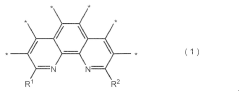
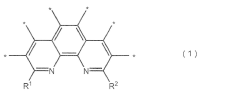
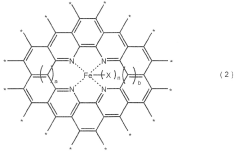
Electrocatalysts CO2 for efficient carbon dioxide reduction reactions
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Electrocatalysis Background and Objectives
Carbon dioxide (CO2) reduction reaction (CO2RR) through electrocatalysis has emerged as a promising approach to address the dual challenges of climate change and sustainable energy production. This technology represents a convergence of environmental remediation and renewable energy utilization by converting CO2, a primary greenhouse gas, into value-added chemicals and fuels using renewable electricity. The historical development of this field traces back to the 1980s when pioneering work demonstrated the feasibility of electrochemical CO2 reduction, though with limited efficiency and selectivity.
Over the past decade, electrocatalytic CO2 reduction has gained significant momentum due to increasing environmental concerns and technological advancements. The evolution of this technology has been marked by progressive improvements in catalyst design, from early metal electrodes to sophisticated nanostructured materials and molecular catalysts. These developments have substantially enhanced conversion efficiencies and product selectivity, making the technology increasingly viable for practical applications.
The primary objective of research in this field is to develop highly efficient, selective, and stable electrocatalysts capable of converting CO2 into specific high-value products at industrially relevant rates and energy efficiencies. Current research aims to overcome the inherent thermodynamic and kinetic barriers associated with CO2 activation, which require significant energy input and often result in multiple competing reaction pathways.
Specific technical goals include achieving Faradaic efficiencies exceeding 90% for target products, reducing overpotentials to below 300 mV, extending catalyst stability to thousands of hours, and developing systems capable of operating at current densities above 200 mA/cm² while maintaining performance metrics. Additionally, there is a growing emphasis on catalyst systems that can function effectively in real-world conditions, including tolerance to impurities in CO2 streams and compatibility with various CO2 sources.
The technology trajectory indicates a shift from fundamental understanding of reaction mechanisms toward practical implementation and scale-up. Recent breakthroughs in in-situ characterization techniques and computational modeling have accelerated catalyst development by providing deeper insights into reaction intermediates and pathways at the molecular level.
Looking forward, the field is moving toward integrated systems that combine CO2 capture and conversion, as well as tandem catalytic processes that can produce more complex molecules in a single step. The ultimate vision is to establish CO2 as a viable carbon feedstock for the chemical industry, creating a circular carbon economy that reduces dependence on fossil resources while mitigating greenhouse gas emissions.
Over the past decade, electrocatalytic CO2 reduction has gained significant momentum due to increasing environmental concerns and technological advancements. The evolution of this technology has been marked by progressive improvements in catalyst design, from early metal electrodes to sophisticated nanostructured materials and molecular catalysts. These developments have substantially enhanced conversion efficiencies and product selectivity, making the technology increasingly viable for practical applications.
The primary objective of research in this field is to develop highly efficient, selective, and stable electrocatalysts capable of converting CO2 into specific high-value products at industrially relevant rates and energy efficiencies. Current research aims to overcome the inherent thermodynamic and kinetic barriers associated with CO2 activation, which require significant energy input and often result in multiple competing reaction pathways.
Specific technical goals include achieving Faradaic efficiencies exceeding 90% for target products, reducing overpotentials to below 300 mV, extending catalyst stability to thousands of hours, and developing systems capable of operating at current densities above 200 mA/cm² while maintaining performance metrics. Additionally, there is a growing emphasis on catalyst systems that can function effectively in real-world conditions, including tolerance to impurities in CO2 streams and compatibility with various CO2 sources.
The technology trajectory indicates a shift from fundamental understanding of reaction mechanisms toward practical implementation and scale-up. Recent breakthroughs in in-situ characterization techniques and computational modeling have accelerated catalyst development by providing deeper insights into reaction intermediates and pathways at the molecular level.
