Evaluating Phenolphthalein’s Impact on Fuel Cell Performance
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein and Fuel Cell Technology Overview
Phenolphthalein, a widely recognized pH indicator, has recently garnered attention in the realm of fuel cell technology. This compound, traditionally used in acid-base titrations, is now being explored for its potential impact on fuel cell performance. Fuel cells, devices that convert chemical energy into electrical energy through electrochemical reactions, have been at the forefront of clean energy research for decades.
The intersection of phenolphthalein and fuel cell technology represents a novel area of investigation. Fuel cells operate on the principle of electrochemical conversion, utilizing hydrogen or other fuels to generate electricity with water and heat as byproducts. The efficiency and durability of these systems are critical factors in their widespread adoption and commercial viability.
Phenolphthalein's role in this context stems from its unique chemical properties. As a pH indicator, it undergoes a dramatic color change in response to changes in hydrogen ion concentration. This characteristic has led researchers to explore its potential applications in monitoring and optimizing fuel cell operations. The compound's sensitivity to pH fluctuations could provide valuable insights into the internal conditions of fuel cells during operation.
The evolution of fuel cell technology has seen significant advancements in materials science, catalysis, and system design. From the early phosphoric acid fuel cells to the more recent proton exchange membrane (PEM) and solid oxide fuel cells (SOFCs), each iteration has brought improvements in efficiency, durability, and cost-effectiveness. The introduction of phenolphthalein into this field represents a potential new avenue for enhancing fuel cell diagnostics and performance.
Current research is focused on understanding how phenolphthalein can be integrated into fuel cell systems without compromising their core functionality. Preliminary studies suggest that the compound could serve as a real-time indicator of electrolyte conditions, potentially alerting operators to imbalances or degradation within the cell. This could lead to more proactive maintenance strategies and improved overall system longevity.
The impact of phenolphthalein on fuel cell performance is multifaceted. While its primary role is envisioned as a diagnostic tool, there are also investigations into its potential effects on reaction kinetics and membrane properties. The compound's interaction with various fuel cell components, including electrodes and electrolyte membranes, is a subject of ongoing research.
As the field progresses, the integration of phenolphthalein into fuel cell technology may open up new possibilities for advanced monitoring systems and smart fuel cells capable of self-diagnosis. This convergence of traditional chemical indicators with cutting-edge energy technology exemplifies the interdisciplinary nature of modern scientific research and its potential to drive innovation in sustainable energy solutions.
The intersection of phenolphthalein and fuel cell technology represents a novel area of investigation. Fuel cells operate on the principle of electrochemical conversion, utilizing hydrogen or other fuels to generate electricity with water and heat as byproducts. The efficiency and durability of these systems are critical factors in their widespread adoption and commercial viability.
Phenolphthalein's role in this context stems from its unique chemical properties. As a pH indicator, it undergoes a dramatic color change in response to changes in hydrogen ion concentration. This characteristic has led researchers to explore its potential applications in monitoring and optimizing fuel cell operations. The compound's sensitivity to pH fluctuations could provide valuable insights into the internal conditions of fuel cells during operation.
The evolution of fuel cell technology has seen significant advancements in materials science, catalysis, and system design. From the early phosphoric acid fuel cells to the more recent proton exchange membrane (PEM) and solid oxide fuel cells (SOFCs), each iteration has brought improvements in efficiency, durability, and cost-effectiveness. The introduction of phenolphthalein into this field represents a potential new avenue for enhancing fuel cell diagnostics and performance.
Current research is focused on understanding how phenolphthalein can be integrated into fuel cell systems without compromising their core functionality. Preliminary studies suggest that the compound could serve as a real-time indicator of electrolyte conditions, potentially alerting operators to imbalances or degradation within the cell. This could lead to more proactive maintenance strategies and improved overall system longevity.
The impact of phenolphthalein on fuel cell performance is multifaceted. While its primary role is envisioned as a diagnostic tool, there are also investigations into its potential effects on reaction kinetics and membrane properties. The compound's interaction with various fuel cell components, including electrodes and electrolyte membranes, is a subject of ongoing research.
