How Phenolphthalein Affects Platelet Aggregation in Blood Studies
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein and Platelets: Background and Objectives
Phenolphthalein, a compound widely known for its use as a pH indicator, has recently garnered attention in the field of hematology for its potential effects on platelet aggregation. This research area represents a fascinating intersection of chemistry and biology, with implications for both diagnostic and therapeutic applications in blood studies.
The study of platelet aggregation is crucial in understanding hemostasis, thrombosis, and various cardiovascular disorders. Platelets play a vital role in blood clotting, and their aggregation is a complex process influenced by numerous factors. The introduction of phenolphthalein into this field opens up new avenues for investigation and potential interventions in platelet-related disorders.
Historically, phenolphthalein has been primarily associated with its colorimetric properties, changing from colorless to pink in alkaline solutions. Its application in blood studies represents a significant shift in focus, highlighting the compound's potential multifaceted nature. This transition from a simple indicator to a bioactive molecule in hematology underscores the importance of cross-disciplinary research in advancing medical science.
The primary objective of investigating phenolphthalein's effects on platelet aggregation is to elucidate the mechanisms by which this compound interacts with platelets and influences their behavior. Researchers aim to determine whether phenolphthalein enhances, inhibits, or modulates platelet aggregation, and under what conditions these effects occur. Understanding these interactions could lead to the development of novel diagnostic tools or therapeutic agents for managing platelet-related disorders.
Another key goal is to explore the potential applications of phenolphthalein in blood studies beyond its traditional use as a pH indicator. This includes assessing its viability as a marker for specific blood conditions, its potential as a platelet function modulator, and its possible role in developing new anticoagulant or antiplatelet therapies.
Furthermore, researchers are interested in comparing the effects of phenolphthalein on platelet aggregation with those of established compounds used in hematology. This comparative analysis aims to identify any unique properties or advantages that phenolphthalein might offer over existing agents, potentially leading to improved diagnostic or therapeutic approaches.
The investigation into phenolphthalein's impact on platelet aggregation also seeks to uncover any potential side effects or limitations of its use in blood studies. This comprehensive evaluation is crucial for determining the compound's safety profile and its suitability for various clinical applications.
The study of platelet aggregation is crucial in understanding hemostasis, thrombosis, and various cardiovascular disorders. Platelets play a vital role in blood clotting, and their aggregation is a complex process influenced by numerous factors. The introduction of phenolphthalein into this field opens up new avenues for investigation and potential interventions in platelet-related disorders.
Historically, phenolphthalein has been primarily associated with its colorimetric properties, changing from colorless to pink in alkaline solutions. Its application in blood studies represents a significant shift in focus, highlighting the compound's potential multifaceted nature. This transition from a simple indicator to a bioactive molecule in hematology underscores the importance of cross-disciplinary research in advancing medical science.
The primary objective of investigating phenolphthalein's effects on platelet aggregation is to elucidate the mechanisms by which this compound interacts with platelets and influences their behavior. Researchers aim to determine whether phenolphthalein enhances, inhibits, or modulates platelet aggregation, and under what conditions these effects occur. Understanding these interactions could lead to the development of novel diagnostic tools or therapeutic agents for managing platelet-related disorders.
Another key goal is to explore the potential applications of phenolphthalein in blood studies beyond its traditional use as a pH indicator. This includes assessing its viability as a marker for specific blood conditions, its potential as a platelet function modulator, and its possible role in developing new anticoagulant or antiplatelet therapies.
Furthermore, researchers are interested in comparing the effects of phenolphthalein on platelet aggregation with those of established compounds used in hematology. This comparative analysis aims to identify any unique properties or advantages that phenolphthalein might offer over existing agents, potentially leading to improved diagnostic or therapeutic approaches.
The investigation into phenolphthalein's impact on platelet aggregation also seeks to uncover any potential side effects or limitations of its use in blood studies. This comprehensive evaluation is crucial for determining the compound's safety profile and its suitability for various clinical applications.