Looking forward, the field is moving toward integrated systems that combine CO2 capture and conversion, as well as tandem catalytic processes that can produce more complex molecules in a single step. The ultimate vision is to establish CO2 as a viable carbon feedstock for the chemical industry, creating a circular carbon economy that reduces dependence on fossil resources while mitigating greenhouse gas emissions.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market size for carbon capture, utilization, and storage (CCUS) technologies was valued at approximately $1.9 billion in 2020 and is projected to reach $3.5 billion by 2025, growing at a CAGR of 13.2%. Within this broader market, electrocatalytic CO2 reduction represents one of the fastest-growing segments due to its potential for producing value-added chemicals and fuels from waste carbon dioxide.
Industrial sectors including chemical manufacturing, energy production, and transportation are the primary target markets for CO2 conversion technologies. Chemical companies are particularly interested in utilizing CO2 as a feedstock for producing methanol, formic acid, carbon monoxide, and various hydrocarbons. The energy sector views these technologies as complementary to renewable energy systems, offering pathways for energy storage and grid stabilization through power-to-X applications.
Regional market analysis reveals that North America and Europe currently lead in research and development investments, while Asia-Pacific demonstrates the fastest growth rate in technology adoption. China has emerged as a significant player, with substantial government funding directed toward carbon neutrality technologies as part of its commitment to reach peak emissions by 2030 and carbon neutrality by 2060.
Market drivers include increasingly stringent carbon pricing mechanisms, with the EU Emissions Trading System price reaching €80 per ton of CO2 in 2022, creating economic incentives for carbon capture and utilization. Additionally, corporate sustainability commitments and ESG (Environmental, Social, and Governance) investment criteria are pushing companies to adopt carbon-neutral or carbon-negative technologies.
Consumer demand for green products is creating pull-through effects in downstream markets. Products manufactured using captured CO2 can command premium prices in certain segments, with surveys indicating that 65% of consumers are willing to pay more for environmentally sustainable products.
Key market challenges include high capital costs for infrastructure development, energy efficiency concerns, and competition from established carbon-intensive processes. The levelized cost of CO2 conversion through electrocatalysis remains 2-3 times higher than conventional production methods for many target chemicals, though this gap is narrowing with technological improvements and economies of scale.
Market forecasts suggest that electrocatalytic CO2 reduction technologies will reach commercial viability for select high-value products by 2025, with broader market penetration expected by 2030 as catalyst performance improves and renewable electricity costs continue to decline.
Industrial sectors including chemical manufacturing, energy production, and transportation are the primary target markets for CO2 conversion technologies. Chemical companies are particularly interested in utilizing CO2 as a feedstock for producing methanol, formic acid, carbon monoxide, and various hydrocarbons. The energy sector views these technologies as complementary to renewable energy systems, offering pathways for energy storage and grid stabilization through power-to-X applications.
Regional market analysis reveals that North America and Europe currently lead in research and development investments, while Asia-Pacific demonstrates the fastest growth rate in technology adoption. China has emerged as a significant player, with substantial government funding directed toward carbon neutrality technologies as part of its commitment to reach peak emissions by 2030 and carbon neutrality by 2060.
Market drivers include increasingly stringent carbon pricing mechanisms, with the EU Emissions Trading System price reaching €80 per ton of CO2 in 2022, creating economic incentives for carbon capture and utilization. Additionally, corporate sustainability commitments and ESG (Environmental, Social, and Governance) investment criteria are pushing companies to adopt carbon-neutral or carbon-negative technologies.
Consumer demand for green products is creating pull-through effects in downstream markets. Products manufactured using captured CO2 can command premium prices in certain segments, with surveys indicating that 65% of consumers are willing to pay more for environmentally sustainable products.
Key market challenges include high capital costs for infrastructure development, energy efficiency concerns, and competition from established carbon-intensive processes. The levelized cost of CO2 conversion through electrocatalysis remains 2-3 times higher than conventional production methods for many target chemicals, though this gap is narrowing with technological improvements and economies of scale.
Market forecasts suggest that electrocatalytic CO2 reduction technologies will reach commercial viability for select high-value products by 2025, with broader market penetration expected by 2030 as catalyst performance improves and renewable electricity costs continue to decline.
Current Electrocatalyst Landscape and Challenges
The current landscape of electrocatalysts for CO2 reduction reaction (CO2RR) is characterized by significant advancements yet persistent challenges. Metal-based catalysts, particularly copper and its derivatives, remain at the forefront due to their unique ability to produce multi-carbon products. Recent developments have focused on nanostructured copper catalysts with controlled facets, defects, and grain boundaries to enhance selectivity and activity. Silver and gold catalysts have demonstrated excellent performance for CO production, while tin and bismuth show promise for formate generation.
