How Phenolphthalein Interacts with Biomolecules in Aqueous Media
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein-Biomolecule Interaction Background
Phenolphthalein, a widely recognized pH indicator, has been a subject of interest in biochemical research due to its unique interactions with biomolecules in aqueous media. The study of these interactions has evolved significantly over the past century, driven by advancements in analytical techniques and a growing understanding of molecular biology.
Initially discovered in the late 19th century, phenolphthalein's color-changing properties in response to pH variations quickly made it a staple in chemical laboratories. However, it wasn't until the mid-20th century that researchers began to explore its potential interactions with biological molecules.
The first investigations into phenolphthalein-biomolecule interactions focused primarily on proteins. Scientists observed that the presence of certain proteins could alter the color transition point of phenolphthalein, suggesting complex formation between the indicator and protein molecules. This discovery opened up new avenues for using phenolphthalein as a tool for protein characterization and quantification.
As research progressed, the scope of study expanded to include other biomolecules such as nucleic acids, lipids, and carbohydrates. Each class of biomolecule was found to interact with phenolphthalein in unique ways, influenced by factors such as pH, temperature, and ionic strength of the aqueous medium.
The advent of spectroscopic techniques in the latter half of the 20th century revolutionized the study of phenolphthalein-biomolecule interactions. UV-visible spectroscopy, fluorescence spectroscopy, and circular dichroism allowed researchers to probe these interactions at a molecular level, providing insights into binding mechanisms and structural changes.
More recently, computational methods have complemented experimental approaches, offering predictions of interaction sites and energetics. Molecular dynamics simulations and quantum mechanical calculations have shed light on the electronic and structural factors governing phenolphthalein's behavior in the presence of biomolecules.
The ongoing research in this field aims to leverage these interactions for various applications. From developing novel biosensors to creating drug delivery systems, the unique properties of phenolphthalein in biological environments continue to inspire innovative solutions in biotechnology and medicine.
As we delve deeper into the intricacies of phenolphthalein-biomolecule interactions, we uncover not only the fundamental principles governing these phenomena but also their potential to address contemporary challenges in biological and medical sciences.
Initially discovered in the late 19th century, phenolphthalein's color-changing properties in response to pH variations quickly made it a staple in chemical laboratories. However, it wasn't until the mid-20th century that researchers began to explore its potential interactions with biological molecules.
The first investigations into phenolphthalein-biomolecule interactions focused primarily on proteins. Scientists observed that the presence of certain proteins could alter the color transition point of phenolphthalein, suggesting complex formation between the indicator and protein molecules. This discovery opened up new avenues for using phenolphthalein as a tool for protein characterization and quantification.
As research progressed, the scope of study expanded to include other biomolecules such as nucleic acids, lipids, and carbohydrates. Each class of biomolecule was found to interact with phenolphthalein in unique ways, influenced by factors such as pH, temperature, and ionic strength of the aqueous medium.
The advent of spectroscopic techniques in the latter half of the 20th century revolutionized the study of phenolphthalein-biomolecule interactions. UV-visible spectroscopy, fluorescence spectroscopy, and circular dichroism allowed researchers to probe these interactions at a molecular level, providing insights into binding mechanisms and structural changes.
More recently, computational methods have complemented experimental approaches, offering predictions of interaction sites and energetics. Molecular dynamics simulations and quantum mechanical calculations have shed light on the electronic and structural factors governing phenolphthalein's behavior in the presence of biomolecules.
The ongoing research in this field aims to leverage these interactions for various applications. From developing novel biosensors to creating drug delivery systems, the unique properties of phenolphthalein in biological environments continue to inspire innovative solutions in biotechnology and medicine.
As we delve deeper into the intricacies of phenolphthalein-biomolecule interactions, we uncover not only the fundamental principles governing these phenomena but also their potential to address contemporary challenges in biological and medical sciences.
Market Applications of Phenolphthalein-Biomolecule Systems
The market applications of phenolphthalein-biomolecule systems span various industries, leveraging the unique interactions between phenolphthalein and biomolecules in aqueous media. One of the most prominent applications is in the field of medical diagnostics. The pH-sensitive properties of phenolphthalein, when combined with specific biomolecules, enable the development of highly sensitive and selective biosensors for detecting various biomarkers in bodily fluids.
