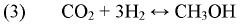Electrocatalysts CO2 in syngas generation and fuel synthesis
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Conversion Electrocatalysis Background and Objectives
Carbon dioxide (CO2) conversion through electrocatalysis represents a critical frontier in sustainable energy research, offering a dual solution to climate change mitigation and renewable energy storage. The evolution of this technology dates back to the 1980s when Honda and Fujishima first demonstrated photocatalytic CO2 reduction. Since then, the field has progressed through several developmental phases, from fundamental electrochemical studies to the current era of advanced catalyst design and system integration.
The technological trajectory has been marked by significant breakthroughs in catalyst materials, from early copper-based systems to modern hybrid catalysts incorporating nanomaterials and molecular complexes. Recent years have witnessed an acceleration in research output, with annual publications on CO2 electrocatalysis increasing nearly tenfold over the past decade, indicating the growing scientific and industrial interest in this field.
Current technological trends point toward several promising directions, including the development of single-atom catalysts with unprecedented selectivity, bimetallic systems that leverage synergistic effects, and the integration of electrocatalysts with renewable energy sources to create carbon-neutral or carbon-negative energy cycles. The emergence of artificial intelligence for catalyst discovery and optimization represents another significant trend that is reshaping the research landscape.
The primary technical objectives in this field center around enhancing three critical performance metrics: energy efficiency, product selectivity, and catalyst stability. Current state-of-the-art systems achieve Faradaic efficiencies of 60-90% for CO and formate production, but efficiency drops significantly for more complex products like ethanol or propanol. Similarly, while laboratory demonstrations have achieved impressive current densities exceeding 300 mA/cm², commercial viability likely requires sustained performance at 500+ mA/cm² with minimal degradation over thousands of hours.
Specifically for syngas generation and fuel synthesis, research objectives include developing catalysts capable of precise H₂/CO ratio control to match downstream synthesis requirements, reducing the overpotential required for CO2 reduction to below 300 mV, and creating integrated systems that can directly convert CO2 to higher-value fuels like methanol or ethanol in a single process. Additionally, there is growing emphasis on developing catalysts from earth-abundant materials to ensure economic viability and scalability.
The ultimate goal of this technological pursuit extends beyond laboratory demonstrations to industrial implementation, aiming to create economically viable pathways for converting waste CO2 into valuable chemical feedstocks and fuels, thereby closing the carbon cycle and contributing to global decarbonization efforts while providing alternative routes to critical industrial chemicals currently derived from fossil resources.
The technological trajectory has been marked by significant breakthroughs in catalyst materials, from early copper-based systems to modern hybrid catalysts incorporating nanomaterials and molecular complexes. Recent years have witnessed an acceleration in research output, with annual publications on CO2 electrocatalysis increasing nearly tenfold over the past decade, indicating the growing scientific and industrial interest in this field.
Current technological trends point toward several promising directions, including the development of single-atom catalysts with unprecedented selectivity, bimetallic systems that leverage synergistic effects, and the integration of electrocatalysts with renewable energy sources to create carbon-neutral or carbon-negative energy cycles. The emergence of artificial intelligence for catalyst discovery and optimization represents another significant trend that is reshaping the research landscape.
The primary technical objectives in this field center around enhancing three critical performance metrics: energy efficiency, product selectivity, and catalyst stability. Current state-of-the-art systems achieve Faradaic efficiencies of 60-90% for CO and formate production, but efficiency drops significantly for more complex products like ethanol or propanol. Similarly, while laboratory demonstrations have achieved impressive current densities exceeding 300 mA/cm², commercial viability likely requires sustained performance at 500+ mA/cm² with minimal degradation over thousands of hours.
Specifically for syngas generation and fuel synthesis, research objectives include developing catalysts capable of precise H₂/CO ratio control to match downstream synthesis requirements, reducing the overpotential required for CO2 reduction to below 300 mV, and creating integrated systems that can directly convert CO2 to higher-value fuels like methanol or ethanol in a single process. Additionally, there is growing emphasis on developing catalysts from earth-abundant materials to ensure economic viability and scalability.
The ultimate goal of this technological pursuit extends beyond laboratory demonstrations to industrial implementation, aiming to create economically viable pathways for converting waste CO2 into valuable chemical feedstocks and fuels, thereby closing the carbon cycle and contributing to global decarbonization efforts while providing alternative routes to critical industrial chemicals currently derived from fossil resources.