Single-atom catalysts (SACs) have emerged as a revolutionary category, offering maximized atom efficiency and tunable electronic structures. These catalysts, typically consisting of isolated metal atoms anchored on carbon or nitrogen-doped supports, have shown remarkable faradaic efficiencies for specific products. Molecular catalysts, including metal porphyrins and metal-organic frameworks (MOFs), provide another avenue with their well-defined active sites and modifiable ligand environments.
Despite these advances, significant challenges persist in the field. Selectivity remains a primary concern, as CO2RR typically yields multiple products simultaneously, complicating downstream separation processes and reducing economic viability. Most catalysts still require high overpotentials to drive the reaction, resulting in substantial energy inefficiency that hinders commercial implementation.
Stability presents another major hurdle, with many promising catalysts showing performance degradation over extended operation periods. This degradation occurs through various mechanisms including metal leaching, surface poisoning, and structural collapse. The complex reaction environment, involving multiple phases (gas, liquid, solid) and competing reactions (particularly hydrogen evolution), further complicates catalyst design and optimization.
Mechanistic understanding remains incomplete, hampering rational catalyst design. The multi-electron, multi-proton transfer nature of CO2RR creates numerous possible reaction pathways and intermediates that are challenging to identify and characterize. Advanced in-situ and operando characterization techniques are being developed but still face limitations in spatial and temporal resolution.
Scalability represents a significant barrier to industrial implementation. Many high-performing catalysts rely on precious metals or complex synthesis procedures that are prohibitively expensive for large-scale applications. Additionally, the translation of performance metrics from laboratory settings (typically using H-cells or flow cells) to industrial electrolyzers introduces unforeseen challenges related to mass transport, bubble formation, and heat management.
The geographic distribution of research efforts shows concentration in North America, Europe, and East Asia, with China, the United States, and Germany leading publication output. This distribution correlates with regions investing heavily in renewable energy infrastructure and carbon neutrality initiatives.
Single-atom catalysts (SACs) have emerged as a revolutionary category, offering maximized atom efficiency and tunable electronic structures. These catalysts, typically consisting of isolated metal atoms anchored on carbon or nitrogen-doped supports, have shown remarkable faradaic efficiencies for specific products. Molecular catalysts, including metal porphyrins and metal-organic frameworks (MOFs), provide another avenue with their well-defined active sites and modifiable ligand environments.
Despite these advances, significant challenges persist in the field. Selectivity remains a primary concern, as CO2RR typically yields multiple products simultaneously, complicating downstream separation processes and reducing economic viability. Most catalysts still require high overpotentials to drive the reaction, resulting in substantial energy inefficiency that hinders commercial implementation.
Stability presents another major hurdle, with many promising catalysts showing performance degradation over extended operation periods. This degradation occurs through various mechanisms including metal leaching, surface poisoning, and structural collapse. The complex reaction environment, involving multiple phases (gas, liquid, solid) and competing reactions (particularly hydrogen evolution), further complicates catalyst design and optimization.
Mechanistic understanding remains incomplete, hampering rational catalyst design. The multi-electron, multi-proton transfer nature of CO2RR creates numerous possible reaction pathways and intermediates that are challenging to identify and characterize. Advanced in-situ and operando characterization techniques are being developed but still face limitations in spatial and temporal resolution.
Scalability represents a significant barrier to industrial implementation. Many high-performing catalysts rely on precious metals or complex synthesis procedures that are prohibitively expensive for large-scale applications. Additionally, the translation of performance metrics from laboratory settings (typically using H-cells or flow cells) to industrial electrolyzers introduces unforeseen challenges related to mass transport, bubble formation, and heat management.
The geographic distribution of research efforts shows concentration in North America, Europe, and East Asia, with China, the United States, and Germany leading publication output. This distribution correlates with regions investing heavily in renewable energy infrastructure and carbon neutrality initiatives.