In the pharmaceutical industry, phenolphthalein-biomolecule systems are utilized in drug delivery mechanisms. The pH-responsive nature of these systems allows for targeted release of therapeutic agents in specific physiological environments, enhancing drug efficacy and reducing side effects. This technology has shown particular promise in the development of oral medications for gastrointestinal disorders and cancer treatments.
Environmental monitoring represents another significant market application. Phenolphthalein-biomolecule systems are employed in the detection of pollutants and contaminants in water sources. These systems offer rapid, on-site testing capabilities, crucial for maintaining water quality standards in both industrial and municipal settings.
The food and beverage industry has also adopted phenolphthalein-biomolecule systems for quality control purposes. These systems are used in the detection of adulterants, monitoring of fermentation processes, and assessment of food freshness. The ability to provide real-time, color-based indicators of chemical changes has made them valuable tools in ensuring food safety and quality.
In the field of forensic science, phenolphthalein-biomolecule interactions are exploited for the detection of blood traces at crime scenes. The Kastle-Meyer test, which utilizes phenolphthalein, remains a standard preliminary test in forensic investigations, demonstrating the enduring relevance of this technology in law enforcement applications.
The textile industry has found innovative uses for phenolphthalein-biomolecule systems in the development of smart fabrics. These materials can change color in response to environmental pH changes, offering applications in protective clothing for workers in hazardous environments or in fashion for creating interactive, color-changing garments.
Emerging applications are being explored in the field of nanotechnology, where phenolphthalein-biomolecule systems are being integrated into nanoparticles for advanced sensing and drug delivery applications. This convergence of technologies promises to open new avenues in personalized medicine and targeted therapies.
In the pharmaceutical industry, phenolphthalein-biomolecule systems are utilized in drug delivery mechanisms. The pH-responsive nature of these systems allows for targeted release of therapeutic agents in specific physiological environments, enhancing drug efficacy and reducing side effects. This technology has shown particular promise in the development of oral medications for gastrointestinal disorders and cancer treatments.
Environmental monitoring represents another significant market application. Phenolphthalein-biomolecule systems are employed in the detection of pollutants and contaminants in water sources. These systems offer rapid, on-site testing capabilities, crucial for maintaining water quality standards in both industrial and municipal settings.
The food and beverage industry has also adopted phenolphthalein-biomolecule systems for quality control purposes. These systems are used in the detection of adulterants, monitoring of fermentation processes, and assessment of food freshness. The ability to provide real-time, color-based indicators of chemical changes has made them valuable tools in ensuring food safety and quality.
In the field of forensic science, phenolphthalein-biomolecule interactions are exploited for the detection of blood traces at crime scenes. The Kastle-Meyer test, which utilizes phenolphthalein, remains a standard preliminary test in forensic investigations, demonstrating the enduring relevance of this technology in law enforcement applications.
The textile industry has found innovative uses for phenolphthalein-biomolecule systems in the development of smart fabrics. These materials can change color in response to environmental pH changes, offering applications in protective clothing for workers in hazardous environments or in fashion for creating interactive, color-changing garments.
Emerging applications are being explored in the field of nanotechnology, where phenolphthalein-biomolecule systems are being integrated into nanoparticles for advanced sensing and drug delivery applications. This convergence of technologies promises to open new avenues in personalized medicine and targeted therapies.
Current Challenges in Phenolphthalein-Biomolecule Studies
The study of phenolphthalein interactions with biomolecules in aqueous media faces several significant challenges that hinder comprehensive understanding and practical applications. One primary obstacle is the complexity of the aqueous environment itself, which introduces numerous variables that can affect the interaction dynamics between phenolphthalein and biomolecules.
The pH-dependent nature of phenolphthalein presents a major challenge in these studies. As phenolphthalein's structure and properties change dramatically with pH variations, researchers struggle to maintain consistent experimental conditions that accurately reflect physiological environments. This pH sensitivity complicates the interpretation of results and makes it difficult to draw conclusive insights about the interaction mechanisms.
Another significant challenge lies in the diverse array of biomolecules present in biological systems. Proteins, nucleic acids, lipids, and carbohydrates all possess unique chemical properties and potential binding sites for phenolphthalein. Isolating and studying specific interactions amidst this complex milieu of biomolecules remains a formidable task for researchers in the field.