Market Analysis for Syngas and Synthetic Fuel Technologies
The global market for syngas and synthetic fuels has experienced significant growth in recent years, driven by increasing energy demands, environmental concerns, and the push for sustainable alternatives to conventional fossil fuels. The syngas market was valued at approximately $43.6 billion in 2022 and is projected to reach $66.5 billion by 2030, growing at a CAGR of 6.2%. This growth is primarily attributed to the rising demand for clean energy solutions and the versatility of syngas as a feedstock for various industrial applications.
The synthetic fuels market, which heavily relies on syngas as an intermediate, is also witnessing substantial expansion. Currently valued at around $22.3 billion, it is expected to grow at a CAGR of 8.7% through 2030. This growth is particularly pronounced in regions with stringent carbon emission regulations and countries seeking to reduce dependence on imported petroleum products.
Geographically, Asia-Pacific dominates the syngas market, accounting for approximately 40% of the global share, with China being the largest contributor due to its extensive coal gasification activities. Europe follows with a 25% market share, driven by strong environmental policies and investments in renewable energy technologies. North America represents about 20% of the market, with significant growth potential due to abundant natural gas resources and increasing focus on carbon capture and utilization technologies.
The demand for electrocatalysts for CO2 conversion in syngas generation is particularly strong in sectors such as chemicals, fertilizers, and transportation fuels. The chemical industry accounts for the largest end-use segment (45%), followed by power generation (30%) and transportation fuels (15%). The remaining 10% is distributed across various other applications including methanol production and ammonia synthesis.
Key market drivers include the increasing focus on carbon neutrality goals, rising natural gas prices, and government incentives for clean energy technologies. The European Union's Green Deal and similar initiatives worldwide are creating favorable market conditions for CO2 conversion technologies. Additionally, the volatility in conventional fuel prices is pushing industries to seek more stable and sustainable alternatives.
Market challenges include high capital investment requirements for syngas and synthetic fuel facilities, technological barriers in achieving efficient CO2 conversion at scale, and competition from increasingly cost-effective renewable energy sources. The economic viability of these technologies remains heavily dependent on carbon pricing mechanisms and regulatory frameworks that incentivize carbon capture and utilization.
The synthetic fuels market, which heavily relies on syngas as an intermediate, is also witnessing substantial expansion. Currently valued at around $22.3 billion, it is expected to grow at a CAGR of 8.7% through 2030. This growth is particularly pronounced in regions with stringent carbon emission regulations and countries seeking to reduce dependence on imported petroleum products.
Geographically, Asia-Pacific dominates the syngas market, accounting for approximately 40% of the global share, with China being the largest contributor due to its extensive coal gasification activities. Europe follows with a 25% market share, driven by strong environmental policies and investments in renewable energy technologies. North America represents about 20% of the market, with significant growth potential due to abundant natural gas resources and increasing focus on carbon capture and utilization technologies.
The demand for electrocatalysts for CO2 conversion in syngas generation is particularly strong in sectors such as chemicals, fertilizers, and transportation fuels. The chemical industry accounts for the largest end-use segment (45%), followed by power generation (30%) and transportation fuels (15%). The remaining 10% is distributed across various other applications including methanol production and ammonia synthesis.
Key market drivers include the increasing focus on carbon neutrality goals, rising natural gas prices, and government incentives for clean energy technologies. The European Union's Green Deal and similar initiatives worldwide are creating favorable market conditions for CO2 conversion technologies. Additionally, the volatility in conventional fuel prices is pushing industries to seek more stable and sustainable alternatives.
Market challenges include high capital investment requirements for syngas and synthetic fuel facilities, technological barriers in achieving efficient CO2 conversion at scale, and competition from increasingly cost-effective renewable energy sources. The economic viability of these technologies remains heavily dependent on carbon pricing mechanisms and regulatory frameworks that incentivize carbon capture and utilization.
Electrocatalyst Development Status and Barriers
The current landscape of electrocatalysts for CO2 conversion reveals significant advancements alongside persistent challenges. Globally, research institutions and industrial players have achieved notable progress in developing catalysts with improved selectivity and efficiency. Metal-based catalysts, particularly copper and its alloys, demonstrate promising performance for CO2 reduction to syngas components and higher-value hydrocarbons. Recent breakthroughs in nanostructured materials have enabled enhanced surface area and active site exposure, contributing to improved catalytic activity.
