Electrocatalysts CO2 for industrial scale carbon utilization
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Conversion Electrocatalysis Background & Objectives
Carbon dioxide (CO2) conversion through electrocatalysis represents a pivotal technological frontier in addressing global climate challenges. This technology has evolved significantly over the past decades, transitioning from theoretical concepts to laboratory demonstrations and now moving toward industrial implementation. The electrocatalytic reduction of CO2 offers a sustainable pathway to transform this greenhouse gas into valuable chemical feedstocks and fuels, effectively closing the carbon cycle while potentially creating economic value.
The historical development of CO2 electrocatalysis can be traced back to the 1980s with pioneering work on metal electrodes. Significant breakthroughs occurred in the early 2000s with the development of more efficient catalysts and improved understanding of reaction mechanisms. Recent years have witnessed exponential growth in research activity, with particular focus on catalyst design, selectivity improvement, and system integration for practical applications.
Current technological trends indicate a shift toward nanostructured catalysts, bimetallic and multimetallic systems, and carbon-based materials with precisely engineered active sites. Additionally, there is growing interest in integrating CO2 electrolysis with renewable energy sources to create truly sustainable carbon utilization pathways. The coupling of CO2 reduction with hydrogen evolution or oxygen evolution reactions in unified systems represents another promising direction.
The primary technical objectives for industrial-scale implementation include developing electrocatalysts with significantly improved energy efficiency, achieving high product selectivity toward targeted chemicals, enhancing catalyst stability for continuous long-term operation, and designing scalable electrode architectures and reactor systems. Specifically, current research aims to reduce overpotentials below 200 mV, increase Faradaic efficiencies above 90%, extend catalyst lifetimes beyond 10,000 hours, and achieve current densities exceeding 200 mA/cm² for practical industrial deployment.
Beyond technical performance, objectives also encompass economic viability and environmental sustainability. This includes reducing capital and operational costs to make electrocatalytic CO2 conversion competitive with conventional production methods, minimizing the environmental footprint of catalyst materials, and ensuring compatibility with existing industrial infrastructure to facilitate adoption.
The ultimate goal is to establish CO2 electrocatalysis as a cornerstone technology in the circular carbon economy, capable of processing gigatons of CO2 annually while producing valuable chemicals and fuels at competitive costs. This would transform CO2 from an environmental liability into a valuable resource, supporting global decarbonization efforts while creating new economic opportunities in sustainable chemical manufacturing.
The historical development of CO2 electrocatalysis can be traced back to the 1980s with pioneering work on metal electrodes. Significant breakthroughs occurred in the early 2000s with the development of more efficient catalysts and improved understanding of reaction mechanisms. Recent years have witnessed exponential growth in research activity, with particular focus on catalyst design, selectivity improvement, and system integration for practical applications.
Current technological trends indicate a shift toward nanostructured catalysts, bimetallic and multimetallic systems, and carbon-based materials with precisely engineered active sites. Additionally, there is growing interest in integrating CO2 electrolysis with renewable energy sources to create truly sustainable carbon utilization pathways. The coupling of CO2 reduction with hydrogen evolution or oxygen evolution reactions in unified systems represents another promising direction.
The primary technical objectives for industrial-scale implementation include developing electrocatalysts with significantly improved energy efficiency, achieving high product selectivity toward targeted chemicals, enhancing catalyst stability for continuous long-term operation, and designing scalable electrode architectures and reactor systems. Specifically, current research aims to reduce overpotentials below 200 mV, increase Faradaic efficiencies above 90%, extend catalyst lifetimes beyond 10,000 hours, and achieve current densities exceeding 200 mA/cm² for practical industrial deployment.
Beyond technical performance, objectives also encompass economic viability and environmental sustainability. This includes reducing capital and operational costs to make electrocatalytic CO2 conversion competitive with conventional production methods, minimizing the environmental footprint of catalyst materials, and ensuring compatibility with existing industrial infrastructure to facilitate adoption.
The ultimate goal is to establish CO2 electrocatalysis as a cornerstone technology in the circular carbon economy, capable of processing gigatons of CO2 annually while producing valuable chemicals and fuels at competitive costs. This would transform CO2 from an environmental liability into a valuable resource, supporting global decarbonization efforts while creating new economic opportunities in sustainable chemical manufacturing.
Market Analysis for Industrial Carbon Utilization
The global market for carbon dioxide utilization technologies is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. The market for CO2 conversion technologies was valued at approximately $1.9 billion in 2020 and is projected to reach $5.7 billion by 2028, representing a compound annual growth rate (CAGR) of 14.8%. Electrocatalytic CO2 conversion specifically is expected to capture an increasing share of this market due to its potential for integration with renewable energy systems.
Industrial sectors including chemicals, fuels, building materials, and food and beverages represent the primary demand centers for converted CO2 products. The chemicals sector currently dominates, accounting for roughly 42% of the market share, with applications in methanol, formic acid, and syngas production. The fuels sector follows at 28%, driven by interest in carbon-neutral synthetic fuels for transportation and industrial applications.
Regionally, Europe leads in CO2 utilization market development with approximately 38% of global market share, supported by stringent carbon regulations and substantial government funding for decarbonization technologies. North America follows at 29%, with Asia-Pacific showing the fastest growth rate at 16.2% annually, primarily driven by China's aggressive carbon neutrality targets and industrial scaling initiatives.
Market demand is further bolstered by carbon pricing mechanisms, which have expanded to cover approximately 22% of global emissions in 2023. The average carbon price in established markets has increased to $35-60 per ton, improving the economic viability of industrial-scale CO2 conversion technologies. Industry analysts project that a carbon price of $75-100 per ton would make most electrocatalytic conversion processes economically competitive without additional subsidies.
End-user industries are increasingly willing to pay premium prices for carbon-neutral or carbon-negative products, with surveys indicating that 67% of industrial chemical buyers would accept a 5-15% price premium for demonstrably low-carbon alternatives. This market pull is complemented by regulatory push factors, including carbon border adjustment mechanisms and mandates for minimum recycled carbon content in certain product categories.
Investment in the sector has grown substantially, with venture capital and corporate investment in CO2 utilization technologies reaching $3.2 billion in 2022, a threefold increase from 2018 levels. Strategic partnerships between technology developers, industrial gas companies, and end-users have become increasingly common, accelerating the commercialization pathway for promising electrocatalytic technologies.
