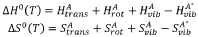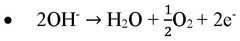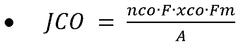Electrocatalysts CO2 enabling green hydrogen and e fuels
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Conversion Electrocatalysis Background and Objectives
The electrocatalytic conversion of carbon dioxide (CO2) represents a pivotal technological frontier in addressing global climate challenges while simultaneously creating valuable energy carriers. This technology has evolved significantly since the 1980s when initial research demonstrated the feasibility of electrochemically reducing CO2 to various products including carbon monoxide, formic acid, and hydrocarbons. The field has witnessed accelerated development in the past decade, driven by increasing urgency to mitigate greenhouse gas emissions and the growing interest in carbon capture and utilization (CCU) technologies.
The evolution of CO2 conversion electrocatalysis has been marked by several key technological breakthroughs, including the development of high-performance catalysts based on transition metals, the engineering of novel nanostructured materials, and the integration of advanced electrochemical systems. Recent advances in computational modeling and in-situ characterization techniques have further enhanced our understanding of reaction mechanisms and catalyst design principles.
Current technological trends point toward the integration of renewable electricity sources with CO2 electrolysis systems, creating a sustainable pathway for converting intermittent renewable energy into storable chemical forms. This approach aligns with the concept of Power-to-X technologies, where excess renewable electricity is utilized for producing hydrogen and carbon-based fuels or chemicals.
The primary objective of research in this field is to develop highly efficient, selective, and stable electrocatalysts capable of converting CO2 into targeted products at industrially relevant rates and energy efficiencies. Specifically, the goals include achieving Faradaic efficiencies exceeding 90% for desired products, reducing overpotentials to below 300 mV, and maintaining catalyst stability for thousands of hours of continuous operation.
Additionally, research aims to enhance the understanding of fundamental reaction mechanisms and structure-property relationships governing catalyst performance. This knowledge is essential for rational catalyst design and optimization, moving beyond empirical approaches toward predictive materials engineering.
From an application perspective, the technology targets the production of green hydrogen and e-fuels as sustainable alternatives to fossil-based counterparts. The ultimate goal is to establish a circular carbon economy where CO2 emissions are captured and recycled into valuable products, thereby closing the carbon loop and reducing net greenhouse gas emissions.
The technological roadmap envisions scaling from laboratory demonstrations to industrial implementation within the next decade, with intermediate milestones including pilot plant operations and demonstration projects. Success in this endeavor would represent a significant contribution to both climate change mitigation strategies and the transition toward renewable energy systems.
The evolution of CO2 conversion electrocatalysis has been marked by several key technological breakthroughs, including the development of high-performance catalysts based on transition metals, the engineering of novel nanostructured materials, and the integration of advanced electrochemical systems. Recent advances in computational modeling and in-situ characterization techniques have further enhanced our understanding of reaction mechanisms and catalyst design principles.
Current technological trends point toward the integration of renewable electricity sources with CO2 electrolysis systems, creating a sustainable pathway for converting intermittent renewable energy into storable chemical forms. This approach aligns with the concept of Power-to-X technologies, where excess renewable electricity is utilized for producing hydrogen and carbon-based fuels or chemicals.
The primary objective of research in this field is to develop highly efficient, selective, and stable electrocatalysts capable of converting CO2 into targeted products at industrially relevant rates and energy efficiencies. Specifically, the goals include achieving Faradaic efficiencies exceeding 90% for desired products, reducing overpotentials to below 300 mV, and maintaining catalyst stability for thousands of hours of continuous operation.
Additionally, research aims to enhance the understanding of fundamental reaction mechanisms and structure-property relationships governing catalyst performance. This knowledge is essential for rational catalyst design and optimization, moving beyond empirical approaches toward predictive materials engineering.
From an application perspective, the technology targets the production of green hydrogen and e-fuels as sustainable alternatives to fossil-based counterparts. The ultimate goal is to establish a circular carbon economy where CO2 emissions are captured and recycled into valuable products, thereby closing the carbon loop and reducing net greenhouse gas emissions.
The technological roadmap envisions scaling from laboratory demonstrations to industrial implementation within the next decade, with intermediate milestones including pilot plant operations and demonstration projects. Success in this endeavor would represent a significant contribution to both climate change mitigation strategies and the transition toward renewable energy systems.