The dynamic nature of biomolecular interactions further complicates these studies. Biomolecules often undergo conformational changes upon binding, and these structural alterations can significantly impact the interaction with phenolphthalein. Capturing and characterizing these transient states requires advanced experimental techniques and sophisticated analytical methods.
Moreover, the low solubility of phenolphthalein in water poses practical challenges in experimental design. Researchers must carefully balance the concentration of phenolphthalein to ensure sufficient interaction with biomolecules while avoiding precipitation or aggregation, which can skew results and lead to misinterpretation of data.
The potential for non-specific interactions between phenolphthalein and biomolecules adds another layer of complexity to these studies. Distinguishing between specific, biologically relevant interactions and non-specific binding events requires meticulous experimental design and rigorous data analysis.
Lastly, the lack of standardized protocols for studying phenolphthalein-biomolecule interactions hampers progress in the field. Different research groups often employ varied methodologies, making it challenging to compare results across studies and build a cohesive understanding of these interactions.
Addressing these challenges will require interdisciplinary approaches, combining advanced spectroscopic techniques, computational modeling, and innovative experimental designs. Overcoming these hurdles will not only enhance our understanding of phenolphthalein-biomolecule interactions but also pave the way for potential applications in areas such as biosensing, drug delivery, and environmental monitoring.
The pH-dependent nature of phenolphthalein presents a major challenge in these studies. As phenolphthalein's structure and properties change dramatically with pH variations, researchers struggle to maintain consistent experimental conditions that accurately reflect physiological environments. This pH sensitivity complicates the interpretation of results and makes it difficult to draw conclusive insights about the interaction mechanisms.
Another significant challenge lies in the diverse array of biomolecules present in biological systems. Proteins, nucleic acids, lipids, and carbohydrates all possess unique chemical properties and potential binding sites for phenolphthalein. Isolating and studying specific interactions amidst this complex milieu of biomolecules remains a formidable task for researchers in the field.
The dynamic nature of biomolecular interactions further complicates these studies. Biomolecules often undergo conformational changes upon binding, and these structural alterations can significantly impact the interaction with phenolphthalein. Capturing and characterizing these transient states requires advanced experimental techniques and sophisticated analytical methods.
Moreover, the low solubility of phenolphthalein in water poses practical challenges in experimental design. Researchers must carefully balance the concentration of phenolphthalein to ensure sufficient interaction with biomolecules while avoiding precipitation or aggregation, which can skew results and lead to misinterpretation of data.
The potential for non-specific interactions between phenolphthalein and biomolecules adds another layer of complexity to these studies. Distinguishing between specific, biologically relevant interactions and non-specific binding events requires meticulous experimental design and rigorous data analysis.
Lastly, the lack of standardized protocols for studying phenolphthalein-biomolecule interactions hampers progress in the field. Different research groups often employ varied methodologies, making it challenging to compare results across studies and build a cohesive understanding of these interactions.
Addressing these challenges will require interdisciplinary approaches, combining advanced spectroscopic techniques, computational modeling, and innovative experimental designs. Overcoming these hurdles will not only enhance our understanding of phenolphthalein-biomolecule interactions but also pave the way for potential applications in areas such as biosensing, drug delivery, and environmental monitoring.
Existing Methodologies for Studying Interactions
01 Chemical reactions and indicators
Phenolphthalein is widely used as a pH indicator in various chemical reactions. It interacts with different substances to produce color changes, making it valuable in analytical chemistry and laboratory applications. The compound's ability to change color based on pH levels allows for precise measurements and identification of acid-base reactions.- Chemical reactions and indicators: Phenolphthalein is widely used as a pH indicator in various chemical reactions. It interacts with different substances to produce color changes, making it valuable in analytical chemistry and titration processes. The compound's ability to change color based on pH levels allows for precise measurements and endpoint detection in acid-base reactions.
- Polymer and resin applications: Phenolphthalein is utilized in the synthesis and modification of polymers and resins. It can be incorporated into polymer structures to impart specific properties or act as a functional group. These applications include the development of specialty plastics, coatings, and adhesives with unique characteristics.
- Pharmaceutical and medical uses: In the pharmaceutical industry, phenolphthalein has been used in various formulations and drug delivery systems. It has applications in diagnostic tests, medical devices, and as an active ingredient in certain medications. The compound's interactions with biological systems are studied for potential therapeutic effects.