Industrial sectors including chemicals, fuels, building materials, and food and beverages represent the primary demand centers for converted CO2 products. The chemicals sector currently dominates, accounting for roughly 42% of the market share, with applications in methanol, formic acid, and syngas production. The fuels sector follows at 28%, driven by interest in carbon-neutral synthetic fuels for transportation and industrial applications.
Regionally, Europe leads in CO2 utilization market development with approximately 38% of global market share, supported by stringent carbon regulations and substantial government funding for decarbonization technologies. North America follows at 29%, with Asia-Pacific showing the fastest growth rate at 16.2% annually, primarily driven by China's aggressive carbon neutrality targets and industrial scaling initiatives.
Market demand is further bolstered by carbon pricing mechanisms, which have expanded to cover approximately 22% of global emissions in 2023. The average carbon price in established markets has increased to $35-60 per ton, improving the economic viability of industrial-scale CO2 conversion technologies. Industry analysts project that a carbon price of $75-100 per ton would make most electrocatalytic conversion processes economically competitive without additional subsidies.
End-user industries are increasingly willing to pay premium prices for carbon-neutral or carbon-negative products, with surveys indicating that 67% of industrial chemical buyers would accept a 5-15% price premium for demonstrably low-carbon alternatives. This market pull is complemented by regulatory push factors, including carbon border adjustment mechanisms and mandates for minimum recycled carbon content in certain product categories.
Investment in the sector has grown substantially, with venture capital and corporate investment in CO2 utilization technologies reaching $3.2 billion in 2022, a threefold increase from 2018 levels. Strategic partnerships between technology developers, industrial gas companies, and end-users have become increasingly common, accelerating the commercialization pathway for promising electrocatalytic technologies.
Current Electrocatalyst Technologies & Barriers
Current electrocatalytic technologies for CO2 conversion have made significant progress but still face substantial barriers for industrial-scale implementation. Metal-based catalysts represent the most extensively studied category, with copper catalysts demonstrating unique capabilities for C-C coupling to produce valuable multi-carbon products. Noble metals like gold and silver excel in CO production, while zinc and tin catalysts favor formate generation. Recent advances in bimetallic and alloy catalysts have shown promising synergistic effects that enhance selectivity and activity beyond single-metal systems.
Molecular catalysts, particularly metal complexes with nitrogen-containing ligands, offer precise control over reaction pathways and product selectivity. These homogeneous catalysts provide valuable mechanistic insights but face challenges in stability and recyclability for industrial applications. Meanwhile, carbon-based catalysts including nitrogen-doped carbon materials and carbon nanotubes have emerged as cost-effective alternatives with tunable properties.
Despite these advances, several critical barriers impede industrial implementation. Low energy efficiency remains a fundamental challenge, with most systems operating at energy efficiencies below 50%, making the process economically unviable at scale. Current density limitations (typically <200 mA/cm²) fall significantly short of the >500 mA/cm² required for industrial viability, while product selectivity rarely exceeds 90% for target compounds.
Catalyst stability presents another major hurdle, with performance degradation occurring over relatively short timeframes (hours to days) compared to the months or years needed for industrial operations. The complex reaction environment, including pH fluctuations and poisoning species, accelerates catalyst deactivation. Additionally, most research focuses on laboratory-scale systems using pure CO2 feeds, whereas industrial applications must handle impure gas streams with varying compositions.
Scale-up challenges are particularly pronounced, as promising lab-scale catalysts often perform poorly when integrated into larger systems. The transition from idealized laboratory conditions to practical industrial environments introduces mass transport limitations, heat management issues, and electrode fabrication complexities that significantly impact performance metrics.
Economic barriers further complicate adoption, with current electrocatalytic CO2 conversion costs exceeding $100/ton of CO2 processed, far above the economically viable threshold of $50/ton. High capital costs for specialized electrodes and membranes, coupled with substantial energy inputs, create unfavorable economics compared to conventional chemical processes.
Molecular catalysts, particularly metal complexes with nitrogen-containing ligands, offer precise control over reaction pathways and product selectivity. These homogeneous catalysts provide valuable mechanistic insights but face challenges in stability and recyclability for industrial applications. Meanwhile, carbon-based catalysts including nitrogen-doped carbon materials and carbon nanotubes have emerged as cost-effective alternatives with tunable properties.
Despite these advances, several critical barriers impede industrial implementation. Low energy efficiency remains a fundamental challenge, with most systems operating at energy efficiencies below 50%, making the process economically unviable at scale. Current density limitations (typically <200 mA/cm²) fall significantly short of the >500 mA/cm² required for industrial viability, while product selectivity rarely exceeds 90% for target compounds.
Catalyst stability presents another major hurdle, with performance degradation occurring over relatively short timeframes (hours to days) compared to the months or years needed for industrial operations. The complex reaction environment, including pH fluctuations and poisoning species, accelerates catalyst deactivation. Additionally, most research focuses on laboratory-scale systems using pure CO2 feeds, whereas industrial applications must handle impure gas streams with varying compositions.
Scale-up challenges are particularly pronounced, as promising lab-scale catalysts often perform poorly when integrated into larger systems. The transition from idealized laboratory conditions to practical industrial environments introduces mass transport limitations, heat management issues, and electrode fabrication complexities that significantly impact performance metrics.
Economic barriers further complicate adoption, with current electrocatalytic CO2 conversion costs exceeding $100/ton of CO2 processed, far above the economically viable threshold of $50/ton. High capital costs for specialized electrodes and membranes, coupled with substantial energy inputs, create unfavorable economics compared to conventional chemical processes.
State-of-the-Art CO2 Conversion Solutions
01 Metal-based electrocatalysts for CO2 reduction
Metal-based catalysts are widely used for electrochemical CO2 reduction due to their high activity and selectivity. Various metals such as copper, silver, gold, and zinc exhibit different product selectivity in CO2 conversion. These catalysts can be optimized through nanostructuring, alloying, or surface modification to enhance their performance in converting CO2 to valuable products like carbon monoxide, formate, or hydrocarbons.- Metal-based electrocatalysts for CO2 reduction: Metal-based catalysts, particularly transition metals like copper, silver, gold, and zinc, are widely used for electrochemical CO2 reduction. These metals can be engineered with specific morphologies, crystal facets, and surface structures to enhance catalytic activity and selectivity toward valuable products such as carbon monoxide, formate, or hydrocarbons. The performance of these catalysts can be further improved through alloying, surface modification, or incorporation into support materials.