State-of-the-Art Electrocatalytic CO2 Reduction Methods
01 Metal-based electrocatalysts for CO2 reduction
Metal-based catalysts, particularly transition metals and their alloys, are widely used for electrochemical CO2 reduction. These catalysts offer tunable selectivity and activity through composition control. Metals like copper, silver, gold, and zinc exhibit different product selectivities, with copper being unique in its ability to produce higher hydrocarbons and alcohols. Metal nanostructures with optimized morphologies can significantly enhance catalytic efficiency by increasing active surface area and exposing more reactive sites.- Metal-based electrocatalysts for CO2 reduction: Metal-based catalysts, particularly transition metals and their alloys, are widely used for electrochemical CO2 reduction. These catalysts can be optimized by controlling their morphology, crystal structure, and surface properties to enhance selectivity and efficiency. Metals such as copper, silver, gold, and zinc exhibit different product selectivities, with copper being particularly notable for its ability to produce higher-value hydrocarbons and alcohols. The performance of these catalysts can be further improved through alloying, surface modification, and nanostructuring techniques.
- Carbon-based and composite electrocatalysts: Carbon-based materials, including graphene, carbon nanotubes, and nitrogen-doped carbon, serve as effective supports or active components in CO2 reduction electrocatalysts. These materials offer high surface area, excellent conductivity, and tunable surface chemistry. Composite catalysts combining carbon materials with metal nanoparticles or metal-organic frameworks demonstrate synergistic effects that enhance catalytic performance. The incorporation of heteroatoms like nitrogen, sulfur, or phosphorus into carbon structures creates active sites that improve CO2 adsorption and activation, leading to higher conversion efficiencies.
- Single-atom catalysts and atomically dispersed active sites: Single-atom catalysts (SACs) represent an emerging class of highly efficient electrocatalysts for CO2 reduction. These catalysts feature isolated metal atoms anchored on various supports, maximizing atom utilization efficiency while providing unique electronic properties that differ from their bulk counterparts. The coordination environment of the metal centers can be precisely tuned to optimize binding energies with reaction intermediates, enhancing both activity and selectivity. These catalysts often demonstrate superior faradaic efficiency and stability compared to traditional nanoparticle catalysts, while requiring significantly less precious metal content.
- Reactor design and system optimization for enhanced efficiency: The design of electrochemical reactors plays a crucial role in determining the overall efficiency of CO2 reduction processes. Factors such as electrode configuration, electrolyte composition, mass transport, and bubble management significantly impact performance. Advanced reactor designs incorporate gas diffusion electrodes, flow cells, and membrane electrode assemblies to overcome mass transfer limitations and improve energy efficiency. Continuous flow systems, pressurized reactors, and integrated approaches that combine electrochemical reduction with other processes can further enhance productivity and economic viability of CO2 conversion technologies.
- Novel electrolyte formulations and operating conditions: The composition of the electrolyte solution and operating conditions significantly influence the efficiency and selectivity of CO2 electroreduction. Innovations include the use of ionic liquids, deep eutectic solvents, and specialized additives that enhance CO2 solubility and stabilize key reaction intermediates. pH control, temperature management, and the application of pulsed potentials or magnetic fields can further optimize catalyst performance. The development of electrolytes that minimize competing hydrogen evolution reactions while promoting desired carbon-carbon coupling pathways represents a key strategy for improving overall process efficiency.