Despite these advances, several critical barriers impede widespread implementation. Catalyst stability remains a primary concern, with many promising materials suffering from deactivation under industrial operating conditions. Long-term durability tests frequently reveal performance degradation after extended operation periods, particularly in the presence of contaminants commonly found in industrial CO2 streams. This degradation often manifests as structural changes, active site poisoning, or leaching of catalyst components.
Energy efficiency presents another significant challenge. Current electrocatalytic systems for CO2 conversion typically require high overpotentials, resulting in substantial energy losses. This inefficiency directly impacts the economic viability of these processes when scaled to industrial levels. The development of catalysts capable of operating at lower overpotentials while maintaining acceptable conversion rates and product selectivity remains an elusive goal.
Product selectivity continues to be problematic for many catalyst systems. The ability to precisely control reaction pathways to favor specific products (CO, CH4, C2+ hydrocarbons) at high Faradaic efficiencies is limited. Many catalysts produce a mixture of products, necessitating costly downstream separation processes that further reduce overall system efficiency.
Scalability issues present formidable barriers to commercialization. Laboratory-scale catalysts often demonstrate promising performance metrics that cannot be maintained when scaled to industrial dimensions. Challenges include maintaining uniform catalyst distribution, managing heat and mass transfer limitations, and ensuring consistent electrical contact across larger electrode surfaces.
The geographical distribution of research expertise shows concentration in North America, Europe, and East Asia, with China, the United States, and Germany leading in patent applications and research publications. This concentration creates potential knowledge gaps in other regions seeking to develop indigenous technologies for CO2 utilization.
Cost factors remain prohibitive for many advanced catalyst formulations. Noble metal catalysts and complex nanostructured materials often involve expensive precursors and sophisticated synthesis methods that limit commercial viability. The development of effective catalysts from earth-abundant materials represents a critical research direction for overcoming this barrier.
Despite these advances, several critical barriers impede widespread implementation. Catalyst stability remains a primary concern, with many promising materials suffering from deactivation under industrial operating conditions. Long-term durability tests frequently reveal performance degradation after extended operation periods, particularly in the presence of contaminants commonly found in industrial CO2 streams. This degradation often manifests as structural changes, active site poisoning, or leaching of catalyst components.
Energy efficiency presents another significant challenge. Current electrocatalytic systems for CO2 conversion typically require high overpotentials, resulting in substantial energy losses. This inefficiency directly impacts the economic viability of these processes when scaled to industrial levels. The development of catalysts capable of operating at lower overpotentials while maintaining acceptable conversion rates and product selectivity remains an elusive goal.
Product selectivity continues to be problematic for many catalyst systems. The ability to precisely control reaction pathways to favor specific products (CO, CH4, C2+ hydrocarbons) at high Faradaic efficiencies is limited. Many catalysts produce a mixture of products, necessitating costly downstream separation processes that further reduce overall system efficiency.
Scalability issues present formidable barriers to commercialization. Laboratory-scale catalysts often demonstrate promising performance metrics that cannot be maintained when scaled to industrial dimensions. Challenges include maintaining uniform catalyst distribution, managing heat and mass transfer limitations, and ensuring consistent electrical contact across larger electrode surfaces.
The geographical distribution of research expertise shows concentration in North America, Europe, and East Asia, with China, the United States, and Germany leading in patent applications and research publications. This concentration creates potential knowledge gaps in other regions seeking to develop indigenous technologies for CO2 utilization.
Cost factors remain prohibitive for many advanced catalyst formulations. Noble metal catalysts and complex nanostructured materials often involve expensive precursors and sophisticated synthesis methods that limit commercial viability. The development of effective catalysts from earth-abundant materials represents a critical research direction for overcoming this barrier.
Current Electrocatalytic Solutions for CO2 Conversion
01 Metal-based electrocatalysts for CO2 reduction
Metal-based catalysts, particularly those containing copper, silver, gold, or zinc, have shown promising performance in electrochemical CO2 reduction. These catalysts can be optimized through various structural modifications, such as nanostructuring, alloying, or surface modification to enhance their selectivity and efficiency. The catalytic activity depends on the binding energy of reaction intermediates, which can be tuned by controlling the electronic structure of the metal catalyst.- Metal-based electrocatalysts for CO2 reduction: Metal-based catalysts, particularly those containing copper, silver, gold, or zinc, have shown promising activity for electrochemical CO2 reduction. These catalysts can be optimized through various structural modifications, such as nanostructuring, alloying, or surface modification, to enhance their selectivity and efficiency for converting CO2 to valuable products like carbon monoxide, formate, or hydrocarbons. The catalyst structure significantly influences the binding energy of intermediates and thus the reaction pathways and product distribution.