As the field progresses, the integration of phenolphthalein into fuel cell technology may open up new possibilities for advanced monitoring systems and smart fuel cells capable of self-diagnosis. This convergence of traditional chemical indicators with cutting-edge energy technology exemplifies the interdisciplinary nature of modern scientific research and its potential to drive innovation in sustainable energy solutions.
Market Analysis for Phenolphthalein in Fuel Cells
The market for phenolphthalein in fuel cells is currently in its nascent stage, with limited commercial applications. However, the potential for growth is significant as researchers and industry players explore innovative ways to enhance fuel cell performance and efficiency. The primary market drivers for phenolphthalein in fuel cells include the increasing demand for clean energy solutions, stringent environmental regulations, and the push for sustainable transportation options.
The global fuel cell market is projected to experience substantial growth in the coming years, with a compound annual growth rate (CAGR) expected to exceed 20% through 2030. This growth is primarily driven by the automotive sector, stationary power generation, and portable electronics. As phenolphthalein's potential impact on fuel cell performance becomes more apparent, its market demand is likely to correlate with the overall fuel cell market expansion.
Currently, the market for phenolphthalein in fuel cells is predominantly research-oriented, with academic institutions and R&D departments of major fuel cell manufacturers showing the most interest. The automotive industry, particularly companies focusing on hydrogen fuel cell vehicles, represents a significant potential market segment. Countries like Japan, South Korea, and Germany, which are at the forefront of fuel cell technology adoption, are likely to be key markets for phenolphthalein applications in fuel cells.
The market potential for phenolphthalein in fuel cells is closely tied to its ability to address key challenges in fuel cell technology, such as improving durability, reducing costs, and enhancing overall system efficiency. If research demonstrates significant performance improvements, the demand for phenolphthalein could see rapid growth across various fuel cell applications.
However, the market faces several challenges. The high cost of fuel cell technology remains a significant barrier to widespread adoption, which in turn limits the immediate market potential for phenolphthalein. Additionally, the lack of established infrastructure for hydrogen fuel distribution poses a challenge to the growth of the fuel cell market, indirectly affecting the demand for phenolphthalein.
Regulatory factors will play a crucial role in shaping the market for phenolphthalein in fuel cells. As governments worldwide implement stricter emissions standards and offer incentives for clean energy technologies, the fuel cell market is expected to expand, potentially creating more opportunities for phenolphthalein applications.
In conclusion, while the current market for phenolphthalein in fuel cells is limited, the potential for growth is substantial. The success of phenolphthalein in this market will largely depend on the outcomes of ongoing research, technological advancements in fuel cell design, and the overall growth trajectory of the fuel cell industry. As the clean energy transition accelerates, the market for innovative solutions like phenolphthalein in fuel cells is poised for significant expansion in the coming years.
The global fuel cell market is projected to experience substantial growth in the coming years, with a compound annual growth rate (CAGR) expected to exceed 20% through 2030. This growth is primarily driven by the automotive sector, stationary power generation, and portable electronics. As phenolphthalein's potential impact on fuel cell performance becomes more apparent, its market demand is likely to correlate with the overall fuel cell market expansion.
Currently, the market for phenolphthalein in fuel cells is predominantly research-oriented, with academic institutions and R&D departments of major fuel cell manufacturers showing the most interest. The automotive industry, particularly companies focusing on hydrogen fuel cell vehicles, represents a significant potential market segment. Countries like Japan, South Korea, and Germany, which are at the forefront of fuel cell technology adoption, are likely to be key markets for phenolphthalein applications in fuel cells.
The market potential for phenolphthalein in fuel cells is closely tied to its ability to address key challenges in fuel cell technology, such as improving durability, reducing costs, and enhancing overall system efficiency. If research demonstrates significant performance improvements, the demand for phenolphthalein could see rapid growth across various fuel cell applications.
However, the market faces several challenges. The high cost of fuel cell technology remains a significant barrier to widespread adoption, which in turn limits the immediate market potential for phenolphthalein. Additionally, the lack of established infrastructure for hydrogen fuel distribution poses a challenge to the growth of the fuel cell market, indirectly affecting the demand for phenolphthalein.
