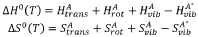Electrocatalysts CO2 for decentralized renewable energy storage
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Conversion Electrocatalysis Background and Objectives
Carbon dioxide (CO2) conversion through electrocatalysis represents a critical frontier in sustainable energy technologies, offering a dual solution to climate change mitigation and renewable energy storage challenges. The evolution of this technology can be traced back to the early 1980s when Honda and Fujishima demonstrated photocatalytic CO2 reduction. However, significant advancements in electrocatalytic CO2 conversion have primarily emerged in the past decade, driven by urgent climate concerns and the increasing penetration of intermittent renewable energy sources.
The technological trajectory has evolved from simple metal electrodes to sophisticated nanostructured catalysts with precisely engineered active sites. Early research focused on copper-based catalysts due to their unique ability to generate multi-carbon products. Recent developments have expanded to include bimetallic systems, metal-organic frameworks, and carbon-based materials that offer improved selectivity and efficiency.
Current global research is increasingly oriented toward addressing the fundamental challenge of CO2 activation—breaking the stable C=O bonds—while maintaining high energy efficiency and product selectivity. The field is witnessing a convergence of materials science, electrochemistry, and computational modeling to design next-generation catalysts with atomic-level precision.
The primary technical objective of this research domain is to develop electrocatalysts capable of converting CO2 into value-added chemicals and fuels with high Faradaic efficiency (>90%), significant energy efficiency (>60%), and extended operational stability (>1000 hours) under industrially relevant conditions. These parameters are essential for practical implementation in decentralized renewable energy storage systems.
Secondary objectives include reducing catalyst costs by minimizing or eliminating precious metal content, enhancing catalyst tolerance to common impurities in industrial CO2 streams, and developing integrated systems that combine CO2 capture and conversion processes. The ultimate goal is to create a technological framework that enables local renewable energy producers to store excess electricity as chemical energy while simultaneously reducing atmospheric CO2 concentrations.
The technological advancement in this field aligns with broader energy transition goals, particularly the need for long-duration energy storage solutions that can address seasonal variations in renewable energy production. By converting CO2 to fuels using renewable electricity during periods of excess generation, this technology could provide a crucial missing link in fully decarbonized energy systems, enabling effective utilization of intermittent renewable resources while creating a circular carbon economy.
The technological trajectory has evolved from simple metal electrodes to sophisticated nanostructured catalysts with precisely engineered active sites. Early research focused on copper-based catalysts due to their unique ability to generate multi-carbon products. Recent developments have expanded to include bimetallic systems, metal-organic frameworks, and carbon-based materials that offer improved selectivity and efficiency.
Current global research is increasingly oriented toward addressing the fundamental challenge of CO2 activation—breaking the stable C=O bonds—while maintaining high energy efficiency and product selectivity. The field is witnessing a convergence of materials science, electrochemistry, and computational modeling to design next-generation catalysts with atomic-level precision.
The primary technical objective of this research domain is to develop electrocatalysts capable of converting CO2 into value-added chemicals and fuels with high Faradaic efficiency (>90%), significant energy efficiency (>60%), and extended operational stability (>1000 hours) under industrially relevant conditions. These parameters are essential for practical implementation in decentralized renewable energy storage systems.
Secondary objectives include reducing catalyst costs by minimizing or eliminating precious metal content, enhancing catalyst tolerance to common impurities in industrial CO2 streams, and developing integrated systems that combine CO2 capture and conversion processes. The ultimate goal is to create a technological framework that enables local renewable energy producers to store excess electricity as chemical energy while simultaneously reducing atmospheric CO2 concentrations.
The technological advancement in this field aligns with broader energy transition goals, particularly the need for long-duration energy storage solutions that can address seasonal variations in renewable energy production. By converting CO2 to fuels using renewable electricity during periods of excess generation, this technology could provide a crucial missing link in fully decarbonized energy systems, enabling effective utilization of intermittent renewable resources while creating a circular carbon economy.