- Carbon-based and composite electrocatalysts: Carbon-based materials, including graphene, carbon nanotubes, and nitrogen-doped carbon, serve as effective supports or active components in electrocatalysts for CO2 reduction. These materials can be combined with metal nanoparticles or metal oxides to create composite catalysts with enhanced activity and stability. The high surface area, electrical conductivity, and tunable surface chemistry of carbon-based materials contribute to improved electron transfer and increased active sites for CO2 conversion, resulting in higher conversion efficiencies.
- Reactor design and system optimization for CO2 conversion: The design of electrochemical reactors plays a crucial role in achieving high CO2 conversion efficiency. Innovations in reactor configuration, electrode arrangement, membrane technology, and electrolyte composition can significantly enhance mass transport, reduce energy losses, and improve overall system performance. Advanced reactor designs, such as flow cells, gas diffusion electrodes, and microfluidic systems, help overcome limitations related to CO2 solubility and mass transfer, leading to higher current densities and conversion rates.
- Process parameters and operating conditions optimization: The efficiency of electrochemical CO2 conversion is heavily influenced by operating parameters such as applied potential, current density, temperature, pressure, and electrolyte composition. Systematic optimization of these parameters can significantly enhance conversion efficiency and product selectivity. Advanced control strategies, including pulsed electrolysis and dynamic potential control, can further improve energy efficiency and reaction kinetics, leading to higher conversion rates and reduced energy consumption.
- Novel catalyst synthesis methods and performance enhancement techniques: Innovative synthesis methods, such as atomic layer deposition, electrodeposition, and sol-gel techniques, enable precise control over catalyst structure and composition. Post-synthesis treatments, including thermal annealing, plasma treatment, and surface functionalization, can further enhance catalyst performance. These approaches allow for the creation of catalysts with optimized electronic properties, increased active site density, and improved stability under reaction conditions, resulting in higher Faradaic efficiency and conversion rates for CO2 reduction.
02 Carbon-based and composite electrocatalysts
Carbon-based materials, including graphene, carbon nanotubes, and nitrogen-doped carbon, serve as effective supports or active components in CO2 reduction electrocatalysts. These materials can be combined with metal nanoparticles or metal oxides to create composite catalysts with enhanced performance. The high surface area, excellent conductivity, and tunable surface chemistry of carbon-based materials contribute to improved CO2 conversion efficiency and product selectivity.Expand Specific Solutions03 Reactor design and system optimization
The design of electrochemical reactors plays a crucial role in determining CO2 conversion efficiency. Factors such as electrode configuration, electrolyte composition, mass transport, and operating conditions (temperature, pressure, applied potential) significantly impact the performance of CO2 reduction systems. Advanced reactor designs, including flow cells, gas diffusion electrodes, and membrane electrode assemblies, can enhance mass transfer and reduce energy consumption, leading to improved conversion efficiency.Expand Specific Solutions04 Catalyst stability and durability enhancement
Improving the stability and durability of electrocatalysts is essential for practical CO2 conversion applications. Strategies include developing corrosion-resistant materials, preventing catalyst poisoning, and designing self-healing catalyst systems. Encapsulation techniques, protective coatings, and support materials that anchor active sites can significantly extend catalyst lifetime while maintaining high conversion efficiency over prolonged operation periods.Expand Specific Solutions05 Novel electrolyte systems and reaction media
The composition and properties of the electrolyte significantly influence CO2 reduction performance. Ionic liquids, deep eutectic solvents, and modified aqueous solutions can enhance CO2 solubility and mass transport while suppressing competing hydrogen evolution reactions. Additives such as organic promoters, pH buffers, and co-catalysts can modify the local reaction environment at the electrode surface, leading to improved faradaic efficiency and product selectivity in CO2 conversion processes.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalyst market for CO2 conversion in syngas generation and fuel synthesis is currently in a growth phase, with increasing commercial interest driven by global decarbonization efforts. The market size is expanding rapidly, projected to reach several billion dollars by 2030 as carbon utilization technologies gain traction. Technologically, the field shows varying maturity levels across different conversion pathways. Leading players include established energy giants like China Petroleum & Chemical Corp. (Sinopec) and SABIC, who leverage their extensive infrastructure and R&D capabilities, alongside specialized innovators such as Infinium Technology and Greyrock Energy focusing on novel catalyst designs. Research institutions like the Chinese Academy of Science and National Institute of Clean & Low Carbon Energy are advancing fundamental breakthroughs, while industrial players including BASF and Dow Global Technologies are scaling up promising technologies for commercial deployment.