- Carbon-based nanomaterials as electrocatalysts: Carbon-based nanomaterials including graphene, carbon nanotubes, and nitrogen-doped carbon structures serve as effective electrocatalysts for CO2 conversion. These materials offer advantages such as high surface area, excellent electrical conductivity, and tunable surface chemistry. Heteroatom doping, particularly with nitrogen, boron, or sulfur, creates active sites that can significantly enhance catalytic performance for CO2 reduction to valuable products while maintaining good stability during long-term operation.
- Metal-organic frameworks and hybrid catalysts: Metal-organic frameworks (MOFs) and hybrid catalyst systems combine the benefits of both metallic and organic components for efficient CO2 electroreduction. These structures feature well-defined active sites, high porosity, and tunable electronic properties. MOF-derived catalysts, where the framework serves as a precursor for metal/carbon composites, have shown exceptional activity and selectivity. Hybrid systems often incorporate multiple catalytic components that work synergistically to overcome limitations of single-component catalysts.
- Nanostructured and bimetallic electrocatalysts: Nanostructured and bimetallic catalysts offer enhanced performance for CO2 electroreduction through increased active surface area and synergistic effects between different metals. These catalysts can be designed with specific architectures such as core-shell structures, nanowires, nanosheets, or porous frameworks to maximize exposure of active sites. Bimetallic combinations allow for electronic modulation and optimized binding energies of reaction intermediates, leading to improved selectivity toward target products and reduced overpotentials.
- Catalyst support materials and reactor design: Support materials and reactor design play crucial roles in enhancing the performance of electrocatalysts for CO2 conversion. Supports such as carbon materials, metal oxides, or conductive polymers can improve catalyst dispersion, stability, and electron transfer properties. Advanced reactor designs, including flow cells, gas diffusion electrodes, and microfluidic systems, address mass transport limitations and improve CO2 solubility at the catalyst interface. These engineering approaches, combined with optimized electrolytes and operating conditions, significantly enhance conversion efficiency and product selectivity.
02 Carbon-based and composite electrocatalysts
Carbon-based materials including graphene, carbon nanotubes, and nitrogen-doped carbons serve as effective electrocatalysts for CO2 reduction. These materials can be combined with metal nanoparticles to create composite catalysts with enhanced activity and stability. The carbon support provides high surface area and electrical conductivity while the metal components offer active sites for CO2 adsorption and conversion, resulting in improved catalytic performance.Expand Specific Solutions03 Ionic liquid and electrolyte systems for CO2 electroreduction
Specialized electrolyte systems including ionic liquids can significantly enhance CO2 electroreduction efficiency. These systems improve CO2 solubility, stabilize reaction intermediates, and modify the local environment at the electrode surface. By carefully selecting electrolyte composition, researchers can control reaction pathways, suppress competing hydrogen evolution reactions, and improve the selectivity toward desired carbon products.Expand Specific Solutions04 Bimetallic and alloy catalysts for selective CO2 conversion
Bimetallic and alloy catalysts offer superior performance in CO2 electroreduction compared to single metal catalysts. By combining two or more metals with complementary properties, these catalysts can achieve higher activity, selectivity, and stability. The synergistic effects between different metal components allow for tuning the binding energies of reaction intermediates, leading to improved product selectivity and energy efficiency in the conversion process.Expand Specific Solutions05 Novel reactor designs and process integration for CO2 electroreduction
Advanced reactor designs and integrated systems are being developed to overcome mass transport limitations and improve the overall efficiency of CO2 electroreduction. These innovations include gas diffusion electrodes, flow cells, and membrane electrode assemblies that enhance CO2 delivery to catalyst surfaces. Integrated approaches combining electrochemical CO2 reduction with renewable energy sources or downstream product separation can improve the economic viability and sustainability of the conversion process.Expand Specific Solutions
Leading Companies & Research Institutions
The CO2 electrocatalysis market is in a growth phase, with increasing industrial interest driven by carbon neutrality goals. The market size is expanding rapidly, projected to reach significant scale as carbon utilization becomes essential for emissions reduction strategies. Technologically, the field shows varying maturity levels across different approaches. Leading players include Dioxide Materials, which specializes in CO2 electrolyzer systems and materials, while established corporations like Siemens Energy, TotalEnergies, and Honda Motor are investing in scalable solutions. Research institutions such as CNRS, Chinese Academy of Sciences, and various universities are advancing fundamental catalytic processes. The competitive landscape features collaboration between academic institutions and industrial partners, with increasing patent activity indicating technology commercialization efforts focused on efficiency improvements and cost reduction for industrial implementation.
Dioxide Materials, Inc.
Technical Solution: Dioxide Materials has developed advanced electrochemical CO2 conversion systems using their proprietary Sustainion® anion exchange membranes and ionomers. Their technology focuses on the electrochemical reduction of CO2 to carbon monoxide and other value-added chemicals through specially designed catalysts. The company has created high-performance electrolyzer stacks that can operate at industrial current densities (>200 mA/cm²) with high energy efficiency. Their approach combines novel catalyst materials with innovative cell designs to overcome traditional limitations in CO2 conversion. Dioxide Materials' systems can achieve Faradaic efficiencies exceeding 95% for CO production, with demonstrated durability over thousands of hours of continuous operation[1]. The company has scaled their technology from laboratory prototypes to commercial-scale units capable of processing tons of CO2 annually, positioning them as leaders in the industrial implementation of CO2 electrocatalysis.
Strengths: Proprietary membrane technology enables higher current densities and selectivity than competitors; established commercial-scale implementation pathway; demonstrated long-term stability. Weaknesses: Higher capital costs compared to traditional carbon utilization methods; requires integration with renewable electricity sources to achieve carbon neutrality; limited product portfolio currently focused primarily on CO production.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has pioneered several breakthrough electrocatalysts for CO2 reduction, focusing on atomic-level catalyst design and in-situ characterization techniques. Their research teams have developed single-atom catalysts dispersed on nitrogen-doped carbon supports that demonstrate exceptional selectivity toward C2+ products. These catalysts feature precisely engineered coordination environments that stabilize key reaction intermediates, lowering activation barriers for C-C coupling. The Institute has also made significant advances in bimetallic catalysts that synergistically enhance CO2 activation and subsequent carbon chain growth. Their work includes innovative approaches to catalyst structure-function relationships, utilizing operando spectroscopy to reveal reaction mechanisms under realistic conditions[2]. Recent developments include hierarchically structured catalysts with optimized mass transport properties that maintain high performance at industrial current densities (>300 mA/cm²) while achieving Faradaic efficiencies above 90% for target products.