Market Analysis for Green Hydrogen and E-Fuels
The global market for green hydrogen and e-fuels is experiencing unprecedented growth, driven by the urgent need to decarbonize energy-intensive sectors. Current market valuations place green hydrogen at approximately $2.5 billion in 2022, with projections indicating a compound annual growth rate of 39% through 2030, potentially reaching $89.1 billion by that time. This exponential growth trajectory reflects the increasing recognition of hydrogen's versatility as an energy carrier and industrial feedstock.
The demand landscape for green hydrogen spans multiple sectors, with industrial applications currently dominating consumption patterns. Heavy industries such as steel manufacturing, ammonia production, and refining represent the largest current market segments, collectively accounting for over 60% of hydrogen demand. Transportation emerges as the fastest-growing segment, particularly in heavy-duty vehicles, shipping, and aviation where battery electrification faces significant challenges.
E-fuels, synthesized using green hydrogen and captured CO2, represent a rapidly expanding market segment valued at approximately $67 million in 2022. Market forecasts suggest this could grow to $20 billion by 2030 as aviation and maritime industries seek drop-in replacements for conventional fuels. The synthetic methanol and e-kerosene segments show particular promise, with projected annual growth rates exceeding 45% through 2028.
Regional analysis reveals Europe leading the market development with ambitious hydrogen strategies and substantial funding commitments. The European Union has committed €470 billion to hydrogen development by 2050, with Germany alone allocating €9 billion to its national hydrogen strategy. Asia-Pacific represents the fastest-growing regional market, driven by Japan's and South Korea's aggressive decarbonization targets and China's emerging leadership in electrolyzer manufacturing.
Market barriers remain significant despite the promising outlook. Production costs for green hydrogen currently range from $3-8 per kilogram, substantially higher than gray hydrogen's $1-2 per kilogram. However, cost reduction pathways are clearly identified, with renewable electricity prices and electrolyzer efficiency improvements as key drivers. Industry projections suggest green hydrogen could reach cost parity with fossil-based alternatives in optimal locations by 2028-2030.
Infrastructure development represents another critical market challenge, with hydrogen transport and storage requiring investments estimated at $200 billion globally by 2030. Policy support mechanisms including carbon pricing, production subsidies, and mandated blend requirements are emerging as essential market enablers across major economies, creating a more favorable investment landscape for electrocatalyst technologies supporting CO2 conversion to valuable e-fuels.
The demand landscape for green hydrogen spans multiple sectors, with industrial applications currently dominating consumption patterns. Heavy industries such as steel manufacturing, ammonia production, and refining represent the largest current market segments, collectively accounting for over 60% of hydrogen demand. Transportation emerges as the fastest-growing segment, particularly in heavy-duty vehicles, shipping, and aviation where battery electrification faces significant challenges.
E-fuels, synthesized using green hydrogen and captured CO2, represent a rapidly expanding market segment valued at approximately $67 million in 2022. Market forecasts suggest this could grow to $20 billion by 2030 as aviation and maritime industries seek drop-in replacements for conventional fuels. The synthetic methanol and e-kerosene segments show particular promise, with projected annual growth rates exceeding 45% through 2028.
Regional analysis reveals Europe leading the market development with ambitious hydrogen strategies and substantial funding commitments. The European Union has committed €470 billion to hydrogen development by 2050, with Germany alone allocating €9 billion to its national hydrogen strategy. Asia-Pacific represents the fastest-growing regional market, driven by Japan's and South Korea's aggressive decarbonization targets and China's emerging leadership in electrolyzer manufacturing.
Market barriers remain significant despite the promising outlook. Production costs for green hydrogen currently range from $3-8 per kilogram, substantially higher than gray hydrogen's $1-2 per kilogram. However, cost reduction pathways are clearly identified, with renewable electricity prices and electrolyzer efficiency improvements as key drivers. Industry projections suggest green hydrogen could reach cost parity with fossil-based alternatives in optimal locations by 2028-2030.
Infrastructure development represents another critical market challenge, with hydrogen transport and storage requiring investments estimated at $200 billion globally by 2030. Policy support mechanisms including carbon pricing, production subsidies, and mandated blend requirements are emerging as essential market enablers across major economies, creating a more favorable investment landscape for electrocatalyst technologies supporting CO2 conversion to valuable e-fuels.