Infinium Technology LLC
Technical Solution: Infinium Technology has developed an innovative electrocatalytic CO2 conversion platform specifically optimized for synthetic fuel production called "Electrofuels Technology." Their approach employs a two-stage process: first converting CO2 to syngas using proprietary silver-based catalysts in a specialized alkaline electrolyzer, then directing this syngas to a modified Fischer-Tropsch synthesis unit. The electrocatalysts achieve remarkable CO selectivity (>95%) at industrially relevant current densities (>200 mA/cm²) while operating at near-ambient conditions. Infinium's system incorporates advanced gas diffusion electrodes with hierarchical porosity that significantly enhances mass transport and reaction kinetics. Their integrated process can produce drop-in replacement fuels including diesel, jet fuel, and gasoline precursors with carbon conversion efficiencies exceeding 70% from CO2 input to final fuel products. The technology has been demonstrated at pilot scale (producing several barrels per day) and shows stable operation over thousands of hours with minimal performance degradation.
Strengths: End-to-end integration from CO2 capture to final fuel products creates a complete carbon utilization solution; demonstrated commercial-scale viability with existing pilot plants; produces drop-in replacement fuels compatible with existing infrastructure. Weaknesses: Process still requires significant electricity input, making economics heavily dependent on low-cost renewable electricity availability; system complexity requires specialized operational expertise.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced electrocatalytic systems for CO2 conversion focusing on syngas production. Their technology employs copper-based catalysts modified with zinc and aluminum oxides to achieve high faradaic efficiency (>85%) for CO reduction. The process operates at relatively low temperatures (40-60°C) and moderate pressures, utilizing a flow-cell reactor design that enhances mass transfer and reaction kinetics. Sinopec's approach integrates renewable electricity sources with their existing refinery infrastructure, creating a circular carbon economy model. Their catalysts demonstrate remarkable selectivity toward CO production with H2/CO ratios that can be tuned between 1:1 and 3:1 by adjusting potential and electrolyte composition, making the syngas suitable for direct Fischer-Tropsch synthesis. Recent developments include incorporating ionic liquid electrolytes to improve CO2 solubility and catalyst stability, extending operational lifetimes to over 1000 hours without significant degradation.
Strengths: Integration with existing petrochemical infrastructure provides immediate industrial scalability; tunable H2/CO ratios offer versatility for downstream applications; demonstrated long-term stability reduces operational costs. Weaknesses: Higher energy consumption compared to some competing technologies; catalyst production involves critical raw materials that may face supply constraints.
Key Innovations in Catalyst Design and Performance
System, apparatus, and method to create synthetic fuel
PatentPendingUS20250263848A1
Innovation
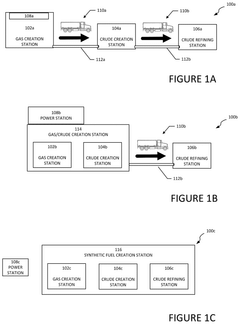
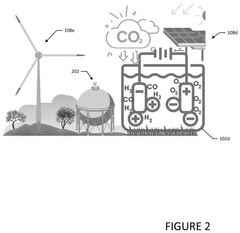
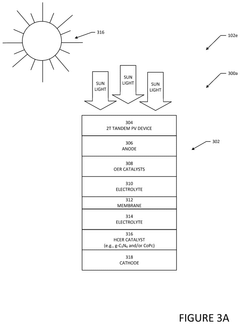
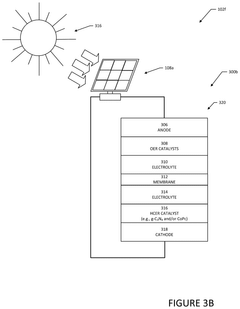
- Utilize PV-driven electrocatalysis systems, specifically PV-integrated and PV-divorced electrocatalysis systems, to convert water and CO2 into syngas, employing catalysts like graphitic carbon nitride (g-C3N4) and co-catalysts to enhance efficiency and durability, and control pH through pressurized CO2 to minimize external electrolyte addition.