Market Analysis for Renewable Energy Storage Solutions
The global market for renewable energy storage solutions is experiencing unprecedented growth, driven by the increasing adoption of intermittent renewable energy sources such as solar and wind. The market was valued at approximately $184 billion in 2022 and is projected to reach $413 billion by 2030, representing a compound annual growth rate (CAGR) of 10.7%. This growth trajectory is supported by declining costs of renewable technologies, favorable government policies, and increasing corporate commitments to carbon neutrality.
Decentralized energy storage systems, particularly those utilizing CO2 conversion technologies, are emerging as a high-potential segment within this broader market. These systems address the critical challenge of energy intermittency while simultaneously contributing to carbon reduction goals. The market for CO2 conversion technologies specifically is expected to grow from $7.5 billion in 2023 to over $25 billion by 2032, reflecting increasing recognition of their dual benefits for energy management and carbon utilization.
Regional analysis reveals varying market dynamics. Europe leads in adoption of CO2-based energy storage solutions, driven by stringent carbon regulations and ambitious climate targets. The European Green Deal has allocated €1 billion specifically for innovative energy storage technologies, including electrocatalytic CO2 conversion. North America follows, with significant investments coming from both government initiatives like the U.S. Department of Energy's $75 million funding for energy storage research and private sector investments.
Asia-Pacific represents the fastest-growing market, with China, Japan, and South Korea making substantial investments in renewable energy infrastructure. China alone has committed $440 billion to renewable energy development in its latest five-year plan, with approximately 15% allocated to storage technologies.
Customer segmentation shows three primary market segments: utility-scale applications (representing 45% of the market), commercial and industrial users (35%), and residential applications (20%). The utility segment currently dominates due to large-scale implementation capabilities, but the commercial segment is growing fastest as businesses seek energy independence and sustainability credentials.
Key market drivers include increasing renewable energy penetration, grid stability concerns, declining costs of electrocatalyst materials, and supportive regulatory frameworks. Barriers to market growth include high initial capital costs, technological limitations in catalyst efficiency and durability, and infrastructure challenges for CO2 capture and transportation.
The competitive landscape features both established energy companies pivoting toward storage solutions and innovative startups focused specifically on CO2 conversion technologies. Strategic partnerships between technology developers, energy providers, and industrial CO2 emitters are becoming increasingly common, creating new business models that monetize both energy storage and carbon utilization.
Decentralized energy storage systems, particularly those utilizing CO2 conversion technologies, are emerging as a high-potential segment within this broader market. These systems address the critical challenge of energy intermittency while simultaneously contributing to carbon reduction goals. The market for CO2 conversion technologies specifically is expected to grow from $7.5 billion in 2023 to over $25 billion by 2032, reflecting increasing recognition of their dual benefits for energy management and carbon utilization.
Regional analysis reveals varying market dynamics. Europe leads in adoption of CO2-based energy storage solutions, driven by stringent carbon regulations and ambitious climate targets. The European Green Deal has allocated €1 billion specifically for innovative energy storage technologies, including electrocatalytic CO2 conversion. North America follows, with significant investments coming from both government initiatives like the U.S. Department of Energy's $75 million funding for energy storage research and private sector investments.
Asia-Pacific represents the fastest-growing market, with China, Japan, and South Korea making substantial investments in renewable energy infrastructure. China alone has committed $440 billion to renewable energy development in its latest five-year plan, with approximately 15% allocated to storage technologies.
Customer segmentation shows three primary market segments: utility-scale applications (representing 45% of the market), commercial and industrial users (35%), and residential applications (20%). The utility segment currently dominates due to large-scale implementation capabilities, but the commercial segment is growing fastest as businesses seek energy independence and sustainability credentials.
Key market drivers include increasing renewable energy penetration, grid stability concerns, declining costs of electrocatalyst materials, and supportive regulatory frameworks. Barriers to market growth include high initial capital costs, technological limitations in catalyst efficiency and durability, and infrastructure challenges for CO2 capture and transportation.
The competitive landscape features both established energy companies pivoting toward storage solutions and innovative startups focused specifically on CO2 conversion technologies. Strategic partnerships between technology developers, energy providers, and industrial CO2 emitters are becoming increasingly common, creating new business models that monetize both energy storage and carbon utilization.