Global Electrocatalyst Technology Status and Barriers
The global landscape of electrocatalysts for CO2 conversion is characterized by significant advancements in recent years, yet substantial challenges remain before widespread commercial implementation. Currently, the field is dominated by metal-based catalysts, with copper, silver, gold, and zinc showing promising selectivity for various carbon products. Transition metal oxides and metal-organic frameworks have also emerged as important catalyst classes with tunable properties and high surface areas.
Research institutions in North America, Europe, and East Asia lead the development efforts, with China, the United States, and Germany accounting for approximately 65% of published research. Industrial adoption remains primarily at the pilot scale, with few full-scale commercial implementations due to persistent technical barriers.
The primary technical challenges include limited catalyst stability under industrial conditions, with most advanced catalysts showing significant degradation after 100-200 hours of operation. Faradaic efficiency for target products rarely exceeds 80% at industrially relevant current densities, and energy efficiency typically remains below 50%, making the process economically challenging compared to conventional fuel production methods.
Selectivity control presents another significant barrier, as most catalysts produce a mixture of products requiring costly separation processes. Current catalyst systems struggle to maintain performance when scaled beyond laboratory dimensions, with mass transport limitations and uneven current distribution affecting larger electrodes.
The integration of CO2 capture with conversion systems remains technically complex, as impurities in industrial CO2 streams can rapidly poison catalyst surfaces. Additionally, the high overpotentials required for CO2 reduction reactions significantly impact energy efficiency, with most systems requiring at least 1V overpotential for meaningful conversion rates.
From a geographical perspective, catalyst technology development shows distinct regional focuses. North American research emphasizes novel materials discovery and fundamental mechanistic understanding. European efforts concentrate on system integration and renewable energy coupling, while East Asian research prioritizes scaling and manufacturing innovations.
The economic barriers are equally challenging, with current noble metal-based catalysts costing $1,000-5,000 per kilogram, making large-scale deployment prohibitively expensive. The capital expenditure for integrated CO2 conversion systems remains 3-5 times higher than conventional fuel production facilities, necessitating significant cost reductions before commercial viability can be achieved.
Research institutions in North America, Europe, and East Asia lead the development efforts, with China, the United States, and Germany accounting for approximately 65% of published research. Industrial adoption remains primarily at the pilot scale, with few full-scale commercial implementations due to persistent technical barriers.
The primary technical challenges include limited catalyst stability under industrial conditions, with most advanced catalysts showing significant degradation after 100-200 hours of operation. Faradaic efficiency for target products rarely exceeds 80% at industrially relevant current densities, and energy efficiency typically remains below 50%, making the process economically challenging compared to conventional fuel production methods.
Selectivity control presents another significant barrier, as most catalysts produce a mixture of products requiring costly separation processes. Current catalyst systems struggle to maintain performance when scaled beyond laboratory dimensions, with mass transport limitations and uneven current distribution affecting larger electrodes.
The integration of CO2 capture with conversion systems remains technically complex, as impurities in industrial CO2 streams can rapidly poison catalyst surfaces. Additionally, the high overpotentials required for CO2 reduction reactions significantly impact energy efficiency, with most systems requiring at least 1V overpotential for meaningful conversion rates.
From a geographical perspective, catalyst technology development shows distinct regional focuses. North American research emphasizes novel materials discovery and fundamental mechanistic understanding. European efforts concentrate on system integration and renewable energy coupling, while East Asian research prioritizes scaling and manufacturing innovations.
The economic barriers are equally challenging, with current noble metal-based catalysts costing $1,000-5,000 per kilogram, making large-scale deployment prohibitively expensive. The capital expenditure for integrated CO2 conversion systems remains 3-5 times higher than conventional fuel production facilities, necessitating significant cost reductions before commercial viability can be achieved.
Current Electrocatalyst Materials and System Designs
01 Metal-based electrocatalysts for CO2 reduction
Metal-based catalysts, particularly transition metals and their alloys, are widely used as electrocatalysts for CO2 conversion. These materials offer tunable electronic properties and active sites that can enhance CO2 reduction efficiency. Various metals such as copper, silver, gold, and zinc exhibit different selectivity toward specific carbon products. Metal nanostructures with optimized morphologies and surface properties can significantly improve conversion rates and product selectivity.- Metal-based electrocatalysts for CO2 reduction: Metal-based catalysts, particularly transition metals like copper, silver, gold, and zinc, are widely used as electrocatalysts for CO2 conversion. These metals can be modified through alloying, nanostructuring, or surface modification to enhance their selectivity and efficiency. The catalytic performance depends on the metal's ability to adsorb CO2 and stabilize reaction intermediates, with different metals favoring different reaction pathways and products.
