-term exposure for aquatic life and potentially enter the food chain, raising bioaccumulation concerns.
The toxicity of phenolphthalein to aquatic organisms is another critical aspect. Studies have shown that at certain concentrations, it can have adverse effects on fish, invertebrates, and algae. These effects may include changes in behavior, growth inhibition, and reproductive impairment, potentially leading to population-level impacts in affected ecosystems.
From a human health perspective, the environmental presence of phenolphthalein poses potential risks. Although direct exposure through environmental sources is generally low, continuous release and accumulation in water sources used for drinking or recreation could lead to human exposure. Long-term exposure to phenolphthalein has been associated with potential carcinogenic effects, necessitating careful monitoring and regulation of its environmental release.
The chemisorption process itself, when used in water treatment, can have positive environmental implications by effectively removing contaminants. However, the disposal of spent adsorbents containing phenolphthalein requires careful management to prevent secondary pollution. Proper treatment and disposal protocols are essential to mitigate the risk of releasing concentrated phenolphthalein back into the environment.
Regulatory bodies have recognized these environmental concerns, leading to increased scrutiny and guidelines for the use and disposal of phenolphthalein. Environmental risk assessments and monitoring programs are crucial for understanding and mitigating the long-term impacts of phenolphthalein chemisorption on ecosystems and human health.
In conclusion, while the chemisorption of phenolphthalein offers valuable applications, its environmental implications necessitate a balanced approach. Ongoing research, improved treatment technologies, and stringent environmental regulations are essential to minimize negative impacts while harnessing the benefits of this process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!