Current Electrocatalyst Technologies and Challenges
The current landscape of electrocatalysts for CO2 conversion is characterized by diverse materials and approaches, each with specific advantages and limitations. Metal-based catalysts, particularly copper and its alloys, have shown promising activity for converting CO2 to hydrocarbons and alcohols. Copper uniquely facilitates C-C coupling reactions necessary for producing valuable multi-carbon products. However, these catalysts often suffer from poor selectivity, with multiple reaction pathways leading to mixed product distributions that complicate downstream separation processes.
Noble metals such as gold, silver, and palladium demonstrate high activity but primarily produce carbon monoxide rather than higher-value products. Their prohibitive cost and limited availability also restrict widespread implementation in decentralized energy storage systems. Transition metal oxides and sulfides offer more economical alternatives with reasonable stability, but typically exhibit lower activity and require higher overpotentials to drive reactions effectively.
Carbon-based materials, including carbon nanotubes, graphene, and nitrogen-doped carbon, represent an emerging class of metal-free catalysts with tunable properties and excellent durability. These materials benefit from high surface areas and customizable functional groups but generally demonstrate lower catalytic activity compared to their metallic counterparts.
A significant challenge across all catalyst types is the competitive hydrogen evolution reaction, which consumes valuable electrons without contributing to CO2 conversion. This parasitic reaction substantially reduces Faradaic efficiency and energy utilization. Additionally, most current catalysts require high overpotentials (>1V) to achieve industrially relevant current densities, severely limiting overall energy efficiency in renewable energy storage applications.
Catalyst stability presents another critical hurdle, with performance degradation occurring through multiple mechanisms including poisoning, leaching, and structural collapse during extended operation. This is particularly problematic for decentralized applications where maintenance capabilities may be limited. Most laboratory demonstrations show promising performance over hours, but commercial viability requires thousands of operational hours.
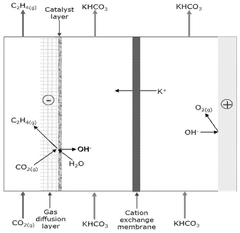
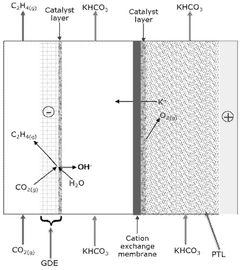
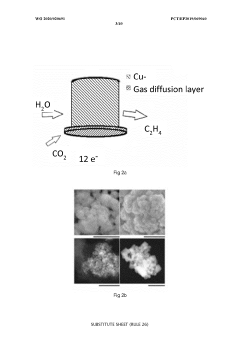
Mass transport limitations also constrain system performance, as CO2's low solubility in aqueous electrolytes creates concentration gradients that limit reaction rates. Various reactor designs attempt to address this through gas diffusion electrodes and flow-cell configurations, but optimal solutions balancing efficiency, complexity, and cost remain elusive.
The integration of catalysts into complete systems introduces additional challenges related to electrode fabrication, membrane compatibility, and system control. These engineering aspects often receive less attention than fundamental catalyst development but are equally critical for practical implementation in decentralized renewable energy storage applications.
Noble metals such as gold, silver, and palladium demonstrate high activity but primarily produce carbon monoxide rather than higher-value products. Their prohibitive cost and limited availability also restrict widespread implementation in decentralized energy storage systems. Transition metal oxides and sulfides offer more economical alternatives with reasonable stability, but typically exhibit lower activity and require higher overpotentials to drive reactions effectively.
Carbon-based materials, including carbon nanotubes, graphene, and nitrogen-doped carbon, represent an emerging class of metal-free catalysts with tunable properties and excellent durability. These materials benefit from high surface areas and customizable functional groups but generally demonstrate lower catalytic activity compared to their metallic counterparts.
A significant challenge across all catalyst types is the competitive hydrogen evolution reaction, which consumes valuable electrons without contributing to CO2 conversion. This parasitic reaction substantially reduces Faradaic efficiency and energy utilization. Additionally, most current catalysts require high overpotentials (>1V) to achieve industrially relevant current densities, severely limiting overall energy efficiency in renewable energy storage applications.