- Carbon-based and composite electrocatalysts: Carbon-based materials such as graphene, carbon nanotubes, and nitrogen-doped carbon serve as effective supports or active components in electrocatalysts for CO2 reduction. These materials can be combined with metal nanoparticles or metal oxides to create composite catalysts with enhanced activity and stability. The high surface area, conductivity, and tunable surface properties of carbon-based materials contribute to improved CO2 conversion efficiency.
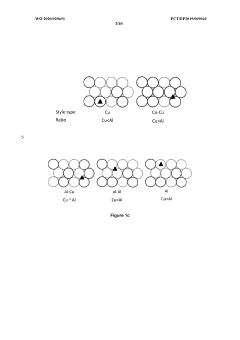
- Reactor design and system optimization for CO2 electroreduction: The design of electrochemical reactors and overall system optimization significantly impact CO2 conversion efficiency. Factors such as electrode configuration, electrolyte composition, mass transport, and operating conditions (temperature, pressure, potential) affect reaction kinetics and product selectivity. Advanced reactor designs, including flow cells, gas diffusion electrodes, and membrane electrode assemblies, can overcome mass transfer limitations and improve energy efficiency in CO2 electroreduction.
- Novel electrolyte systems for enhanced CO2 reduction: Electrolyte composition plays a crucial role in CO2 electroreduction performance. Ionic liquids, deep eutectic solvents, and modified aqueous electrolytes can enhance CO2 solubility, stabilize intermediates, and influence product selectivity. The addition of specific ions, pH control, and buffer systems can suppress competing hydrogen evolution reactions and direct the reaction pathway toward desired products, thereby improving overall conversion efficiency.
- Catalyst surface engineering and interface control: Engineering the catalyst surface structure and controlling the catalyst-electrolyte interface are effective strategies for enhancing CO2 conversion efficiency. Techniques include creating defects, exposing specific crystal facets, introducing functional groups, and developing core-shell structures. These approaches modify the local electronic environment, binding energies, and reaction pathways, leading to improved activity, selectivity, and stability of electrocatalysts for CO2 reduction.
02 Carbon-based and composite electrocatalysts
Carbon-based materials, including graphene, carbon nanotubes, and doped carbon structures, serve as effective supports or active catalysts for CO2 electroreduction. These materials can be functionalized or combined with metal nanoparticles to create composite catalysts with enhanced activity and stability. The high surface area and conductivity of carbon-based materials facilitate electron transfer during the CO2 reduction process, while heteroatom doping (N, S, P) creates active sites that improve catalytic performance and product selectivity.Expand Specific Solutions03 Catalyst design for improved selectivity and efficiency
Advanced catalyst design strategies focus on controlling the reaction pathway to enhance selectivity toward desired products. This includes engineering the catalyst structure at the atomic and molecular level, creating defect sites, and tuning the local chemical environment. Strategies such as alloying, surface modification, and the creation of single-atom catalysts can significantly improve CO2 conversion efficiency by lowering activation barriers and stabilizing key reaction intermediates. The rational design of catalysts with specific binding energies for reaction intermediates enables better control over product distribution.Expand Specific Solutions04 Electrolyte composition and reaction conditions
The composition of the electrolyte and reaction conditions significantly impact CO2 conversion efficiency. Factors such as pH, temperature, pressure, and the presence of specific ions can alter reaction pathways and product selectivity. Ionic liquids and specialized electrolytes can enhance CO2 solubility and stabilize reaction intermediates. Optimizing operating parameters such as applied potential, current density, and gas flow rates is crucial for maximizing conversion efficiency and maintaining catalyst stability during long-term operation.Expand Specific Solutions05 Novel reactor designs and system integration
Innovative reactor designs and integrated systems can overcome mass transport limitations and improve overall CO2 conversion efficiency. Gas diffusion electrodes, flow cells, and microfluidic reactors enable better control of reactant delivery to catalyst surfaces. Membrane-electrode assemblies and integrated systems that combine CO2 capture with electrochemical reduction offer pathways for continuous operation with higher energy efficiency. Advanced reactor configurations also facilitate the separation of products and recycling of unreacted CO2, which is essential for practical applications.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Electrocatalysis