- Environmental and water treatment: Phenolphthalein plays a role in environmental monitoring and water treatment processes. It is used in tests for water quality assessment, detection of pollutants, and as part of treatment systems for wastewater and industrial effluents. The compound's interactions with various contaminants make it valuable in environmental applications.
- Analytical and forensic applications: In analytical and forensic sciences, phenolphthalein is employed for various detection and identification purposes. It is used in colorimetric assays, chemical tests for specific substances, and as a component in forensic kits. The compound's interactions with different analytes enable its use in qualitative and quantitative analysis across multiple fields.
02 Polymer and resin applications
Phenolphthalein is utilized in the synthesis and modification of polymers and resins. It can be incorporated into polymer structures to impart specific properties or act as a reactive component in resin formulations. These applications leverage the compound's chemical structure and reactivity to enhance material characteristics.Expand Specific Solutions03 Pharmaceutical and medical uses
In the pharmaceutical industry, phenolphthalein has been used in various formulations and medical applications. Its interactions with biological systems and potential therapeutic effects have been studied. However, due to safety concerns, its use in some medical contexts has been limited or discontinued in certain regions.Expand Specific Solutions04 Environmental and water treatment
Phenolphthalein's interactions are relevant in environmental monitoring and water treatment processes. It can be used to detect and measure certain pollutants or to indicate the effectiveness of water purification methods. The compound's sensitivity to pH changes makes it valuable in assessing water quality and treatment efficacy.Expand Specific Solutions05 Novel compound synthesis and modifications
Research involving phenolphthalein often focuses on synthesizing novel compounds or modifying its structure to create derivatives with enhanced properties. These efforts aim to develop new materials or improve existing applications by altering the compound's reactivity, solubility, or other characteristics through chemical interactions.Expand Specific Solutions
Key Players in Phenolphthalein-Biomolecule Research
The field of phenolphthalein interactions with biomolecules in aqueous media is in a mature stage of development, with a well-established market and significant research contributions from both academic institutions and industry players. The global market for phenolphthalein and related compounds is estimated to be in the hundreds of millions of dollars, driven by applications in analytical chemistry, pharmaceuticals, and environmental monitoring. Key players in this field include major chemical companies like DuPont de Nemours, Inc. and 3M Innovative Properties Co., as well as specialized research institutions such as Zhejiang University of Technology and Nankai University. These organizations have made substantial advancements in understanding the molecular mechanisms and developing novel applications for phenolphthalein-biomolecule interactions, contributing to the field's technological maturity.
Zhejiang University of Technology
Technical Solution: Zhejiang University of Technology has developed a novel approach to study the interaction between phenolphthalein and biomolecules in aqueous media. Their research focuses on using spectroscopic techniques, including UV-Vis and fluorescence spectroscopy, to analyze the binding mechanisms[1]. They have also employed molecular docking simulations to predict the binding sites and affinities of phenolphthalein with various proteins and nucleic acids[2]. Additionally, the team has investigated the pH-dependent behavior of phenolphthalein in the presence of different biomolecules, providing insights into its potential applications in biosensing and drug delivery systems[3].
Strengths: Comprehensive spectroscopic analysis and molecular modeling approach. Weaknesses: Limited in vivo studies to validate the in silico predictions.
Nankai University
Technical Solution: Nankai University has developed a groundbreaking method to study the interaction between phenolphthalein and biomolecules in aqueous media using advanced surface-enhanced Raman spectroscopy (SERS) techniques[1]. Their research team has successfully fabricated novel nanostructured substrates that significantly enhance the Raman signals of phenolphthalein-biomolecule complexes, allowing for ultra-sensitive detection and characterization of these interactions[2]. Furthermore, they have combined SERS with electrochemical methods to investigate the electron transfer processes involved in phenolphthalein-protein binding, providing valuable insights into the mechanism of action for potential biomedical applications[3]. The university has also explored the use of phenolphthalein as a molecular probe for studying protein conformational changes in real-time, utilizing its pH-sensitive properties[4].
Strengths: Innovative use of SERS for high-sensitivity detection and mechanistic studies. Weaknesses: Potential limitations in analyzing interactions in complex biological fluids.