Strengths: World-leading fundamental research capabilities; sophisticated characterization techniques for mechanistic understanding; strong focus on catalyst stability and scalability. Weaknesses: Some technologies remain at laboratory scale; challenges in translating academic research to industrial implementation; requires further engineering development for full-scale deployment.
Key Patents & Scientific Breakthroughs
Electrocatalytic Process for Carbon Dioxide Conversion
PatentActiveUS20190211463A1
Innovation
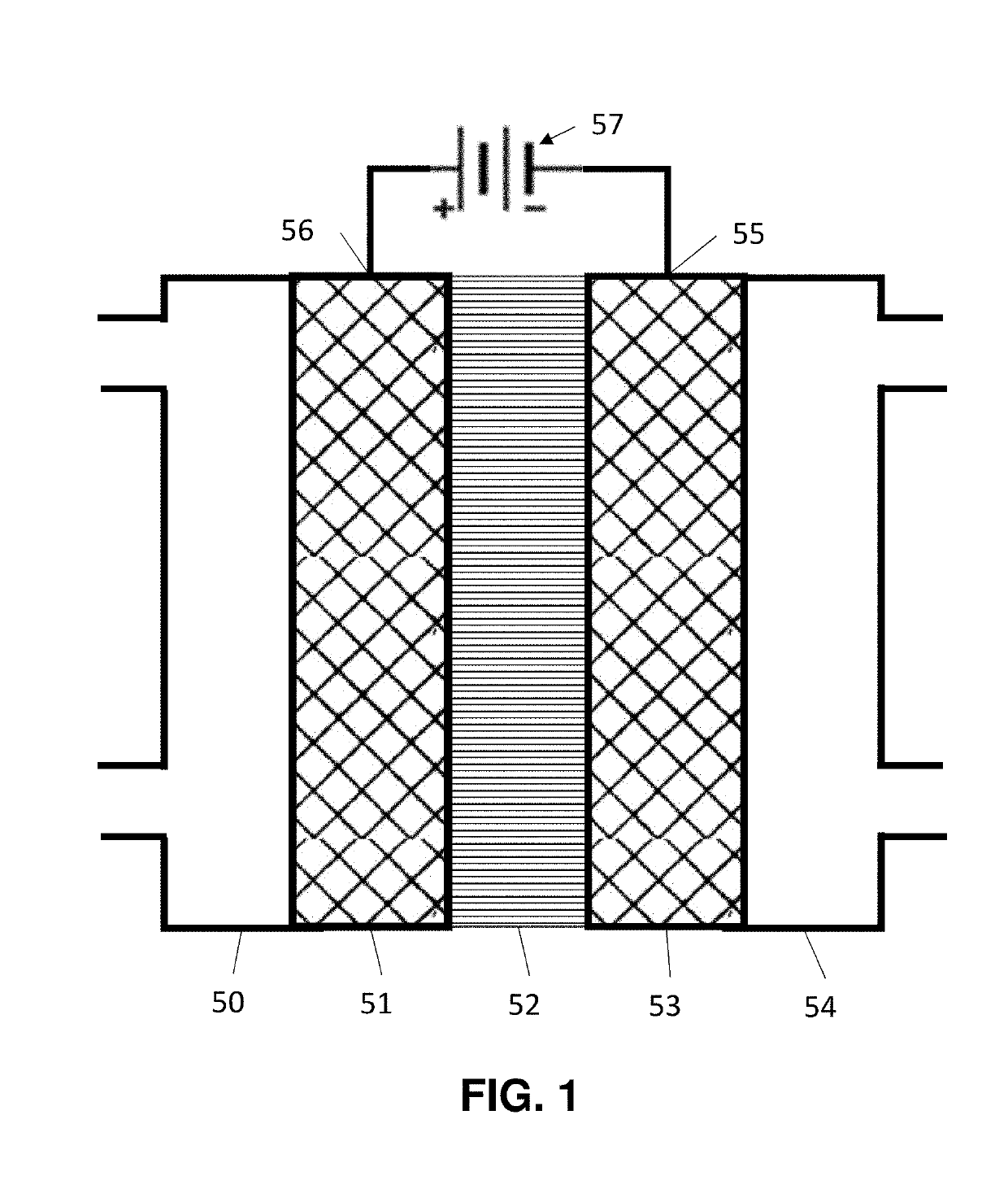
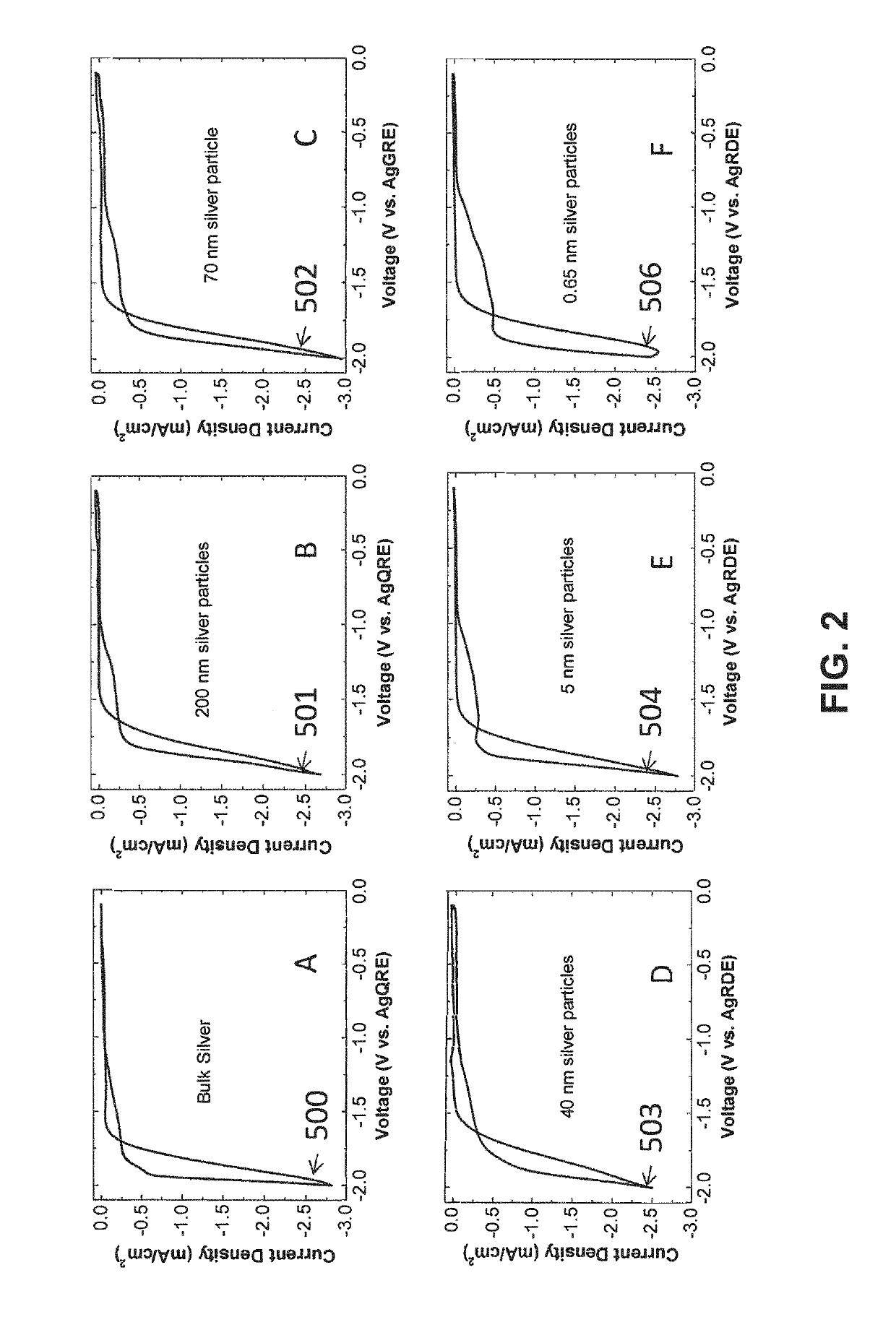
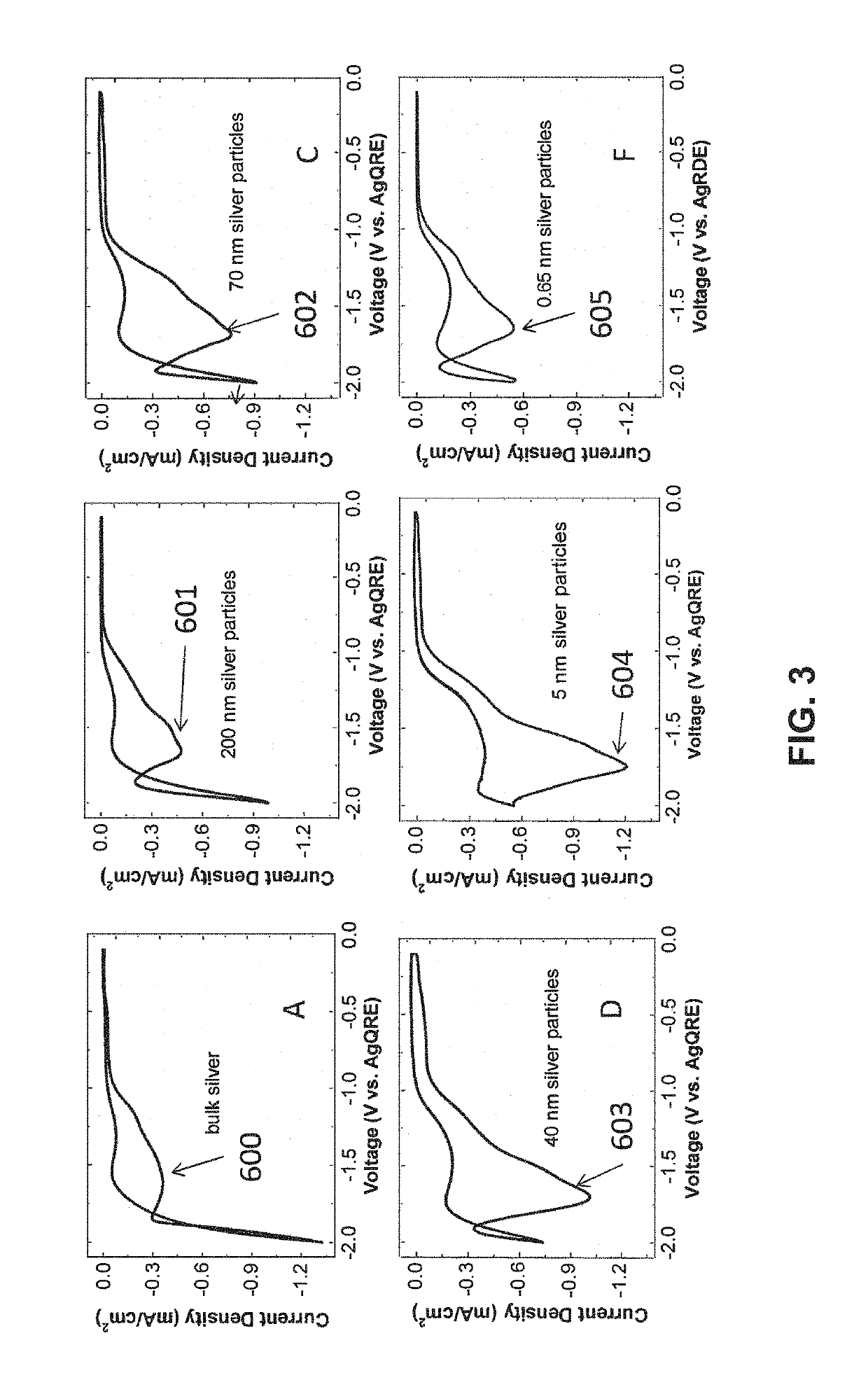