Market Analysis: Blood Study Reagents
The blood study reagents market has experienced significant growth in recent years, driven by the increasing prevalence of blood disorders and the rising demand for advanced diagnostic technologies. This market segment encompasses a wide range of products, including phenolphthalein, which is used in various blood studies, particularly those involving platelet aggregation.
The global market for blood study reagents is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is primarily attributed to the expanding geriatric population, the rising incidence of chronic diseases, and the growing awareness of early disease detection and prevention.
Phenolphthalein, traditionally known for its use as a pH indicator, has found applications in blood studies due to its unique properties. Its potential effects on platelet aggregation have garnered interest from researchers and pharmaceutical companies alike, contributing to the overall market demand for specialized blood study reagents.
The market for blood study reagents is highly fragmented, with numerous players competing for market share. Key market participants include established pharmaceutical companies, biotechnology firms, and specialized reagent manufacturers. These companies are investing heavily in research and development to introduce innovative products and gain a competitive edge in the market.
Geographically, North America holds the largest share of the blood study reagents market, followed by Europe and Asia-Pacific. The dominance of North America can be attributed to its advanced healthcare infrastructure, high healthcare expenditure, and the presence of major market players. However, the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare facilities, increasing healthcare awareness, and rising disposable incomes.
The market for blood study reagents, including those used in platelet aggregation studies, is influenced by several factors. These include technological advancements in blood analysis techniques, increasing automation in laboratory processes, and the growing trend towards personalized medicine. Additionally, the COVID-19 pandemic has highlighted the importance of blood studies in understanding and managing various health conditions, further driving market growth.
Challenges in the blood study reagents market include stringent regulatory requirements, high costs associated with research and development, and the need for skilled professionals to conduct complex blood analyses. However, these challenges also present opportunities for innovation and market differentiation for companies willing to invest in overcoming these obstacles.
The global market for blood study reagents is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is primarily attributed to the expanding geriatric population, the rising incidence of chronic diseases, and the growing awareness of early disease detection and prevention.
Phenolphthalein, traditionally known for its use as a pH indicator, has found applications in blood studies due to its unique properties. Its potential effects on platelet aggregation have garnered interest from researchers and pharmaceutical companies alike, contributing to the overall market demand for specialized blood study reagents.
The market for blood study reagents is highly fragmented, with numerous players competing for market share. Key market participants include established pharmaceutical companies, biotechnology firms, and specialized reagent manufacturers. These companies are investing heavily in research and development to introduce innovative products and gain a competitive edge in the market.
Geographically, North America holds the largest share of the blood study reagents market, followed by Europe and Asia-Pacific. The dominance of North America can be attributed to its advanced healthcare infrastructure, high healthcare expenditure, and the presence of major market players. However, the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare facilities, increasing healthcare awareness, and rising disposable incomes.
The market for blood study reagents, including those used in platelet aggregation studies, is influenced by several factors. These include technological advancements in blood analysis techniques, increasing automation in laboratory processes, and the growing trend towards personalized medicine. Additionally, the COVID-19 pandemic has highlighted the importance of blood studies in understanding and managing various health conditions, further driving market growth.
Challenges in the blood study reagents market include stringent regulatory requirements, high costs associated with research and development, and the need for skilled professionals to conduct complex blood analyses. However, these challenges also present opportunities for innovation and market differentiation for companies willing to invest in overcoming these obstacles.
Current Challenges in Platelet Aggregation Studies
Platelet aggregation studies face several significant challenges that hinder progress in understanding blood clotting mechanisms and developing effective treatments for related disorders. One of the primary obstacles is the complexity of platelet activation and aggregation processes, which involve multiple signaling pathways and molecular interactions. This intricate network of factors makes it difficult to isolate and study individual components without disrupting the overall system.
Another major challenge is the lack of standardization in platelet aggregation assays. Different laboratories often use varying methodologies, reagents, and equipment, leading to inconsistent results and difficulties in comparing findings across studies. This lack of uniformity hampers the reproducibility of experiments and slows down the translation of research findings into clinical applications.