Catalyst stability presents another critical hurdle, with performance degradation occurring through multiple mechanisms including poisoning, leaching, and structural collapse during extended operation. This is particularly problematic for decentralized applications where maintenance capabilities may be limited. Most laboratory demonstrations show promising performance over hours, but commercial viability requires thousands of operational hours.
Mass transport limitations also constrain system performance, as CO2's low solubility in aqueous electrolytes creates concentration gradients that limit reaction rates. Various reactor designs attempt to address this through gas diffusion electrodes and flow-cell configurations, but optimal solutions balancing efficiency, complexity, and cost remain elusive.
The integration of catalysts into complete systems introduces additional challenges related to electrode fabrication, membrane compatibility, and system control. These engineering aspects often receive less attention than fundamental catalyst development but are equally critical for practical implementation in decentralized renewable energy storage applications.
State-of-the-Art CO2 Conversion Catalyst Solutions
01 Metal-based electrocatalysts for CO2 reduction
Metal-based catalysts are widely used for electrochemical CO2 reduction due to their excellent conductivity and catalytic properties. Various metals such as copper, silver, gold, and zinc have been developed as electrocatalysts with different selectivity toward CO2 reduction products. These catalysts can be optimized through structural engineering, alloying, or surface modification to enhance their activity, selectivity, and stability for converting CO2 into valuable chemicals and fuels.- Metal-based electrocatalysts for CO2 reduction: Various metal-based catalysts can be used for electrochemical CO2 reduction. These include noble metals (gold, silver), transition metals (copper, nickel, iron), and their alloys. These catalysts facilitate the electron transfer to CO2 molecules, enabling their conversion to valuable products like carbon monoxide, formic acid, or hydrocarbons. The catalytic performance depends on the metal's electronic structure, surface morphology, and binding energy with reaction intermediates.
- Nanostructured electrocatalysts for enhanced CO2 conversion: Nanostructured materials offer advantages for CO2 electroreduction due to their high surface area, abundant active sites, and tunable properties. These include nanoparticles, nanowires, nanosheets, and porous structures that can significantly improve catalytic activity and selectivity. The nanoscale architecture allows for better mass transport, enhanced electron transfer, and optimized binding of intermediates, resulting in higher conversion efficiency and product selectivity compared to bulk materials.
- Carbon-supported and carbon-based electrocatalysts: Carbon materials serve both as supports for metal catalysts and as catalysts themselves for CO2 electroreduction. Carbon supports (graphene, carbon nanotubes, porous carbon) provide high conductivity, large surface area, and stability. Nitrogen-doped or heteroatom-doped carbon materials can act as metal-free catalysts with tunable selectivity. The carbon structure influences the dispersion of active sites, electron transfer pathways, and interaction with CO2 molecules, affecting overall catalytic performance.
- Bimetallic and multi-component electrocatalysts: Combining two or more metals or components creates synergistic effects for CO2 electroreduction. Bimetallic catalysts can optimize binding energies of intermediates, alter electronic structures, and create unique active sites at interfaces. Core-shell structures, alloys, and supported catalysts with multiple components allow for tuning product selectivity and activity. These multi-component systems can overcome limitations of single-metal catalysts by balancing competing reaction pathways.
- Electrocatalyst systems with ionic liquids and electrolyte engineering: The electrolyte environment significantly impacts CO2 electroreduction performance. Ionic liquids can enhance CO2 solubility, stabilize intermediates, and modify the local reaction environment. Electrolyte engineering through pH control, buffer selection, and additives can direct reaction pathways and improve efficiency. Advanced electrocatalyst systems integrate catalyst design with optimized electrolytes to overcome mass transport limitations, suppress competing reactions, and enhance overall conversion rates.