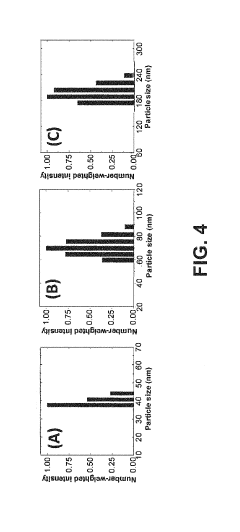
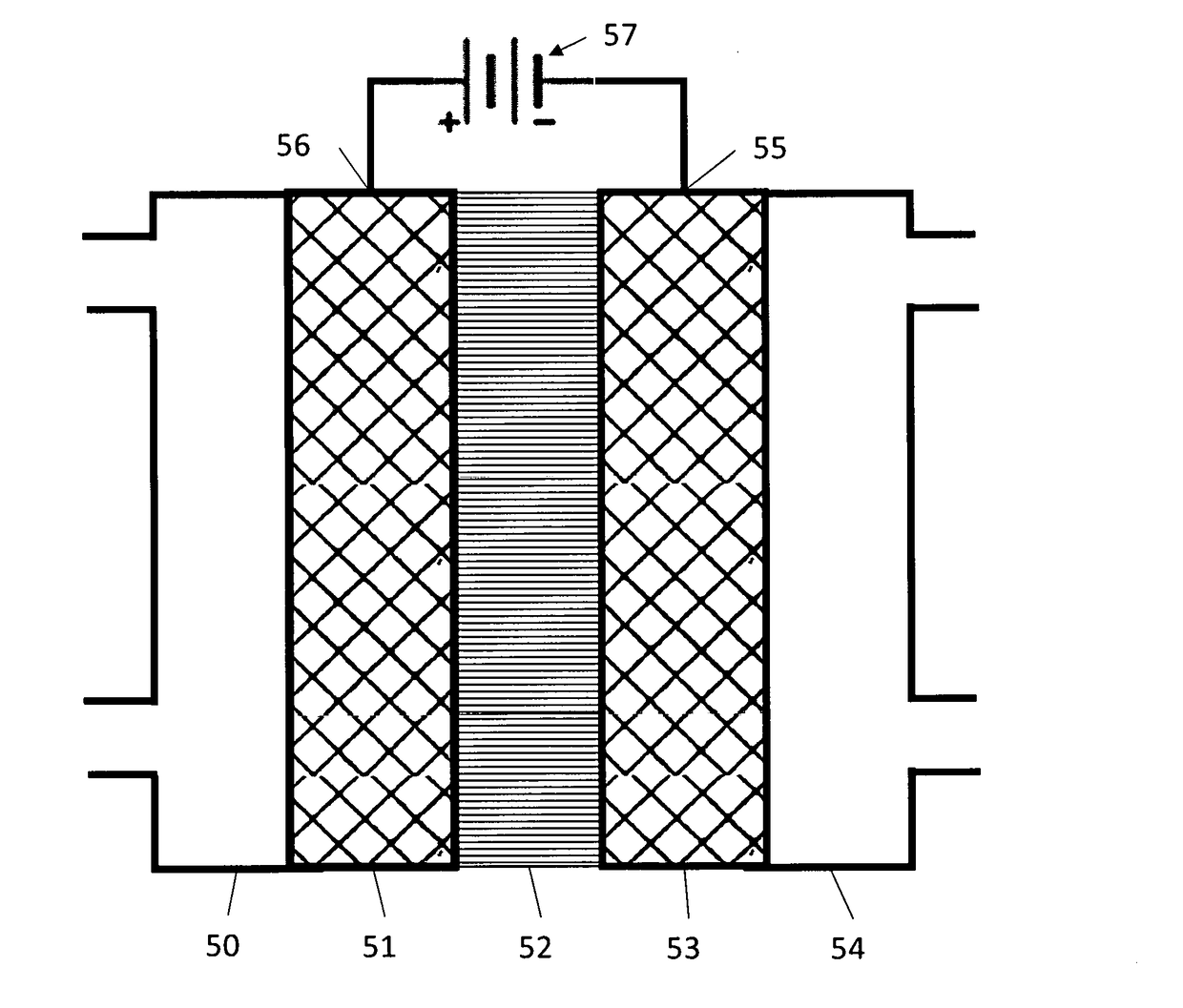
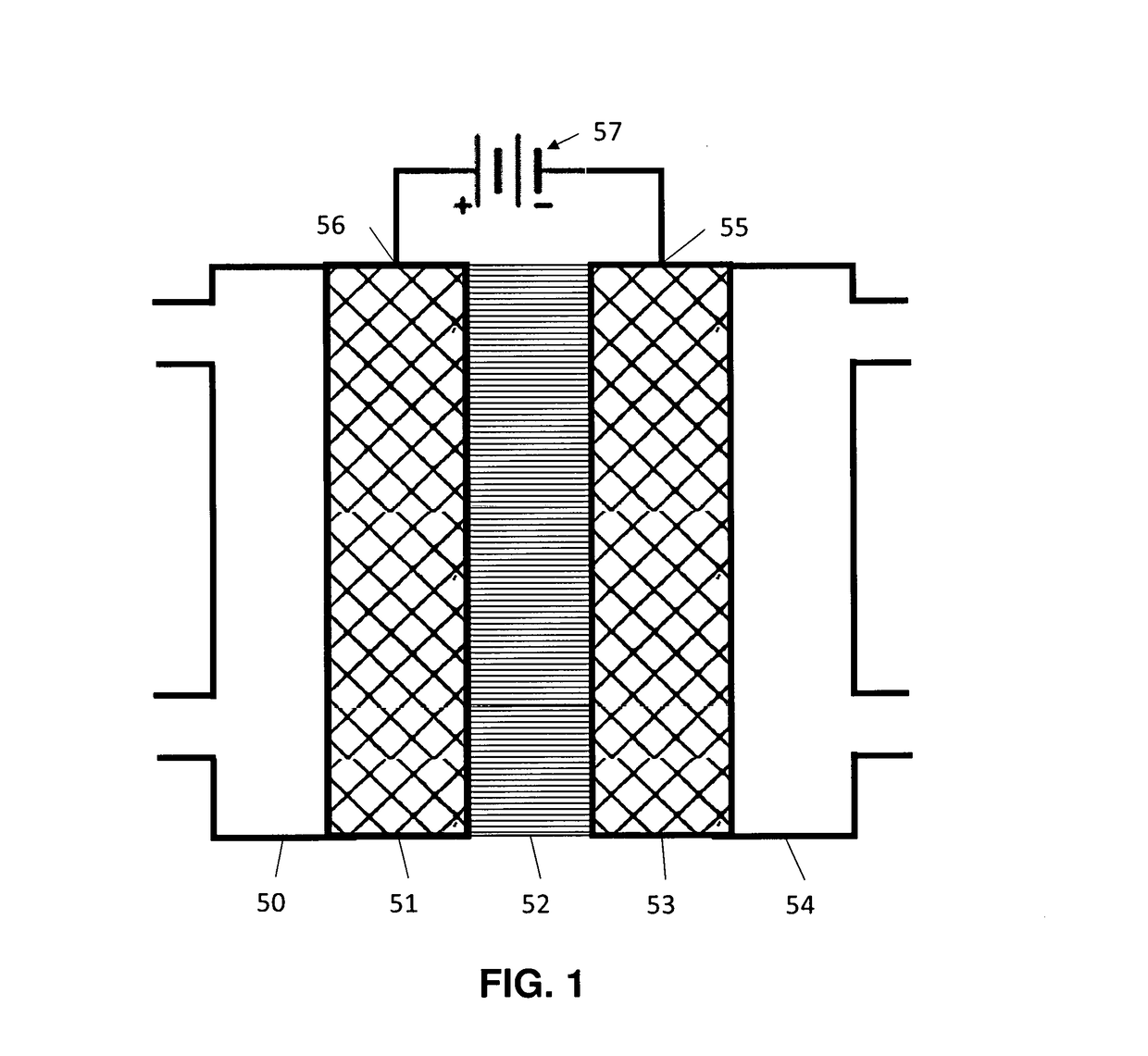
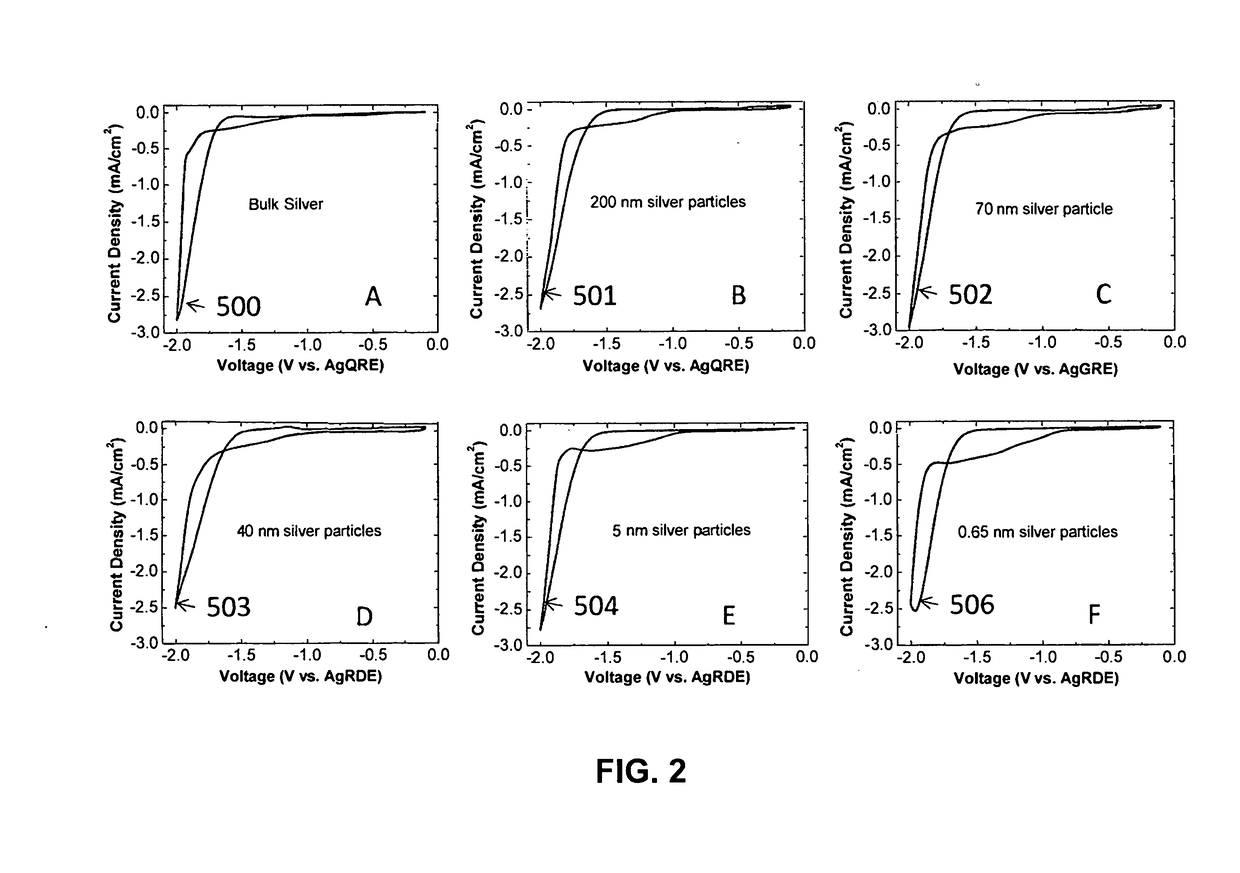
- A novel catalyst combination featuring a Catalytically Active Element with supported or unsupported particles of specific sizes, combined with a Helper Polymer containing positively charged cyclic amine groups, is used in the cathode catalyst layer of an electrochemical cell to enhance CO2 conversion efficiency and reduce power requirements.
Devices For Electrocatalytic Conversion Of Carbon Dioxide
PatentActiveUS20180111083A1
Innovation
- A novel catalyst combination featuring a Catalytically Active Element with a particle size between 0.6 nm and 100 nm, supported or unsupported, paired with a Helper Polymer containing positively charged cyclic amine groups, enhances the rate and efficiency of CO2 conversion reactions in electrochemical cells.
Techno-economic Assessment
The techno-economic assessment of CO2 electrocatalytic conversion technologies reveals significant challenges for industrial-scale implementation despite promising laboratory results. Current capital expenditure (CAPEX) for electrolyzer systems ranges from $800-1,500/kW, substantially higher than the $200-300/kW target needed for commercial viability. Operating expenses (OPEX) are dominated by electricity costs, representing 60-70% of total operational expenditure, with current consumption averaging 6-8 kWh per kg of CO2 converted.
Energy efficiency remains a critical barrier, with most systems achieving only 40-60% Faradaic efficiency at industrially relevant current densities. This translates to high energy costs of $0.15-0.25 per kg of product, compared to conventional production methods at $0.08-0.12 per kg. The economic viability threshold appears to be around 80% Faradaic efficiency combined with current densities exceeding 200 mA/cm² - parameters rarely achieved simultaneously in existing systems.