The sensitivity of platelets to external factors poses another significant hurdle. Platelets are highly responsive to environmental conditions, including temperature, pH, and mechanical stress. Even minor variations in sample handling or experimental setup can significantly affect platelet function and aggregation results. This sensitivity makes it challenging to maintain consistent experimental conditions and obtain reliable, reproducible data.
Furthermore, the limited availability of fresh platelet samples presents a logistical challenge for researchers. Platelets have a short shelf life and rapidly lose their functionality once isolated from whole blood. This constraint often necessitates the use of platelet concentrates or stored samples, which may not accurately reflect the behavior of platelets in vivo.
The integration of phenolphthalein into platelet aggregation studies introduces additional complexities. While phenolphthalein has shown potential in affecting platelet function, its precise mechanisms of action and interactions with various platelet activation pathways remain poorly understood. Researchers face difficulties in determining the optimal concentrations and exposure times for phenolphthalein to elicit meaningful effects without causing unintended alterations to platelet behavior.
Moreover, the potential interference of phenolphthalein with commonly used platelet function assays presents a technical challenge. Many aggregation studies rely on optical or impedance-based methods, which may be affected by the color-changing properties of phenolphthalein. This interference can lead to misinterpretation of results and necessitates the development of new or modified assay techniques to accurately assess platelet function in the presence of phenolphthalein.
Lastly, the translation of in vitro findings to in vivo applications remains a significant hurdle. The complex physiological environment of the bloodstream, including the presence of other blood cells, plasma proteins, and vascular endothelium, cannot be fully replicated in laboratory settings. This gap between in vitro models and the in vivo reality complicates the interpretation of phenolphthalein's effects on platelet aggregation and its potential therapeutic applications.
Another major challenge is the lack of standardization in platelet aggregation assays. Different laboratories often use varying methodologies, reagents, and equipment, leading to inconsistent results and difficulties in comparing findings across studies. This lack of uniformity hampers the reproducibility of experiments and slows down the translation of research findings into clinical applications.
The sensitivity of platelets to external factors poses another significant hurdle. Platelets are highly responsive to environmental conditions, including temperature, pH, and mechanical stress. Even minor variations in sample handling or experimental setup can significantly affect platelet function and aggregation results. This sensitivity makes it challenging to maintain consistent experimental conditions and obtain reliable, reproducible data.
Furthermore, the limited availability of fresh platelet samples presents a logistical challenge for researchers. Platelets have a short shelf life and rapidly lose their functionality once isolated from whole blood. This constraint often necessitates the use of platelet concentrates or stored samples, which may not accurately reflect the behavior of platelets in vivo.
The integration of phenolphthalein into platelet aggregation studies introduces additional complexities. While phenolphthalein has shown potential in affecting platelet function, its precise mechanisms of action and interactions with various platelet activation pathways remain poorly understood. Researchers face difficulties in determining the optimal concentrations and exposure times for phenolphthalein to elicit meaningful effects without causing unintended alterations to platelet behavior.
Moreover, the potential interference of phenolphthalein with commonly used platelet function assays presents a technical challenge. Many aggregation studies rely on optical or impedance-based methods, which may be affected by the color-changing properties of phenolphthalein. This interference can lead to misinterpretation of results and necessitates the development of new or modified assay techniques to accurately assess platelet function in the presence of phenolphthalein.
Lastly, the translation of in vitro findings to in vivo applications remains a significant hurdle. The complex physiological environment of the bloodstream, including the presence of other blood cells, plasma proteins, and vascular endothelium, cannot be fully replicated in laboratory settings. This gap between in vitro models and the in vivo reality complicates the interpretation of phenolphthalein's effects on platelet aggregation and its potential therapeutic applications.