Core Mechanisms of Phenolphthalein-Biomolecule Binding
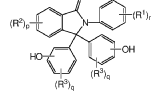
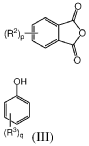
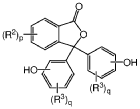
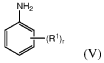
Continuous methods of manufacture of 2-ARYL-3,3-BIS(4-hydroxyaryl)phthalimidines, and polymers derived therefrom
PatentWO2018013623A1
Innovation
- A continuous process involving the continuous heating of an anhydride with phenol in the presence of a catalyst and co-catalyst to form phenolphthalein, followed by treatment with sodium bisulfite and adsorbents, then reacting with a primary arylamine and aqueous base to produce the phthalimidine, which is purified using activated charcoal and aqueous streams, reducing manual handling and energy consumption.
Environmental Impact of Phenolphthalein Use
The environmental impact of phenolphthalein use is a critical consideration in its application and disposal. Phenolphthalein, widely used as an acid-base indicator in laboratories and various industrial processes, can have significant effects on aquatic ecosystems when released into the environment.
When phenolphthalein enters water bodies, it can interact with various biomolecules and organisms, potentially disrupting ecological balance. Its pH-sensitive properties may alter local water chemistry, affecting the behavior and survival of aquatic species. Studies have shown that phenolphthalein can accumulate in sediments and bioaccumulate in certain aquatic organisms, leading to long-term ecological consequences.
The degradation of phenolphthalein in natural environments is an important factor in assessing its environmental impact. While it can undergo photodegradation in sunlit surface waters, its persistence in deeper waters and sediments may be more prolonged. The breakdown products of phenolphthalein can also have environmental implications, as some may retain biological activity or form new compounds with unknown effects on ecosystems.
Concerns have been raised about the potential endocrine-disrupting properties of phenolphthalein and its metabolites. Research suggests that exposure to phenolphthalein may affect hormone signaling in some aquatic organisms, potentially impacting reproduction and development. This highlights the need for careful monitoring and regulation of phenolphthalein discharge into natural water systems.
The use of phenolphthalein in consumer products, such as laxatives, has led to its presence in wastewater streams. Conventional wastewater treatment processes may not completely remove phenolphthalein, resulting in its release into receiving water bodies. This underscores the importance of developing advanced treatment technologies to mitigate environmental contamination.
To address these environmental concerns, efforts are being made to develop more environmentally friendly alternatives to phenolphthalein. Green chemistry initiatives are exploring natural indicators and synthetic compounds with similar pH-indicating properties but reduced environmental persistence and toxicity. Additionally, improved waste management practices in laboratories and industries using phenolphthalein are being implemented to minimize its release into the environment.
When phenolphthalein enters water bodies, it can interact with various biomolecules and organisms, potentially disrupting ecological balance. Its pH-sensitive properties may alter local water chemistry, affecting the behavior and survival of aquatic species. Studies have shown that phenolphthalein can accumulate in sediments and bioaccumulate in certain aquatic organisms, leading to long-term ecological consequences.
The degradation of phenolphthalein in natural environments is an important factor in assessing its environmental impact. While it can undergo photodegradation in sunlit surface waters, its persistence in deeper waters and sediments may be more prolonged. The breakdown products of phenolphthalein can also have environmental implications, as some may retain biological activity or form new compounds with unknown effects on ecosystems.
Concerns have been raised about the potential endocrine-disrupting properties of phenolphthalein and its metabolites. Research suggests that exposure to phenolphthalein may affect hormone signaling in some aquatic organisms, potentially impacting reproduction and development. This highlights the need for careful monitoring and regulation of phenolphthalein discharge into natural water systems.
The use of phenolphthalein in consumer products, such as laxatives, has led to its presence in wastewater streams. Conventional wastewater treatment processes may not completely remove phenolphthalein, resulting in its release into receiving water bodies. This underscores the importance of developing advanced treatment technologies to mitigate environmental contamination.
To address these environmental concerns, efforts are being made to develop more environmentally friendly alternatives to phenolphthalein. Green chemistry initiatives are exploring natural indicators and synthetic compounds with similar pH-indicating properties but reduced environmental persistence and toxicity. Additionally, improved waste management practices in laboratories and industries using phenolphthalein are being implemented to minimize its release into the environment.