Infrastructure requirements present additional economic challenges. Industrial implementation demands significant modifications to existing facilities, including integration with renewable energy sources to maximize carbon reduction benefits. Initial assessments indicate infrastructure adaptation costs of $2-4 million for medium-scale facilities, with payback periods currently exceeding 8-10 years without policy incentives.
Product value streams show promising diversity but uncertain market stability. High-value chemicals like ethylene and ethanol ($800-1,200/ton) offer better economic returns than simpler products like carbon monoxide or formic acid ($300-500/ton). However, market volatility for these products introduces significant risk factors for long-term investment planning.
Policy support mechanisms significantly impact economic feasibility. Carbon pricing mechanisms of at least $50-70/ton CO2 would be required to make most electrocatalytic processes competitive with conventional routes. Current carbon prices in most markets remain below this threshold, though projections suggest they may reach these levels by 2030 in advanced economies.
Sensitivity analysis indicates that electricity price reductions to below $0.04/kWh, combined with catalyst improvements extending operational lifetimes to >20,000 hours, would dramatically improve economic viability. Under these conditions, levelized cost of production could decrease by 30-40%, potentially reaching parity with conventional processes for select product streams by 2028-2030.
Energy efficiency remains a critical barrier, with most systems achieving only 40-60% Faradaic efficiency at industrially relevant current densities. This translates to high energy costs of $0.15-0.25 per kg of product, compared to conventional production methods at $0.08-0.12 per kg. The economic viability threshold appears to be around 80% Faradaic efficiency combined with current densities exceeding 200 mA/cm² - parameters rarely achieved simultaneously in existing systems.
Infrastructure requirements present additional economic challenges. Industrial implementation demands significant modifications to existing facilities, including integration with renewable energy sources to maximize carbon reduction benefits. Initial assessments indicate infrastructure adaptation costs of $2-4 million for medium-scale facilities, with payback periods currently exceeding 8-10 years without policy incentives.
Product value streams show promising diversity but uncertain market stability. High-value chemicals like ethylene and ethanol ($800-1,200/ton) offer better economic returns than simpler products like carbon monoxide or formic acid ($300-500/ton). However, market volatility for these products introduces significant risk factors for long-term investment planning.
Policy support mechanisms significantly impact economic feasibility. Carbon pricing mechanisms of at least $50-70/ton CO2 would be required to make most electrocatalytic processes competitive with conventional routes. Current carbon prices in most markets remain below this threshold, though projections suggest they may reach these levels by 2030 in advanced economies.
Sensitivity analysis indicates that electricity price reductions to below $0.04/kWh, combined with catalyst improvements extending operational lifetimes to >20,000 hours, would dramatically improve economic viability. Under these conditions, levelized cost of production could decrease by 30-40%, potentially reaching parity with conventional processes for select product streams by 2028-2030.
Regulatory & Environmental Impact
The regulatory landscape for CO2 conversion technologies is rapidly evolving as governments worldwide implement policies to address climate change. The European Union's Carbon Border Adjustment Mechanism (CBAM) and Emissions Trading System (ETS) create economic incentives for industrial carbon capture and utilization, while the United States' 45Q tax credits offer up to $85 per metric ton for captured carbon used in industrial processes. These frameworks are driving industrial adoption of electrocatalytic CO2 conversion technologies.
Environmental regulations increasingly mandate emissions reductions across industrial sectors, with many jurisdictions establishing carbon neutrality targets between 2030-2050. This regulatory pressure creates both compliance requirements and market opportunities for electrocatalyst technologies that can convert waste CO2 into valuable products. Companies implementing such solutions may qualify for regulatory credits, tax incentives, and improved environmental compliance standings.
The environmental impact assessment of electrocatalytic CO2 conversion must consider the full lifecycle analysis. While these technologies offer significant carbon reduction potential, their environmental footprint depends on electricity sources, catalyst materials, and manufacturing processes. Electrocatalysts powered by renewable energy demonstrate substantially better environmental performance than those relying on fossil fuel electricity. Additionally, the toxicity and scarcity of catalyst materials (particularly precious metals) present environmental trade-offs that must be carefully evaluated.
Standardization efforts for measuring and verifying CO2 conversion efficiency and environmental benefits are still developing. Organizations like ISO and ASTM are working to establish protocols for quantifying carbon utilization impacts, which will be crucial for regulatory compliance and accessing incentive programs. The lack of universally accepted standards currently creates market uncertainty and complicates cross-border implementation.
Regional regulatory differences significantly impact technology deployment strategies. China's focus on industrial decarbonization through its national emissions trading scheme creates different market conditions than the EU's more consumer-facing approach. Companies developing electrocatalyst technologies must navigate these varying regulatory environments, often requiring customized deployment strategies for different markets to maximize both environmental benefits and economic returns.
Environmental regulations increasingly mandate emissions reductions across industrial sectors, with many jurisdictions establishing carbon neutrality targets between 2030-2050. This regulatory pressure creates both compliance requirements and market opportunities for electrocatalyst technologies that can convert waste CO2 into valuable products. Companies implementing such solutions may qualify for regulatory credits, tax incentives, and improved environmental compliance standings.
The environmental impact assessment of electrocatalytic CO2 conversion must consider the full lifecycle analysis. While these technologies offer significant carbon reduction potential, their environmental footprint depends on electricity sources, catalyst materials, and manufacturing processes. Electrocatalysts powered by renewable energy demonstrate substantially better environmental performance than those relying on fossil fuel electricity. Additionally, the toxicity and scarcity of catalyst materials (particularly precious metals) present environmental trade-offs that must be carefully evaluated.
Standardization efforts for measuring and verifying CO2 conversion efficiency and environmental benefits are still developing. Organizations like ISO and ASTM are working to establish protocols for quantifying carbon utilization impacts, which will be crucial for regulatory compliance and accessing incentive programs. The lack of universally accepted standards currently creates market uncertainty and complicates cross-border implementation.
Regional regulatory differences significantly impact technology deployment strategies. China's focus on industrial decarbonization through its national emissions trading scheme creates different market conditions than the EU's more consumer-facing approach. Companies developing electrocatalyst technologies must navigate these varying regulatory environments, often requiring customized deployment strategies for different markets to maximize both environmental benefits and economic returns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!