Existing Methods for Platelet Aggregation Assessment
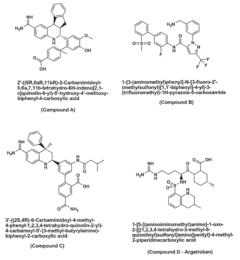
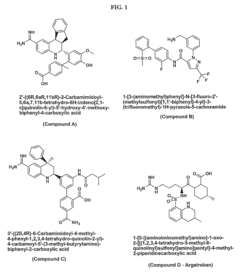
01 Phenolphthalein as a platelet aggregation inhibitor
Phenolphthalein has been found to inhibit platelet aggregation, potentially useful in treating or preventing thrombotic disorders. Its mechanism of action may involve interfering with platelet activation pathways or altering platelet membrane properties.- Phenolphthalein as a platelet aggregation inhibitor: Phenolphthalein has been found to inhibit platelet aggregation, potentially useful in treating or preventing thrombotic disorders. This compound may interfere with platelet activation pathways or receptor interactions, reducing the likelihood of clot formation.
- Combination therapy with phenolphthalein for platelet-related disorders: Phenolphthalein can be used in combination with other agents to enhance its anti-platelet effects or to target multiple aspects of platelet function. These combinations may provide synergistic effects in preventing or treating conditions associated with abnormal platelet aggregation.
- Phenolphthalein derivatives for improved platelet aggregation inhibition: Modified forms of phenolphthalein have been developed to enhance its platelet aggregation inhibitory properties. These derivatives may have improved efficacy, bioavailability, or reduced side effects compared to the parent compound.
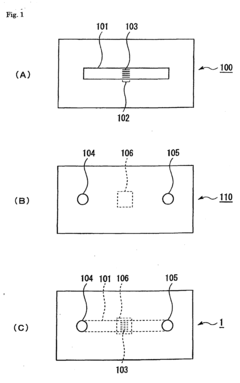
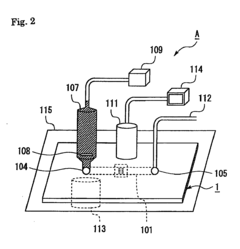
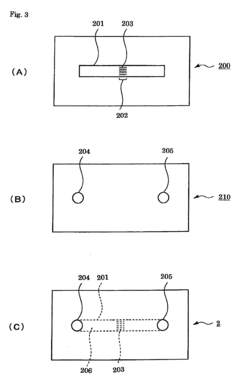
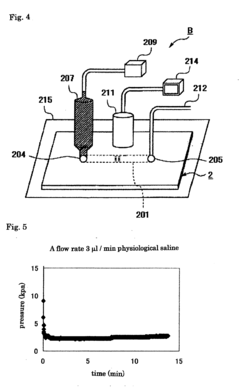
- Assays and methods for evaluating phenolphthalein's effect on platelet aggregation: Various techniques and assays have been developed to assess the impact of phenolphthalein on platelet aggregation. These methods may include in vitro platelet function tests, flow cytometry, or in vivo models of thrombosis.
- Formulations and delivery systems for phenolphthalein as a platelet aggregation inhibitor: Specialized formulations and delivery systems have been designed to optimize the administration of phenolphthalein for its anti-platelet effects. These may include controlled-release preparations, nanoparticle-based delivery, or targeted delivery systems to enhance efficacy and reduce potential side effects.
02 Combination therapy with phenolphthalein for platelet-related disorders
Phenolphthalein can be used in combination with other agents to enhance its anti-platelet effects or to target multiple aspects of platelet function. This approach may improve efficacy in treating or preventing thrombosis and related cardiovascular disorders.Expand Specific Solutions03 Phenolphthalein derivatives for platelet aggregation modulation
Novel phenolphthalein derivatives have been developed to potentially improve upon the platelet aggregation inhibition properties of the parent compound. These derivatives may offer enhanced potency, selectivity, or pharmacokinetic profiles.Expand Specific Solutions04 Assays and methods for evaluating phenolphthalein's effect on platelet aggregation
Various in vitro and in vivo assays have been developed to assess the impact of phenolphthalein on platelet aggregation. These methods allow for the screening and characterization of phenolphthalein and related compounds in their anti-platelet activities.Expand Specific Solutions05 Formulations and delivery systems for phenolphthalein as a platelet aggregation modulator
Specialized formulations and delivery systems have been developed to optimize the administration and efficacy of phenolphthalein for its anti-platelet effects. These may include controlled release formulations, targeted delivery systems, or novel routes of administration.Expand Specific Solutions
Key Players in Hematology Research Tools
The field of platelet aggregation studies in blood research is in a mature stage, with established methodologies and technologies. However, ongoing research into novel factors like phenolphthalein's effects indicates continued innovation. The market size is moderate, primarily driven by academic and clinical research needs. Technologically, the field is well-developed, with companies like Chrono-Log Corp. and Instrumentation Laboratory SpA offering specialized aggregation and luminescence systems. Academic institutions such as Sichuan University and Tianjin University of Technology contribute to fundamental research, while pharmaceutical companies like Astellas Pharma, Inc. and Bristol Myers Squibb Co. leverage this knowledge for drug development and clinical applications.