02 Carbon-based and composite electrocatalysts
Carbon-based materials, including graphene, carbon nanotubes, and nitrogen-doped carbon, serve as effective supports or catalysts for CO2 reduction. These materials offer high conductivity, large surface area, and tunable electronic properties. Composite catalysts combining carbon materials with metal nanoparticles create synergistic effects that enhance catalytic performance. The carbon support stabilizes metal particles, prevents aggregation, and sometimes participates directly in the catalytic process, improving overall efficiency and selectivity.Expand Specific Solutions03 Single-atom catalysts and atomically dispersed active sites
Single-atom catalysts (SACs) represent an emerging class of highly efficient electrocatalysts for CO2 reduction. By isolating individual metal atoms on supports, SACs maximize atom utilization efficiency and provide unique electronic properties that differ from bulk materials. These catalysts often demonstrate superior activity and selectivity due to their well-defined, uniform active sites. The coordination environment around the single atoms can be precisely engineered to optimize binding energies with reaction intermediates, leading to enhanced catalytic performance.Expand Specific Solutions04 Electrolyte engineering and reaction environment optimization
The composition and properties of the electrolyte significantly impact CO2 reduction efficiency. Factors such as pH, ionic strength, buffer capacity, and the presence of specific ions can dramatically alter reaction pathways and product distributions. Advanced electrolyte engineering approaches include using ionic liquids, deep eutectic solvents, or specially designed additives to enhance CO2 solubility and stabilize key intermediates. Optimizing the local reaction environment near the catalyst surface through electrolyte design is crucial for achieving high Faradaic efficiency and product selectivity.Expand Specific Solutions05 Catalyst interface and structural engineering
The design and engineering of catalyst interfaces and structures play a critical role in determining CO2 reduction performance. Creating defects, vacancies, grain boundaries, and specific crystal facets can generate highly active sites for CO2 activation. Hierarchical structures combining macro, micro, and nanoscale features optimize mass transport while maintaining high surface area. Core-shell architectures, 2D materials, and precisely engineered surface ligands can be used to tune the electronic properties of active sites and stabilize key reaction intermediates, leading to improved efficiency and selectivity.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalyst market for CO2 reduction reactions is currently in an early growth phase, characterized by intensive R&D activities across academic and industrial sectors. The global market size is projected to expand significantly as carbon capture technologies gain traction amid climate change concerns. Leading research institutions like KIST (South Korea), Zhejiang University, and University of Science & Technology of China are advancing fundamental catalyst science, while industrial players including Siemens Energy, Panasonic, and Toshiba are focusing on commercial applications. Technology maturity varies considerably, with most solutions at TRL 3-6. Major corporations such as Mitsubishi Electric, Hitachi, and NTT are investing in scalable systems, while specialized entities like Avantium Knowledge Centre are developing novel catalyst formulations. International collaboration between universities and industry is accelerating development toward economically viable solutions.
Zhejiang University
Technical Solution: Zhejiang University has developed innovative single-atom catalysts (SACs) for electrochemical CO2 reduction, focusing on atomically dispersed metal centers on nitrogen-doped carbon supports. Their M-N-C catalysts (where M includes Fe, Co, Ni) demonstrate remarkable CO2 conversion efficiency with near 100% selectivity for specific products. Their breakthrough involves precise control of the coordination environment around metal centers, creating isolated active sites with optimized binding energies for CO2 activation[3]. They've pioneered in-situ X-ray absorption spectroscopy techniques to monitor catalyst structural changes during reaction, enabling rational design improvements. Recent work includes developing bimetallic single-atom catalysts that leverage synergistic effects between different metal centers to lower energy barriers and improve product selectivity. Their catalysts achieve current densities exceeding 300 mA/cm² with stable operation for over 200 hours in flow cell configurations[4], representing significant progress toward practical applications.
Strengths: Maximized atom efficiency through single-atom design; exceptional selectivity for target products; detailed mechanistic understanding enabling rational catalyst improvement; relatively low precious metal content reducing costs. Weaknesses: Challenges in maintaining single-atom dispersion during long-term operation; complex synthesis procedures potentially limiting large-scale production; some systems still require significant overpotentials for practical reaction rates.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy has developed an integrated CO2 electrolysis system focused on industrial scalability and integration with renewable energy sources. Their approach centers on high-temperature solid oxide electrolysis cells (SOECs) operating at 700-850°C, which significantly reduces the electrical energy required for CO2 reduction compared to low-temperature approaches. The company's proprietary ceramic-based electrocatalysts incorporate mixed ionic-electronic conducting materials with tailored perovskite structures that demonstrate exceptional stability under harsh operating conditions[5]. Their system architecture includes innovative heat management solutions that utilize waste heat from industrial processes to maintain operating temperatures, further improving overall energy efficiency. Siemens has demonstrated pilot-scale systems producing syngas (CO/H2 mixtures) with conversion efficiencies exceeding 85% and minimal degradation over thousands of operating hours. Their modular design allows for flexible scaling from kilowatt to megawatt installations, with integrated power electronics specifically designed to handle variable renewable energy inputs[6].
Strengths: Industrial-scale system integration expertise; high-temperature operation reducing electrical energy requirements; robust catalyst formulations with exceptional long-term stability; ability to directly utilize industrial CO2 streams without extensive purification. Weaknesses: High-temperature operation requires specialized materials and thermal management; higher capital costs compared to some low-temperature approaches; primarily focused on syngas rather than higher-value liquid products; longer start-up times limiting responsiveness to intermittent renewable energy.