Regulatory factors will play a crucial role in shaping the market for phenolphthalein in fuel cells. As governments worldwide implement stricter emissions standards and offer incentives for clean energy technologies, the fuel cell market is expected to expand, potentially creating more opportunities for phenolphthalein applications.
In conclusion, while the current market for phenolphthalein in fuel cells is limited, the potential for growth is substantial. The success of phenolphthalein in this market will largely depend on the outcomes of ongoing research, technological advancements in fuel cell design, and the overall growth trajectory of the fuel cell industry. As the clean energy transition accelerates, the market for innovative solutions like phenolphthalein in fuel cells is poised for significant expansion in the coming years.
Current Challenges in Fuel Cell Performance
Fuel cell technology has made significant strides in recent years, yet several challenges persist in optimizing their performance. One of the primary obstacles is the durability and longevity of fuel cell components. The harsh operating conditions, including high temperatures and corrosive environments, lead to degradation of catalysts, membranes, and other critical elements. This degradation results in reduced efficiency and shortened lifespan of fuel cells, limiting their widespread adoption in various applications.
Another significant challenge is the cost-effectiveness of fuel cell systems. Despite advancements in manufacturing processes, the use of expensive materials such as platinum catalysts continues to drive up production costs. This economic barrier hinders the competitiveness of fuel cells against traditional energy technologies, particularly in price-sensitive markets.
The management of water and heat within fuel cell systems presents another complex challenge. Proper water balance is crucial for optimal proton conductivity in the membrane, yet excess water can lead to flooding and reduced performance. Similarly, efficient heat management is essential to maintain optimal operating temperatures and prevent thermal degradation of components.
Fuel purity and contaminant sensitivity remain ongoing concerns in fuel cell operation. Impurities in the fuel stream, even in trace amounts, can significantly impact cell performance and longevity. This necessitates the development of more robust fuel processing and purification technologies, as well as fuel cell designs that are more tolerant to contaminants.
The integration of fuel cells into existing energy infrastructures poses additional challenges. Issues such as fuel storage, distribution, and compatibility with current power systems need to be addressed for widespread implementation. This includes developing efficient hydrogen production, storage, and transportation methods for hydrogen fuel cells.
In the context of evaluating phenolphthalein's impact on fuel cell performance, researchers face the challenge of understanding how this compound interacts with various fuel cell components. The potential effects on membrane conductivity, catalyst activity, and overall system efficiency need to be thoroughly investigated. Moreover, the long-term implications of phenolphthalein exposure on fuel cell durability and performance stability require extensive study.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, electrochemistry, and system engineering. Innovations in nanotechnology and advanced manufacturing techniques offer promising avenues for overcoming some of these obstacles. However, significant research and development efforts are still needed to fully realize the potential of fuel cell technology and its widespread adoption across various sectors.
Another significant challenge is the cost-effectiveness of fuel cell systems. Despite advancements in manufacturing processes, the use of expensive materials such as platinum catalysts continues to drive up production costs. This economic barrier hinders the competitiveness of fuel cells against traditional energy technologies, particularly in price-sensitive markets.
The management of water and heat within fuel cell systems presents another complex challenge. Proper water balance is crucial for optimal proton conductivity in the membrane, yet excess water can lead to flooding and reduced performance. Similarly, efficient heat management is essential to maintain optimal operating temperatures and prevent thermal degradation of components.
Fuel purity and contaminant sensitivity remain ongoing concerns in fuel cell operation. Impurities in the fuel stream, even in trace amounts, can significantly impact cell performance and longevity. This necessitates the development of more robust fuel processing and purification technologies, as well as fuel cell designs that are more tolerant to contaminants.
The integration of fuel cells into existing energy infrastructures poses additional challenges. Issues such as fuel storage, distribution, and compatibility with current power systems need to be addressed for widespread implementation. This includes developing efficient hydrogen production, storage, and transportation methods for hydrogen fuel cells.