The electrocatalyst market for CO2 conversion enabling green hydrogen and e-fuels production is in an early growth phase, characterized by intensive R&D activities across academic institutions and industry players. The global market is expanding rapidly, driven by decarbonization initiatives and estimated to reach several billion dollars by 2030. Leading companies like Johnson Matthey, TotalEnergies, and Honda are investing heavily in this technology, while research institutions such as Dalian Institute of Chemical Physics, National Institute of Clean & Low Carbon Energy, and various universities are advancing catalyst innovations. Emerging players like Dioxycle are developing specialized electrolyser solutions. The technology remains at varying maturity levels, with most commercial applications still in demonstration phases, though significant progress in catalyst efficiency and selectivity has been achieved in recent years.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed a comprehensive portfolio of advanced electrocatalysts for CO2 conversion, leveraging their extensive expertise in precious metal catalysis. Their proprietary PGM-based (Platinum Group Metals) catalysts feature precisely engineered nanostructures that maximize active site density while minimizing precious metal loading. Their flagship technology employs silver-based catalysts supported on functionalized carbon materials that achieve CO Faradaic efficiencies exceeding 95% at industrially relevant current densities (>300 mA/cm²)[1]. Johnson Matthey has also pioneered bimetallic catalyst systems combining copper with palladium that demonstrate enhanced selectivity toward multi-carbon products like ethanol and ethylene. Their catalyst manufacturing processes incorporate scalable methods including controlled precipitation and advanced impregnation techniques that ensure consistent performance across production batches. The company has developed complete electrode assemblies optimized for gas-diffusion electrode configurations that address mass transport limitations in CO2 reduction systems[2]. Their integrated approach includes membrane electrode assembly design that minimizes crossover issues while maintaining high conductivity.
Strengths: Unparalleled expertise in precious metal catalyst manufacturing at industrial scale; robust catalyst formulations with exceptional stability under real-world conditions; comprehensive intellectual property portfolio. Weaknesses: Higher cost associated with precious metal components; some systems still face challenges with product selectivity control, particularly for specific higher-value products.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed advanced metal-organic framework (MOF) based electrocatalysts for CO2 conversion. Their approach focuses on atomically dispersed metal sites within MOF structures that provide precise control over catalytic active centers. They've pioneered the development of copper-based single-atom catalysts embedded in nitrogen-doped carbon matrices that achieve Faradaic efficiencies exceeding 90% for CO2 reduction to CO and formate[1]. Their recent breakthrough involves bimetallic catalysts combining copper with zinc or silver atoms that synergistically enhance C-C coupling for higher-value multi-carbon products. The institute has also developed innovative in-situ characterization techniques to monitor catalyst structural changes during reaction conditions, enabling rational catalyst design based on reaction mechanism insights[2]. Their electrocatalysts operate at industrially relevant current densities (>200 mA/cm²) while maintaining stability over 1000+ hours of continuous operation.
Strengths: Exceptional atomic-level control over catalyst structure enabling precise tuning of product selectivity; strong integration of computational modeling with experimental validation; extensive characterization capabilities. Weaknesses: Some systems still require relatively high overpotentials (>0.8V) for efficient conversion; scale-up challenges from laboratory to industrial implementation remain significant.
Key Patents and Scientific Breakthroughs in CO2 Conversion
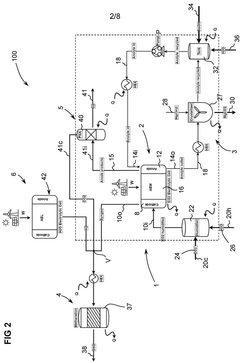
Catalysts for electrochemical co2 reduction and associated methods
PatentWO2020020691A1
Innovation
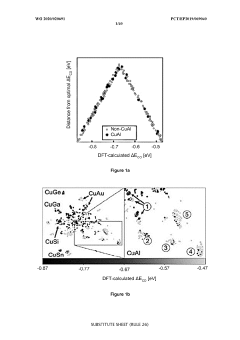
- Development of multi-metal electrocatalysts comprising copper (Cu) and at least one enhancer metal from Ge, Ga, Sn, Si, Ag, Au, Zn, or Al, with a preferred Cu-Al material, where Al is ion-implanted or evaporated into Cu and chemically etched to form a de-alloyed nanoporous structure, enhancing CO2 reduction activity and selectivity.