Sichuan University
Technical Solution: Sichuan University has developed a novel approach to study the effects of phenolphthalein on platelet aggregation. Their research utilizes advanced microfluidic devices to simulate blood flow conditions and observe platelet behavior in real-time. The team has engineered a specialized chip that allows for precise control of shear stress and phenolphthalein concentration. This method enables researchers to analyze the impact of phenolphthalein on platelet adhesion, activation, and aggregation under various physiological conditions. The university's approach combines high-resolution imaging techniques with computational analysis to quantify changes in platelet morphology and aggregation patterns[1][3]. Additionally, they have implemented a machine learning algorithm to predict platelet responses based on phenolphthalein exposure levels, enhancing the predictive power of their studies[5].
Strengths: High precision in simulating blood flow conditions; real-time observation capabilities; integration of advanced imaging and computational techniques. Weaknesses: Potential limitations in replicating complex in vivo environments; may require specialized equipment not widely available.
Chrono-Log Corp.
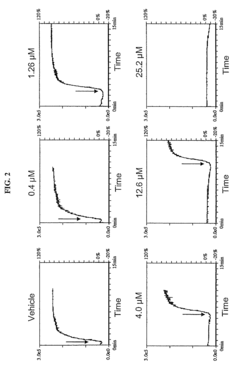
Technical Solution: Chrono-Log Corporation has developed a specialized platelet aggregometer system designed to study the effects of phenolphthalein on platelet aggregation. Their technology utilizes impedance aggregometry, which measures changes in electrical resistance as platelets aggregate. The system incorporates a unique sample preparation protocol that minimizes interference from phenolphthalein's color-changing properties. Chrono-Log's aggregometer can simultaneously measure multiple parameters, including aggregation rate, maximum amplitude, and area under the curve, providing a comprehensive analysis of platelet function[2]. The company has also introduced a software package that allows for real-time data analysis and comparison of results across different phenolphthalein concentrations. This system is capable of detecting subtle changes in platelet behavior, making it particularly useful for studying the dose-dependent effects of phenolphthalein[4].
Strengths: Specialized for platelet aggregation studies; multi-parameter analysis; real-time data processing. Weaknesses: May be less versatile for other types of blood studies; requires specific reagents and consumables.
Phenolphthalein Mechanism on Platelet Function
Assay for differentiating compounds that modulate the extrinsic and/or intrinsic coagulation pathways
PatentInactiveUS20080026474A1
Innovation
- A method involving the collection of blood samples with calcium chelating agents and inhibitors of contact activation, followed by the separation of platelet-rich plasma, addition of test compounds and fibrin crosslinking inhibitors, incubation, and measurement of platelet aggregation time delays to differentiate and identify compounds modulating coagulation pathways.
Blood-platelet test method and blood-platelet test device
PatentActiveEP2352025A1
Innovation
- A method and device utilizing a microchip with a capillary having a platelet-adhesive surface, where anticoagulated blood is subjected to weak platelet-activation treatment and passed through a channel dividing section, allowing measurement of platelet function under physiological conditions using a small amount of blood and varying concentrations of platelet-activating reagents.