In the context of evaluating phenolphthalein's impact on fuel cell performance, researchers face the challenge of understanding how this compound interacts with various fuel cell components. The potential effects on membrane conductivity, catalyst activity, and overall system efficiency need to be thoroughly investigated. Moreover, the long-term implications of phenolphthalein exposure on fuel cell durability and performance stability require extensive study.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, electrochemistry, and system engineering. Innovations in nanotechnology and advanced manufacturing techniques offer promising avenues for overcoming some of these obstacles. However, significant research and development efforts are still needed to fully realize the potential of fuel cell technology and its widespread adoption across various sectors.
Existing Methods for Fuel Cell Performance Enhancement
01 Fuel cell stack design optimization
Improving fuel cell performance through optimized stack design, including enhanced electrode structures, membrane configurations, and flow field patterns. This approach focuses on maximizing power density, improving reactant distribution, and reducing internal resistance within the fuel cell stack.- Fuel cell stack design optimization: Improving fuel cell performance through optimized stack design, including enhanced electrode structures, membrane configurations, and flow field patterns. This approach focuses on maximizing power density, improving reactant distribution, and minimizing internal resistance to enhance overall efficiency.
- Advanced control systems for fuel cells: Implementing sophisticated control systems to monitor and regulate fuel cell operation in real-time. These systems optimize performance by adjusting parameters such as fuel flow, temperature, and humidity based on current operating conditions and power demands.
- Novel catalyst materials and structures: Developing and utilizing advanced catalyst materials and structures to enhance electrochemical reactions within fuel cells. This includes exploring nanostructured catalysts, alloy compositions, and support materials to improve catalytic activity, durability, and overall cell performance.
- Thermal management and heat recovery: Implementing effective thermal management strategies and heat recovery systems to maintain optimal operating temperatures and improve overall system efficiency. This includes innovative cooling methods, waste heat utilization, and temperature distribution optimization within the fuel cell stack.
- Fuel cell diagnostics and performance monitoring: Developing advanced diagnostic tools and performance monitoring techniques to assess fuel cell health, identify potential issues, and optimize maintenance schedules. This includes in-situ and ex-situ measurement methods, predictive modeling, and data analytics to enhance long-term performance and reliability.
02 Advanced materials for fuel cell components
Developing and utilizing advanced materials for fuel cell components, such as catalysts, membranes, and bipolar plates. These materials aim to enhance durability, conductivity, and overall efficiency of the fuel cell system, leading to improved performance and longer operational lifetimes.Expand Specific Solutions03 Fuel cell system control and monitoring
Implementing sophisticated control and monitoring systems to optimize fuel cell performance. This includes real-time diagnostics, adaptive control algorithms, and predictive maintenance strategies to ensure optimal operating conditions and prevent performance degradation over time.Expand Specific Solutions04 Thermal management and water balance
Developing effective thermal management and water balance strategies to maintain optimal operating temperatures and humidity levels within the fuel cell. This approach aims to prevent membrane dehydration, flooding, and other issues that can negatively impact fuel cell performance.Expand Specific Solutions05 Integration of auxiliary systems
Optimizing the integration of auxiliary systems, such as air supply, fuel processing, and power conditioning units, to enhance overall fuel cell system performance. This includes improving the efficiency of balance-of-plant components and reducing parasitic losses to maximize net power output.Expand Specific Solutions
Key Players in Fuel Cell Industry
The competitive landscape for evaluating phenolphthalein's impact on fuel cell performance is in an early development stage, with a relatively small but growing market. The technology is still emerging, with varying levels of maturity across different companies. Key players like Toyota Motor Corp., Ballard Power Systems, and Hyundai Motor Co. are leading research efforts, leveraging their automotive expertise. Academic institutions such as Tongji University and Wuhan University of Technology are contributing to fundamental research. Specialized firms like FuelCell Energy and Intelligent Energy are focusing on niche applications. As the technology advances, collaboration between industry leaders and research institutions will likely drive innovation and commercialization in this promising field.
Toyota Motor Corp.