Key Patents and Scientific Breakthroughs in CO2RR
Catalyst for carbon dioxide electroreduction reaction or nitrogen electroreduction reaction, method for producing catalyst for carbon dioxide electroreduction reaction or nitrogen electroreduction reaction, and electrode for carbon dioxide electroreduction reaction or nitrogen electroreduction reaction
PatentInactiveJP2021115501A
Innovation
- Incorporating Fe-N4 structures with a high active point density of 3.0 × 10^-5 to 1.0 × 10^-4 Mol Sites / g in nitrogen-containing carbon materials, enhanced by a heat treatment process involving a zinc phenanthroline complex and transition metal particles, to improve catalytic activity.
Environmental Impact and Sustainability Assessment
The development of electrocatalysts for CO2 reduction represents a critical technological pathway toward mitigating climate change impacts. These catalytic systems offer a dual environmental benefit: reducing atmospheric CO2 concentrations while simultaneously producing valuable chemical feedstocks and fuels. Life cycle assessment (LCA) studies indicate that well-designed electrocatalytic CO2 reduction processes can achieve net carbon negativity when powered by renewable electricity sources, potentially removing more CO2 than is emitted during catalyst production and operation.
Current environmental impact analyses reveal that copper-based catalysts, while effective for multi-carbon product formation, often involve energy-intensive synthesis methods and use of toxic reagents. In contrast, transition metal-nitrogen-carbon (M-N-C) catalysts demonstrate lower embodied energy and reduced environmental toxicity during production, though their long-term stability remains a concern for sustainable implementation.
Water consumption represents another significant environmental consideration, particularly for gas-diffusion electrode systems that require precise water management. Advanced membrane electrode assemblies have shown promise in reducing water requirements by up to 40% compared to traditional H-cell configurations, thereby enhancing the sustainability profile of these systems in water-stressed regions.
The end-of-life management of spent electrocatalysts presents both challenges and opportunities. Noble metal catalysts (Ag, Au, Pt) offer excellent recyclability potential, with recovery rates exceeding 90% through established hydrometallurgical processes. However, composite catalysts with complex structures often lack standardized recycling protocols, creating potential waste management issues that require further research attention.
From a sustainability perspective, the energy return on investment (EROI) for CO2 reduction systems continues to improve. Recent advances in catalyst design have reduced overpotentials significantly, with state-of-the-art copper-based catalysts achieving Faradaic efficiencies above 90% for specific products at energy inputs approaching theoretical minimums. This translates to improved sustainability metrics when integrated with renewable energy sources.
The environmental footprint of catalyst production remains an area requiring optimization. Current manufacturing routes for high-performance nanomaterials often involve multiple energy-intensive steps and hazardous chemicals. Green chemistry approaches, including aqueous synthesis methods and biomass-derived precursors, have demonstrated promising results in reducing environmental impact while maintaining catalytic performance, though scale-up challenges persist.
Current environmental impact analyses reveal that copper-based catalysts, while effective for multi-carbon product formation, often involve energy-intensive synthesis methods and use of toxic reagents. In contrast, transition metal-nitrogen-carbon (M-N-C) catalysts demonstrate lower embodied energy and reduced environmental toxicity during production, though their long-term stability remains a concern for sustainable implementation.
Water consumption represents another significant environmental consideration, particularly for gas-diffusion electrode systems that require precise water management. Advanced membrane electrode assemblies have shown promise in reducing water requirements by up to 40% compared to traditional H-cell configurations, thereby enhancing the sustainability profile of these systems in water-stressed regions.
The end-of-life management of spent electrocatalysts presents both challenges and opportunities. Noble metal catalysts (Ag, Au, Pt) offer excellent recyclability potential, with recovery rates exceeding 90% through established hydrometallurgical processes. However, composite catalysts with complex structures often lack standardized recycling protocols, creating potential waste management issues that require further research attention.
From a sustainability perspective, the energy return on investment (EROI) for CO2 reduction systems continues to improve. Recent advances in catalyst design have reduced overpotentials significantly, with state-of-the-art copper-based catalysts achieving Faradaic efficiencies above 90% for specific products at energy inputs approaching theoretical minimums. This translates to improved sustainability metrics when integrated with renewable energy sources.