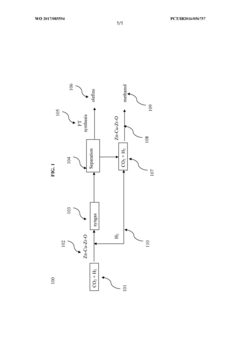
Process and catalyst for conversion of co2 to syngas for a simultaneous production of olefins and methanol
PatentWO2017085594A2
Innovation
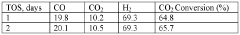
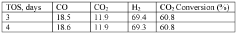
- A method using a Zn-Cu-Zr-O catalyst in a reaction chamber to convert H2 and CO2 into syngas, followed by separation and subsequent Fischer-Tropsch synthesis for olefins and methanol production, with the same catalyst facilitating both reactions at controlled temperatures and pressures.
Techno-economic Assessment of CO2 Conversion Processes
The techno-economic assessment of CO2 conversion processes reveals significant variations in economic viability across different technological pathways. Current electrochemical CO2 reduction technologies demonstrate production costs ranging from $0.75 to $2.50 per kilogram of syngas, depending on catalyst efficiency, electricity costs, and system scale. Notably, copper-based catalysts show promising economics for C2+ product formation, while silver and gold catalysts excel in CO production but face prohibitive material costs for large-scale deployment.
Capital expenditure for industrial-scale CO2 conversion facilities ranges between $500-1,500 per kilowatt of installed capacity, with electrolyzer systems representing 40-60% of total investment. Operational expenses are dominated by electricity costs (50-70%), followed by maintenance (15-20%) and catalyst replacement (10-15%). Renewable electricity integration can significantly improve the economic profile, potentially reducing operational costs by 20-30% when coupled with optimized load management systems.
Sensitivity analysis indicates that electricity price fluctuations create the most substantial economic risk, with a $0.01/kWh change potentially altering production costs by 8-12%. Catalyst durability presents another critical economic factor, as degradation rates exceeding 1% per 1000 operating hours can increase annual operational costs by 15-25% due to replacement requirements and efficiency losses.
The levelized cost of syngas production via CO2 electroreduction currently exceeds conventional fossil-based routes by 30-80%, presenting a significant commercialization barrier. However, projected technology improvements suggest potential cost parity by 2030-2035, assuming catalyst performance improvements of 2-3x current densities and 50% reductions in overpotential requirements.
Carbon pricing mechanisms significantly impact economic feasibility, with models indicating that carbon prices exceeding $50-70 per ton CO2 would make several electrochemical pathways competitive with conventional processes. Integration with existing industrial infrastructure, particularly cement and steel manufacturing, offers the most promising near-term deployment scenarios with potential internal rates of return exceeding 15% under favorable policy conditions.
Market analysis projects that CO2-derived syngas could capture 5-8% of the global syngas market by 2030, representing a $4-7 billion opportunity, with growth accelerating as technology matures and regulatory frameworks increasingly favor carbon-neutral production methods.
Capital expenditure for industrial-scale CO2 conversion facilities ranges between $500-1,500 per kilowatt of installed capacity, with electrolyzer systems representing 40-60% of total investment. Operational expenses are dominated by electricity costs (50-70%), followed by maintenance (15-20%) and catalyst replacement (10-15%). Renewable electricity integration can significantly improve the economic profile, potentially reducing operational costs by 20-30% when coupled with optimized load management systems.
Sensitivity analysis indicates that electricity price fluctuations create the most substantial economic risk, with a $0.01/kWh change potentially altering production costs by 8-12%. Catalyst durability presents another critical economic factor, as degradation rates exceeding 1% per 1000 operating hours can increase annual operational costs by 15-25% due to replacement requirements and efficiency losses.
The levelized cost of syngas production via CO2 electroreduction currently exceeds conventional fossil-based routes by 30-80%, presenting a significant commercialization barrier. However, projected technology improvements suggest potential cost parity by 2030-2035, assuming catalyst performance improvements of 2-3x current densities and 50% reductions in overpotential requirements.
Carbon pricing mechanisms significantly impact economic feasibility, with models indicating that carbon prices exceeding $50-70 per ton CO2 would make several electrochemical pathways competitive with conventional processes. Integration with existing industrial infrastructure, particularly cement and steel manufacturing, offers the most promising near-term deployment scenarios with potential internal rates of return exceeding 15% under favorable policy conditions.