Technical Solution: Toyota's approach likely leverages their expertise in both fuel cell electric vehicles (FCEVs) and hybrid systems. They may be investigating phenolphthalein as a potential additive in their proprietary electrolyte formulations or as a diagnostic tool for membrane degradation. Toyota's advanced testing facilities allow for in-situ analysis of operating fuel cells, potentially using spectroscopic methods to observe phenolphthalein's behavior under real-world conditions[3]. Their research may also extend to how phenolphthalein interacts with Toyota's unique catalyst designs, which have shown improved platinum utilization and durability[4].
Strengths: Vast resources and experience in FCEV commercialization. Weaknesses: Research may be heavily biased towards automotive applications, potentially overlooking other use cases.
Ballard Power Systems, Inc.
Technical Solution: Ballard Power Systems has developed advanced proton exchange membrane (PEM) fuel cell technology for various applications. Their approach to evaluating phenolphthalein's impact on fuel cell performance likely involves incorporating it into membrane electrode assemblies (MEAs) and conducting extensive electrochemical testing. They may use techniques such as cyclic voltammetry and impedance spectroscopy to assess how phenolphthalein affects key performance metrics like power density, efficiency, and durability[1]. Ballard's expertise in catalyst layer optimization could allow them to fine-tune the interaction between phenolphthalein and platinum catalysts to potentially enhance oxygen reduction kinetics[2].
Strengths: Extensive experience in PEM fuel cell development and testing. Weaknesses: May be primarily focused on automotive and heavy-duty applications rather than broader phenolphthalein research.
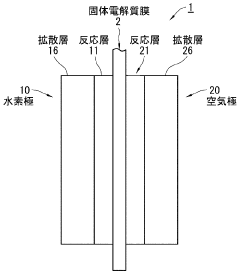
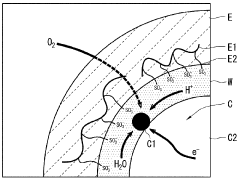
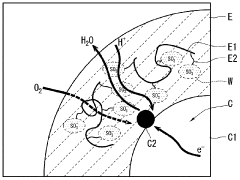
Phenolphthalein's Mechanism in Fuel Cells
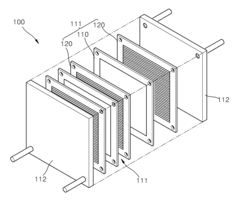
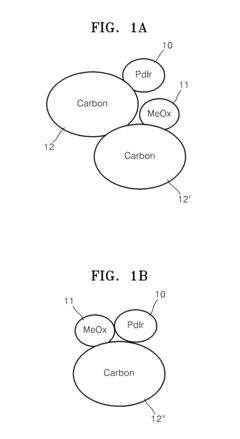
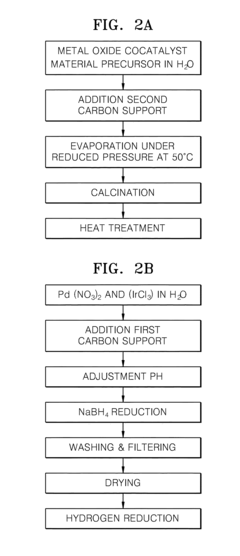
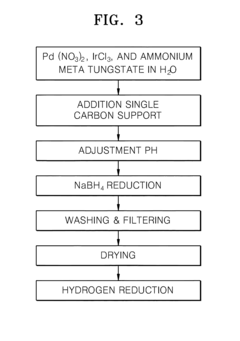
Electrode catalyst for fuel cells, method of preparing the same, and fuel cell including electrode containing the electrode catalyst
PatentInactiveUS20110294038A1
Innovation
- A non-platinum (Pt) based electrode catalyst is developed, comprising a carbon support, a non-Pt metal catalyst material alloy (such as Pd with Ir, Co, Ni, or Ru), and a metal oxide cocatalyst material, supported on carbon, which are mixed and reduced to create a catalyst with enhanced hydrogen oxidation activity.
Fuel cell reaction layer production method and catalyst paste production method
PatentWO2011125716A1
Innovation
- A method for producing a reaction layer with a PFF structure, where hydrophilic functional groups of a polymer electrolyte are oriented towards the catalyst, reducing electronic and ionic conduction resistance, and enhancing water management through pulverization and modification of catalyst metal particles with hydrophilic groups, allowing efficient water transfer.