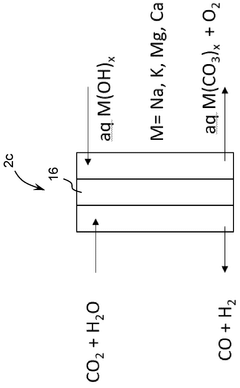
Co 2 and h 2o co-electrolyser system
PatentWO2024235958A1
Innovation
- A CO2 and H2O co-electrolyser system utilizing an anion-exchange membrane (AEM) electrolyser with a mineralization system that converts bicarbonate and carbonate ions into stable metal carbonates, preventing ion crossover and enhancing CO2 storage and conversion, while producing syngas for further conversion to methane.
Techno-Economic Assessment of CO2-to-Fuel Pathways
The techno-economic assessment of CO2-to-fuel pathways reveals significant variations in economic viability across different conversion technologies. Current analysis indicates that capital expenditure for electrochemical CO2 reduction facilities ranges from $1,500-3,000 per kW of installed capacity, with operating costs heavily influenced by electricity prices, typically representing 60-70% of total production costs.
Production costs for e-fuels derived from CO2 conversion currently range from $3.50-7.00 per gallon equivalent, substantially higher than conventional fossil fuels. However, sensitivity analyses suggest that with continued technological improvements in electrocatalyst efficiency and declining renewable electricity costs, production costs could decrease by 40-50% by 2030.
The economic competitiveness of different pathways varies significantly. CO2-to-methanol pathways demonstrate levelized costs of $0.90-1.20 per kilogram, while CO2-to-ethanol pathways show higher costs at $1.40-2.00 per kilogram. Syngas production (CO/H2) represents the most economically favorable pathway at present, with production costs of $0.70-0.90 per kilogram.
Scale economies play a crucial role in pathway viability. Modeling indicates that facilities processing at least 50,000 tons of CO2 annually achieve significantly better economics than smaller installations, with unit costs decreasing by approximately 30% when scaling from pilot to commercial scale.
Policy support mechanisms significantly impact economic feasibility. Carbon pricing mechanisms of $50-100 per ton CO2 would make several pathways competitive with conventional alternatives. Tax incentives, such as the 45Q tax credit in the United States, improve project economics but are generally insufficient as standalone measures to achieve market competitiveness.
Regional variations in electricity prices create substantial differences in pathway economics. Locations with abundant low-cost renewable electricity (below $0.04/kWh) demonstrate production costs 30-40% lower than regions with higher electricity prices, highlighting the importance of facility siting decisions.
The integration of CO2 conversion facilities with existing industrial infrastructure (steel mills, cement plants) improves economics through reduced carbon capture costs and potential thermal integration benefits, potentially reducing overall production costs by 15-25% compared to standalone facilities.
Production costs for e-fuels derived from CO2 conversion currently range from $3.50-7.00 per gallon equivalent, substantially higher than conventional fossil fuels. However, sensitivity analyses suggest that with continued technological improvements in electrocatalyst efficiency and declining renewable electricity costs, production costs could decrease by 40-50% by 2030.
The economic competitiveness of different pathways varies significantly. CO2-to-methanol pathways demonstrate levelized costs of $0.90-1.20 per kilogram, while CO2-to-ethanol pathways show higher costs at $1.40-2.00 per kilogram. Syngas production (CO/H2) represents the most economically favorable pathway at present, with production costs of $0.70-0.90 per kilogram.
Scale economies play a crucial role in pathway viability. Modeling indicates that facilities processing at least 50,000 tons of CO2 annually achieve significantly better economics than smaller installations, with unit costs decreasing by approximately 30% when scaling from pilot to commercial scale.
Policy support mechanisms significantly impact economic feasibility. Carbon pricing mechanisms of $50-100 per ton CO2 would make several pathways competitive with conventional alternatives. Tax incentives, such as the 45Q tax credit in the United States, improve project economics but are generally insufficient as standalone measures to achieve market competitiveness.
Regional variations in electricity prices create substantial differences in pathway economics. Locations with abundant low-cost renewable electricity (below $0.04/kWh) demonstrate production costs 30-40% lower than regions with higher electricity prices, highlighting the importance of facility siting decisions.