Market analysis projects that CO2-derived syngas could capture 5-8% of the global syngas market by 2030, representing a $4-7 billion opportunity, with growth accelerating as technology matures and regulatory frameworks increasingly favor carbon-neutral production methods.
Sustainability Impact and Carbon Neutrality Implications
Electrocatalytic CO2 conversion technologies represent a critical pathway toward achieving global carbon neutrality goals. By transforming carbon dioxide into valuable syngas and synthetic fuels, these processes directly contribute to closing the carbon cycle and reducing net greenhouse gas emissions. The sustainability impact of these technologies extends beyond simple carbon capture, offering a regenerative approach that transforms waste CO2 into energy carriers and chemical feedstocks.
The carbon neutrality implications are particularly significant when considering the potential for creating a circular carbon economy. Electrocatalytic CO2 conversion powered by renewable electricity enables the production of carbon-neutral fuels that can replace fossil-derived alternatives in hard-to-decarbonize sectors such as aviation, shipping, and heavy industry. This approach effectively recycles atmospheric carbon rather than introducing additional fossil carbon into the active carbon cycle.
Life cycle assessments of electrocatalytic CO2 conversion systems demonstrate their potential to achieve substantial net carbon emission reductions compared to conventional fossil fuel production pathways. However, these benefits are heavily dependent on the source of electricity used to power the electrolysis process. When coupled with renewable energy sources, the carbon footprint can approach true neutrality, whereas grid electricity with high fossil fuel content may significantly diminish the climate benefits.
The scalability of these technologies presents both opportunities and challenges for sustainability. While laboratory demonstrations show promising conversion efficiencies, industrial-scale implementation requires careful consideration of catalyst durability, selectivity, and resource requirements. Particularly important is the avoidance of critical raw materials and rare earth elements in catalyst design, which could otherwise create new sustainability challenges through resource depletion and mining impacts.
Water consumption represents another important sustainability consideration, as electrocatalytic processes typically require ultrapure water. In water-stressed regions, this could create competition with other essential water uses. Integrated systems that recycle process water and utilize seawater or wastewater as feedstock are being developed to address this concern.
Beyond climate impacts, these technologies offer additional environmental co-benefits, including reduced air pollution compared to conventional fuel production and potential integration with industrial processes to capture and utilize point-source emissions. The economic sustainability dimension is equally important, with decreasing renewable electricity costs gradually improving the cost-competitiveness of electrocatalytic pathways against conventional fossil-based production methods.
The carbon neutrality implications are particularly significant when considering the potential for creating a circular carbon economy. Electrocatalytic CO2 conversion powered by renewable electricity enables the production of carbon-neutral fuels that can replace fossil-derived alternatives in hard-to-decarbonize sectors such as aviation, shipping, and heavy industry. This approach effectively recycles atmospheric carbon rather than introducing additional fossil carbon into the active carbon cycle.
Life cycle assessments of electrocatalytic CO2 conversion systems demonstrate their potential to achieve substantial net carbon emission reductions compared to conventional fossil fuel production pathways. However, these benefits are heavily dependent on the source of electricity used to power the electrolysis process. When coupled with renewable energy sources, the carbon footprint can approach true neutrality, whereas grid electricity with high fossil fuel content may significantly diminish the climate benefits.
The scalability of these technologies presents both opportunities and challenges for sustainability. While laboratory demonstrations show promising conversion efficiencies, industrial-scale implementation requires careful consideration of catalyst durability, selectivity, and resource requirements. Particularly important is the avoidance of critical raw materials and rare earth elements in catalyst design, which could otherwise create new sustainability challenges through resource depletion and mining impacts.
Water consumption represents another important sustainability consideration, as electrocatalytic processes typically require ultrapure water. In water-stressed regions, this could create competition with other essential water uses. Integrated systems that recycle process water and utilize seawater or wastewater as feedstock are being developed to address this concern.
Beyond climate impacts, these technologies offer additional environmental co-benefits, including reduced air pollution compared to conventional fuel production and potential integration with industrial processes to capture and utilize point-source emissions. The economic sustainability dimension is equally important, with decreasing renewable electricity costs gradually improving the cost-competitiveness of electrocatalytic pathways against conventional fossil-based production methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!