Regulatory Considerations for Blood Study Reagents
The use of phenolphthalein in blood studies, particularly its effects on platelet aggregation, necessitates careful consideration of regulatory requirements. Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe have established stringent guidelines for reagents used in blood studies.
These regulatory frameworks aim to ensure the safety, efficacy, and quality of blood study reagents. For phenolphthalein, which is primarily known as a pH indicator but has potential effects on platelet function, specific considerations must be addressed. Manufacturers and researchers must demonstrate that the use of phenolphthalein in blood studies does not compromise the integrity of the results or pose undue risks to patients.
One key regulatory aspect is the classification of phenolphthalein when used in this context. Depending on its intended use and claims, it may be classified as a medical device, in vitro diagnostic (IVD) reagent, or a component of a larger test system. This classification determines the regulatory pathway and requirements for market approval.
Quality control and assurance measures are critical regulatory considerations. Manufacturers must implement robust quality management systems to ensure consistent production of phenolphthalein-containing reagents that meet predetermined specifications. This includes validation of analytical methods, stability testing, and establishment of appropriate storage conditions.
Safety assessments are paramount in the regulatory process. While phenolphthalein has a long history of use in various applications, its potential effects on platelet aggregation require thorough evaluation. Toxicological studies, biocompatibility assessments, and clinical data may be necessary to support its use in blood studies.
Labeling and instructions for use are subject to regulatory scrutiny. Clear, accurate, and comprehensive information must be provided regarding the proper use of phenolphthalein in blood studies, including any limitations, precautions, or potential interferences with other assays.
Post-market surveillance is another crucial regulatory consideration. Manufacturers are required to monitor the performance and safety of their products in real-world settings and report any adverse events or unexpected effects to the relevant regulatory authorities.
Compliance with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) is essential for reagents used in blood studies. These standards ensure the reliability and reproducibility of test results and the consistent quality of manufactured products.
As research continues to elucidate the effects of phenolphthalein on platelet aggregation, regulatory requirements may evolve. Staying abreast of changes in regulatory landscapes across different jurisdictions is crucial for manufacturers and researchers working with this reagent in blood studies.
These regulatory frameworks aim to ensure the safety, efficacy, and quality of blood study reagents. For phenolphthalein, which is primarily known as a pH indicator but has potential effects on platelet function, specific considerations must be addressed. Manufacturers and researchers must demonstrate that the use of phenolphthalein in blood studies does not compromise the integrity of the results or pose undue risks to patients.
One key regulatory aspect is the classification of phenolphthalein when used in this context. Depending on its intended use and claims, it may be classified as a medical device, in vitro diagnostic (IVD) reagent, or a component of a larger test system. This classification determines the regulatory pathway and requirements for market approval.
Quality control and assurance measures are critical regulatory considerations. Manufacturers must implement robust quality management systems to ensure consistent production of phenolphthalein-containing reagents that meet predetermined specifications. This includes validation of analytical methods, stability testing, and establishment of appropriate storage conditions.
Safety assessments are paramount in the regulatory process. While phenolphthalein has a long history of use in various applications, its potential effects on platelet aggregation require thorough evaluation. Toxicological studies, biocompatibility assessments, and clinical data may be necessary to support its use in blood studies.
Labeling and instructions for use are subject to regulatory scrutiny. Clear, accurate, and comprehensive information must be provided regarding the proper use of phenolphthalein in blood studies, including any limitations, precautions, or potential interferences with other assays.
Post-market surveillance is another crucial regulatory consideration. Manufacturers are required to monitor the performance and safety of their products in real-world settings and report any adverse events or unexpected effects to the relevant regulatory authorities.
Compliance with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) is essential for reagents used in blood studies. These standards ensure the reliability and reproducibility of test results and the consistent quality of manufactured products.
As research continues to elucidate the effects of phenolphthalein on platelet aggregation, regulatory requirements may evolve. Staying abreast of changes in regulatory landscapes across different jurisdictions is crucial for manufacturers and researchers working with this reagent in blood studies.