The integration of CO2 conversion facilities with existing industrial infrastructure (steel mills, cement plants) improves economics through reduced carbon capture costs and potential thermal integration benefits, potentially reducing overall production costs by 15-25% compared to standalone facilities.
Policy Frameworks Supporting Carbon Capture Utilization Technologies
The global policy landscape for carbon capture utilization (CCU) technologies has evolved significantly in recent years, reflecting growing recognition of their importance in climate change mitigation strategies. Major economies including the United States, European Union, China, and Japan have established comprehensive policy frameworks that specifically target CO2 conversion technologies for producing green hydrogen and e-fuels.
In the United States, the Inflation Reduction Act of 2022 expanded the 45Q tax credit for carbon capture to $85 per metric ton for CO2 utilized in products including e-fuels, substantially improving the economic viability of electrocatalytic CO2 conversion projects. This is complemented by the Department of Energy's Carbon Negative Shot initiative, which aims to accelerate research and deployment of carbon removal technologies through targeted funding programs.
The European Union has positioned itself as a policy leader through its Renewable Energy Directive II (RED II), which mandates increasing shares of renewable fuels in transportation, creating significant market pull for e-fuels derived from captured CO2. The EU Innovation Fund specifically allocates resources to breakthrough technologies in CO2 utilization, while the European Green Deal provides a comprehensive framework supporting the entire value chain of carbon capture and conversion technologies.
Regulatory frameworks in Asia show varying approaches, with China's 14th Five-Year Plan explicitly targeting carbon neutrality through technological innovation, including substantial investments in electrocatalytic research. Japan's Green Growth Strategy similarly prioritizes carbon recycling technologies with specific subsidies for e-fuel production facilities.
Cross-cutting policy instruments gaining traction globally include carbon pricing mechanisms, which create economic incentives for CO2 utilization by establishing a cost for carbon emissions. Currently, over 40 countries have implemented some form of carbon pricing, though price levels vary significantly and often remain below the threshold needed to make electrocatalytic conversion economically competitive without additional support.
Public procurement policies are emerging as powerful market creation tools, with several jurisdictions implementing mandates for government agencies to purchase products derived from captured carbon, including synthetic fuels for public transportation fleets. These demand-side policies complement supply-side incentives like tax credits and research grants.
International collaboration frameworks, including Mission Innovation and the Carbon Sequestration Leadership Forum, facilitate knowledge sharing and coordinate research efforts across borders, accelerating technology development through pooled resources and expertise in electrocatalyst development for CO2 conversion.
In the United States, the Inflation Reduction Act of 2022 expanded the 45Q tax credit for carbon capture to $85 per metric ton for CO2 utilized in products including e-fuels, substantially improving the economic viability of electrocatalytic CO2 conversion projects. This is complemented by the Department of Energy's Carbon Negative Shot initiative, which aims to accelerate research and deployment of carbon removal technologies through targeted funding programs.
The European Union has positioned itself as a policy leader through its Renewable Energy Directive II (RED II), which mandates increasing shares of renewable fuels in transportation, creating significant market pull for e-fuels derived from captured CO2. The EU Innovation Fund specifically allocates resources to breakthrough technologies in CO2 utilization, while the European Green Deal provides a comprehensive framework supporting the entire value chain of carbon capture and conversion technologies.
Regulatory frameworks in Asia show varying approaches, with China's 14th Five-Year Plan explicitly targeting carbon neutrality through technological innovation, including substantial investments in electrocatalytic research. Japan's Green Growth Strategy similarly prioritizes carbon recycling technologies with specific subsidies for e-fuel production facilities.
Cross-cutting policy instruments gaining traction globally include carbon pricing mechanisms, which create economic incentives for CO2 utilization by establishing a cost for carbon emissions. Currently, over 40 countries have implemented some form of carbon pricing, though price levels vary significantly and often remain below the threshold needed to make electrocatalytic conversion economically competitive without additional support.
Public procurement policies are emerging as powerful market creation tools, with several jurisdictions implementing mandates for government agencies to purchase products derived from captured carbon, including synthetic fuels for public transportation fleets. These demand-side policies complement supply-side incentives like tax credits and research grants.
International collaboration frameworks, including Mission Innovation and the Carbon Sequestration Leadership Forum, facilitate knowledge sharing and coordinate research efforts across borders, accelerating technology development through pooled resources and expertise in electrocatalyst development for CO2 conversion.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!