Environmental Impact of Phenolphthalein Use
The use of phenolphthalein in fuel cell research raises important environmental considerations that must be carefully evaluated. While phenolphthalein itself is not typically considered a major environmental hazard, its production, use, and disposal can have potential impacts on ecosystems and human health.
Phenolphthalein is primarily synthesized through the condensation of phthalic anhydride and phenol, both of which are derived from petrochemical sources. The manufacturing process involves energy-intensive steps and may generate chemical waste streams that require proper treatment and disposal. Efforts to develop more sustainable synthesis routes using bio-based feedstocks or green chemistry principles could help mitigate these upstream environmental impacts.
In fuel cell applications, phenolphthalein is often used as a pH indicator or in small quantities for analytical purposes. Its direct environmental impact during fuel cell operation is likely minimal due to the small volumes involved. However, proper handling and disposal protocols are essential to prevent accidental releases or contamination of water systems.
The biodegradability and environmental fate of phenolphthalein are important factors to consider. Studies have shown that phenolphthalein can undergo photodegradation in aquatic environments, with half-lives ranging from hours to days depending on conditions. Microbial degradation pathways have also been identified, suggesting that the compound does not persist indefinitely in the environment.
Ecotoxicological assessments of phenolphthalein have yielded mixed results. While acute toxicity to aquatic organisms is generally low, some studies have reported potential endocrine-disrupting effects at higher concentrations. The long-term ecological impacts of chronic, low-level exposure remain an area of ongoing research and debate.
From a lifecycle perspective, the environmental footprint of phenolphthalein use in fuel cell research extends beyond direct impacts. Considerations include the energy and resources required for its production, transportation, and eventual disposal or treatment of waste materials containing the compound.
As the field of fuel cell technology advances, there is an opportunity to explore more environmentally benign alternatives to phenolphthalein for pH indication and other analytical purposes. Developing and adopting greener substitutes could contribute to the overall sustainability profile of fuel cell research and development efforts.
In conclusion, while the environmental impact of phenolphthalein use in fuel cell performance evaluation is likely limited due to small quantities involved, a holistic approach considering its entire lifecycle is warranted. Implementing best practices for handling, minimizing waste, and exploring eco-friendly alternatives can help mitigate potential environmental risks associated with its use in research settings.
Phenolphthalein is primarily synthesized through the condensation of phthalic anhydride and phenol, both of which are derived from petrochemical sources. The manufacturing process involves energy-intensive steps and may generate chemical waste streams that require proper treatment and disposal. Efforts to develop more sustainable synthesis routes using bio-based feedstocks or green chemistry principles could help mitigate these upstream environmental impacts.
In fuel cell applications, phenolphthalein is often used as a pH indicator or in small quantities for analytical purposes. Its direct environmental impact during fuel cell operation is likely minimal due to the small volumes involved. However, proper handling and disposal protocols are essential to prevent accidental releases or contamination of water systems.
The biodegradability and environmental fate of phenolphthalein are important factors to consider. Studies have shown that phenolphthalein can undergo photodegradation in aquatic environments, with half-lives ranging from hours to days depending on conditions. Microbial degradation pathways have also been identified, suggesting that the compound does not persist indefinitely in the environment.
Ecotoxicological assessments of phenolphthalein have yielded mixed results. While acute toxicity to aquatic organisms is generally low, some studies have reported potential endocrine-disrupting effects at higher concentrations. The long-term ecological impacts of chronic, low-level exposure remain an area of ongoing research and debate.
From a lifecycle perspective, the environmental footprint of phenolphthalein use in fuel cell research extends beyond direct impacts. Considerations include the energy and resources required for its production, transportation, and eventual disposal or treatment of waste materials containing the compound.
As the field of fuel cell technology advances, there is an opportunity to explore more environmentally benign alternatives to phenolphthalein for pH indication and other analytical purposes. Developing and adopting greener substitutes could contribute to the overall sustainability profile of fuel cell research and development efforts.