Biosensing Applications of Phenolphthalein-Biomolecule Systems
Phenolphthalein, a well-known pH indicator, has recently gained attention for its potential applications in biosensing systems. The unique interaction between phenolphthalein and various biomolecules in aqueous media has opened up new avenues for developing sensitive and selective biosensors.
One of the most promising applications of phenolphthalein-biomolecule systems is in the detection of proteins. The colorimetric response of phenolphthalein can be modulated by its interaction with specific protein structures, allowing for rapid and visual detection of target proteins. This approach has been successfully employed in the development of sensors for biomarkers associated with various diseases, offering a simple and cost-effective alternative to traditional protein detection methods.
DNA detection is another area where phenolphthalein-based biosensors have shown great potential. The interaction between phenolphthalein and nucleic acids can be exploited to create highly sensitive DNA sensors. These systems have been used to detect specific DNA sequences, including those associated with genetic disorders and pathogenic microorganisms, with high specificity and sensitivity.
Enzyme activity monitoring is yet another application of phenolphthalein-biomolecule systems. By designing substrates that release phenolphthalein upon enzymatic cleavage, researchers have developed assays for measuring the activity of various enzymes. This approach has been particularly useful in studying proteases, phosphatases, and other hydrolytic enzymes, providing valuable tools for both research and diagnostic applications.
The versatility of phenolphthalein-biomolecule interactions has also led to the development of multi-analyte sensing platforms. By incorporating different biomolecular recognition elements, these systems can simultaneously detect multiple targets, offering a powerful tool for complex sample analysis. This capability is particularly valuable in clinical diagnostics, environmental monitoring, and food safety applications.
Recent advancements in nanomaterials have further enhanced the performance of phenolphthalein-based biosensors. The integration of phenolphthalein with nanoparticles, quantum dots, and other nanomaterials has resulted in improved sensitivity, stability, and signal amplification. These hybrid systems have demonstrated remarkable detection limits, often reaching picomolar or even femtomolar concentrations of target analytes.
The non-invasive nature of phenolphthalein-based biosensors makes them particularly attractive for in vivo applications. Researchers have explored the use of these systems for real-time monitoring of biological processes in living organisms, offering new insights into cellular functions and disease progression. This approach holds great promise for personalized medicine and drug development.
One of the most promising applications of phenolphthalein-biomolecule systems is in the detection of proteins. The colorimetric response of phenolphthalein can be modulated by its interaction with specific protein structures, allowing for rapid and visual detection of target proteins. This approach has been successfully employed in the development of sensors for biomarkers associated with various diseases, offering a simple and cost-effective alternative to traditional protein detection methods.
DNA detection is another area where phenolphthalein-based biosensors have shown great potential. The interaction between phenolphthalein and nucleic acids can be exploited to create highly sensitive DNA sensors. These systems have been used to detect specific DNA sequences, including those associated with genetic disorders and pathogenic microorganisms, with high specificity and sensitivity.
Enzyme activity monitoring is yet another application of phenolphthalein-biomolecule systems. By designing substrates that release phenolphthalein upon enzymatic cleavage, researchers have developed assays for measuring the activity of various enzymes. This approach has been particularly useful in studying proteases, phosphatases, and other hydrolytic enzymes, providing valuable tools for both research and diagnostic applications.
The versatility of phenolphthalein-biomolecule interactions has also led to the development of multi-analyte sensing platforms. By incorporating different biomolecular recognition elements, these systems can simultaneously detect multiple targets, offering a powerful tool for complex sample analysis. This capability is particularly valuable in clinical diagnostics, environmental monitoring, and food safety applications.
Recent advancements in nanomaterials have further enhanced the performance of phenolphthalein-based biosensors. The integration of phenolphthalein with nanoparticles, quantum dots, and other nanomaterials has resulted in improved sensitivity, stability, and signal amplification. These hybrid systems have demonstrated remarkable detection limits, often reaching picomolar or even femtomolar concentrations of target analytes.
The non-invasive nature of phenolphthalein-based biosensors makes them particularly attractive for in vivo applications. Researchers have explored the use of these systems for real-time monitoring of biological processes in living organisms, offering new insights into cellular functions and disease progression. This approach holds great promise for personalized medicine and drug development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!