The environmental footprint of catalyst production remains an area requiring optimization. Current manufacturing routes for high-performance nanomaterials often involve multiple energy-intensive steps and hazardous chemicals. Green chemistry approaches, including aqueous synthesis methods and biomass-derived precursors, have demonstrated promising results in reducing environmental impact while maintaining catalytic performance, though scale-up challenges persist.
Scale-up and Commercialization Pathways
The commercialization of CO2 reduction electrocatalysts requires strategic scaling from laboratory prototypes to industrial applications. Current pilot projects demonstrate promising pathways, with companies like Siemens, Opus 12, and Carbon Recycling International implementing demonstration plants that convert CO2 into valuable products such as methanol, ethylene, and formic acid. These early commercial ventures typically operate at kilowatt scales, with plans to expand to megawatt capacity within the next five years.
Key technical challenges in scaling include maintaining catalyst performance at industrial scales, where issues of mass transport, heat management, and structural integrity become increasingly significant. The degradation mechanisms that appear negligible in laboratory settings often become critical barriers when operating continuously at commercial scales. Engineering solutions focusing on electrode architecture optimization and reactor design innovations are addressing these challenges.
Economic viability remains a central concern, with current production costs for electrocatalytic CO2 reduction products generally 2-3 times higher than conventional fossil-based alternatives. However, techno-economic analyses project cost parity could be achieved by 2030 through improvements in catalyst efficiency, renewable electricity cost reduction, and economies of scale. Carbon pricing mechanisms and regulatory frameworks supporting low-carbon technologies will significantly influence commercialization timelines.
Manufacturing scale-up follows several distinct pathways depending on catalyst type. Metal-based catalysts leverage existing electroplating and coating infrastructure, while more complex materials like metal-organic frameworks require specialized production techniques. Several companies are developing modular electrolyzer designs that can be deployed incrementally, reducing initial capital requirements while allowing for gradual capacity expansion.
Strategic partnerships between technology developers, industrial gas suppliers, and end-users of carbon-derived products are emerging as the dominant commercialization model. These collaborations create value chains that connect CO2 sources with conversion technologies and product markets. Notable examples include partnerships between cement manufacturers and CO2 utilization startups, where captured emissions become feedstock for electrocatalytic conversion.
Standardization efforts are underway to establish performance metrics, safety protocols, and quality standards for CO2 reduction systems. These standards will facilitate market adoption by providing confidence to investors and customers while enabling meaningful comparisons between competing technologies. Industry consortia and international organizations are collaboratively developing these frameworks to support the emerging CO2 utilization economy.
Key technical challenges in scaling include maintaining catalyst performance at industrial scales, where issues of mass transport, heat management, and structural integrity become increasingly significant. The degradation mechanisms that appear negligible in laboratory settings often become critical barriers when operating continuously at commercial scales. Engineering solutions focusing on electrode architecture optimization and reactor design innovations are addressing these challenges.
Economic viability remains a central concern, with current production costs for electrocatalytic CO2 reduction products generally 2-3 times higher than conventional fossil-based alternatives. However, techno-economic analyses project cost parity could be achieved by 2030 through improvements in catalyst efficiency, renewable electricity cost reduction, and economies of scale. Carbon pricing mechanisms and regulatory frameworks supporting low-carbon technologies will significantly influence commercialization timelines.
Manufacturing scale-up follows several distinct pathways depending on catalyst type. Metal-based catalysts leverage existing electroplating and coating infrastructure, while more complex materials like metal-organic frameworks require specialized production techniques. Several companies are developing modular electrolyzer designs that can be deployed incrementally, reducing initial capital requirements while allowing for gradual capacity expansion.
Strategic partnerships between technology developers, industrial gas suppliers, and end-users of carbon-derived products are emerging as the dominant commercialization model. These collaborations create value chains that connect CO2 sources with conversion technologies and product markets. Notable examples include partnerships between cement manufacturers and CO2 utilization startups, where captured emissions become feedstock for electrocatalytic conversion.
Standardization efforts are underway to establish performance metrics, safety protocols, and quality standards for CO2 reduction systems. These standards will facilitate market adoption by providing confidence to investors and customers while enabling meaningful comparisons between competing technologies. Industry consortia and international organizations are collaboratively developing these frameworks to support the emerging CO2 utilization economy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!