In conclusion, while the environmental impact of phenolphthalein use in fuel cell performance evaluation is likely limited due to small quantities involved, a holistic approach considering its entire lifecycle is warranted. Implementing best practices for handling, minimizing waste, and exploring eco-friendly alternatives can help mitigate potential environmental risks associated with its use in research settings.
Cost-Benefit Analysis of Phenolphthalein Integration
The integration of phenolphthalein into fuel cell systems presents a complex cost-benefit scenario that requires careful analysis. On the cost side, the primary consideration is the additional expense of incorporating phenolphthalein into the fuel cell components. This includes not only the raw material costs but also potential modifications to manufacturing processes and quality control measures to ensure proper integration.
Furthermore, there may be indirect costs associated with potential changes in fuel cell performance characteristics. These could include adjustments to power output, efficiency, or durability, which might necessitate redesigns in other system components or operational parameters. The long-term stability of phenolphthalein within the fuel cell environment is another factor that could impact maintenance schedules and overall system lifespan.
On the benefit side, the primary advantage of phenolphthalein integration lies in its potential to enhance fuel cell diagnostics and monitoring capabilities. By acting as a visual pH indicator, phenolphthalein can provide real-time feedback on the internal conditions of the fuel cell, potentially allowing for early detection of performance issues or degradation.
This improved diagnostic capability could lead to significant cost savings in maintenance and operational efficiency. Early detection of problems can prevent catastrophic failures, reduce downtime, and extend the overall lifespan of fuel cell systems. Additionally, the enhanced monitoring capabilities could provide valuable data for further research and development, potentially leading to innovations in fuel cell design and operation.
Another potential benefit is the possibility of simplified quality control processes during manufacturing. Phenolphthalein's color-changing properties could allow for easier visual inspection of fuel cell components, potentially reducing the need for more complex and expensive testing procedures.
When weighing these costs and benefits, it's crucial to consider the specific application and scale of fuel cell deployment. For large-scale industrial applications, the initial costs of integration may be more easily offset by long-term operational benefits. In contrast, for smaller or more cost-sensitive applications, the added expense might outweigh the potential advantages.
In conclusion, while the integration of phenolphthalein into fuel cells does incur additional costs, the potential benefits in terms of improved diagnostics, maintenance, and operational efficiency could provide significant value, particularly in large-scale or critical applications. Further research and pilot studies would be beneficial to quantify these costs and benefits more precisely in various operational contexts.
Furthermore, there may be indirect costs associated with potential changes in fuel cell performance characteristics. These could include adjustments to power output, efficiency, or durability, which might necessitate redesigns in other system components or operational parameters. The long-term stability of phenolphthalein within the fuel cell environment is another factor that could impact maintenance schedules and overall system lifespan.
On the benefit side, the primary advantage of phenolphthalein integration lies in its potential to enhance fuel cell diagnostics and monitoring capabilities. By acting as a visual pH indicator, phenolphthalein can provide real-time feedback on the internal conditions of the fuel cell, potentially allowing for early detection of performance issues or degradation.
This improved diagnostic capability could lead to significant cost savings in maintenance and operational efficiency. Early detection of problems can prevent catastrophic failures, reduce downtime, and extend the overall lifespan of fuel cell systems. Additionally, the enhanced monitoring capabilities could provide valuable data for further research and development, potentially leading to innovations in fuel cell design and operation.
Another potential benefit is the possibility of simplified quality control processes during manufacturing. Phenolphthalein's color-changing properties could allow for easier visual inspection of fuel cell components, potentially reducing the need for more complex and expensive testing procedures.
When weighing these costs and benefits, it's crucial to consider the specific application and scale of fuel cell deployment. For large-scale industrial applications, the initial costs of integration may be more easily offset by long-term operational benefits. In contrast, for smaller or more cost-sensitive applications, the added expense might outweigh the potential advantages.
In conclusion, while the integration of phenolphthalein into fuel cells does incur additional costs, the potential benefits in terms of improved diagnostics, maintenance, and operational efficiency could provide significant value, particularly in large-scale or critical applications. Further research and pilot studies would be beneficial to quantify these costs and benefits more precisely in various operational contexts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!