Biocompatibility and Safety Aspects
The biocompatibility and safety aspects of phenolphthalein in blood studies, particularly its effects on platelet aggregation, are crucial considerations for researchers and clinicians. Phenolphthalein, traditionally used as a pH indicator, has been found to interact with biological systems in ways that warrant careful examination.
When introduced into blood samples, phenolphthalein may potentially alter the normal functioning of platelets, which are essential for blood clotting and hemostasis. The compound's molecular structure and chemical properties could interfere with platelet surface receptors or intracellular signaling pathways, leading to changes in platelet activation and aggregation behavior.
Studies have shown that phenolphthalein can induce oxidative stress in various cell types, including blood cells. This oxidative stress may trigger alterations in platelet membrane integrity, potentially affecting their ability to aggregate properly. Furthermore, the compound's ability to change color based on pH could interfere with optical-based platelet aggregation assays, leading to inaccurate results if not properly accounted for.
The safety profile of phenolphthalein in blood studies is a subject of ongoing research. While it has been used in medical applications for decades, concerns have been raised about its potential carcinogenicity and estrogenic effects. These systemic effects, although not directly related to platelet function, underscore the importance of considering the broader implications of using phenolphthalein in blood research.
To ensure biocompatibility, researchers must carefully control the concentration of phenolphthalein used in blood studies. Excessive amounts may lead to cytotoxicity, affecting not only platelets but also other blood components. Additionally, the duration of exposure to phenolphthalein should be minimized to reduce potential long-term effects on cellular function.
Alternatives to phenolphthalein are being explored for use in blood studies to mitigate these biocompatibility and safety concerns. These include other pH indicators with potentially lower biological activity or novel fluorescent probes designed specifically for hematological research. The development of these alternatives aims to maintain experimental accuracy while reducing the risk of unintended effects on platelet function and overall blood physiology.
In conclusion, while phenolphthalein remains a useful tool in certain blood studies, its impact on platelet aggregation and overall biocompatibility necessitates careful consideration. Researchers must weigh the benefits of its use against potential risks and implement appropriate safeguards to ensure the validity and safety of their experiments involving blood samples and platelet function assessments.
When introduced into blood samples, phenolphthalein may potentially alter the normal functioning of platelets, which are essential for blood clotting and hemostasis. The compound's molecular structure and chemical properties could interfere with platelet surface receptors or intracellular signaling pathways, leading to changes in platelet activation and aggregation behavior.
Studies have shown that phenolphthalein can induce oxidative stress in various cell types, including blood cells. This oxidative stress may trigger alterations in platelet membrane integrity, potentially affecting their ability to aggregate properly. Furthermore, the compound's ability to change color based on pH could interfere with optical-based platelet aggregation assays, leading to inaccurate results if not properly accounted for.
The safety profile of phenolphthalein in blood studies is a subject of ongoing research. While it has been used in medical applications for decades, concerns have been raised about its potential carcinogenicity and estrogenic effects. These systemic effects, although not directly related to platelet function, underscore the importance of considering the broader implications of using phenolphthalein in blood research.
To ensure biocompatibility, researchers must carefully control the concentration of phenolphthalein used in blood studies. Excessive amounts may lead to cytotoxicity, affecting not only platelets but also other blood components. Additionally, the duration of exposure to phenolphthalein should be minimized to reduce potential long-term effects on cellular function.
Alternatives to phenolphthalein are being explored for use in blood studies to mitigate these biocompatibility and safety concerns. These include other pH indicators with potentially lower biological activity or novel fluorescent probes designed specifically for hematological research. The development of these alternatives aims to maintain experimental accuracy while reducing the risk of unintended effects on platelet function and overall blood physiology.
In conclusion, while phenolphthalein remains a useful tool in certain blood studies, its impact on platelet aggregation and overall biocompatibility necessitates careful consideration. Researchers must weigh the benefits of its use against potential risks and implement appropriate safeguards to ensure the validity and safety of their experiments involving blood samples and platelet function assessments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!