02 Nanostructured catalysts for enhanced CO2 electroreduction
Nanostructured materials offer advantages for CO2 electroreduction due to their high surface area, abundant active sites, and tunable electronic properties. These catalysts include nanoparticles, nanowires, nanosheets, and porous structures that can significantly improve catalytic performance compared to bulk materials. The controlled synthesis of nanostructured catalysts with specific morphologies, sizes, and compositions enables more efficient CO2 conversion with higher selectivity toward target products.Expand Specific Solutions03 Carbon-based and hybrid electrocatalysts
Carbon-based materials such as graphene, carbon nanotubes, and doped carbons serve as effective supports or catalysts for CO2 electroreduction. These materials can be functionalized or doped with heteroatoms (N, S, P) to create active sites for CO2 adsorption and conversion. Hybrid catalysts combining carbon materials with metals or metal oxides show synergistic effects, enhancing catalytic activity while providing stability and conductivity for efficient electron transfer during the electrochemical process.Expand Specific Solutions04 Electrocatalytic systems and reactor designs
Advanced electrocatalytic systems incorporate innovative reactor designs, electrode configurations, and membrane technologies to optimize CO2 conversion efficiency. These systems address challenges such as mass transport limitations, product separation, and energy efficiency. Continuous flow reactors, gas diffusion electrodes, and integrated systems that combine CO2 capture with electroreduction represent significant advancements in scaling up the electrochemical conversion of CO2 to valuable products.Expand Specific Solutions05 Catalyst performance enhancement strategies
Various strategies have been developed to enhance electrocatalyst performance for CO2 reduction, including interface engineering, defect creation, and electrolyte optimization. The introduction of promoters, stabilizers, or co-catalysts can significantly improve activity and selectivity. Advanced characterization techniques and computational methods are employed to understand reaction mechanisms and guide rational catalyst design, leading to more efficient and selective CO2 conversion processes.Expand Specific Solutions
Leading Organizations in CO2 Electrocatalysis Research
The electrocatalyst market for CO2 conversion in renewable energy storage is in its early growth phase, characterized by intensive R&D activities across academic institutions and industry players. The global market is projected to expand significantly as decarbonization efforts accelerate, with current estimates in the multi-billion dollar range. Leading companies like Johnson Matthey, Siemens Energy, and TotalEnergies are advancing commercial applications, while Chinese entities including Sinopec and Wanhua Chemical are rapidly scaling up their technological capabilities. Academic powerhouses such as California Institute of Technology, Dalian Institute of Chemical Physics, and Centre National de la Recherche Scientifique are driving fundamental breakthroughs. The technology remains at mid-maturity level, with most solutions at TRL 4-6, indicating promising commercial potential but requiring further development for widespread deployment.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced copper-based electrocatalysts for CO2 reduction with enhanced selectivity towards multi-carbon products. Their technology employs nanostructured Cu catalysts with precisely controlled morphology and surface properties to achieve Faradaic efficiencies exceeding 60% for C2+ products. Sinopec's approach incorporates ionic liquid-modified electrode interfaces that stabilize key reaction intermediates, significantly improving reaction kinetics. Their integrated system combines renewable electricity sources with modular electrolyzer stacks designed for distributed deployment at various scales, from industrial parks to remote locations with renewable energy access. The technology includes proprietary membrane electrode assemblies that minimize energy losses and enable operation at industrially relevant current densities (>200 mA/cm²), making it suitable for decentralized renewable energy storage applications where excess electricity can be converted to valuable chemical feedstocks.
Strengths: Extensive industrial infrastructure and scaling capabilities; integration with existing petrochemical value chains; significant R&D resources. Weaknesses: Traditional focus on fossil fuels may slow transition to renewable-based systems; technology still requires further development to reach commercial viability in terms of catalyst stability and overall system efficiency.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has pioneered advanced precious metal-based electrocatalysts for CO2 conversion, focusing on distributed renewable energy storage applications. Their technology utilizes bimetallic nanoparticle catalysts combining silver with transition metals that achieve high selectivity (>85%) for CO reduction to carbon monoxide at low overpotentials. This CO can then be utilized in established Fischer-Tropsch processes to produce liquid fuels. The company has developed proprietary catalyst deposition techniques that maximize active site density while minimizing precious metal loading, addressing cost concerns for large-scale deployment. Their modular "eCO2nversion" system integrates with intermittent renewable energy sources, featuring rapid response capabilities (<30 seconds) to fluctuating power inputs, making it ideal for grid-balancing applications. Johnson Matthey's technology includes specialized gas diffusion electrodes that maintain performance even under varying load conditions, with demonstrated durability exceeding 5,000 operating hours in pilot installations across Europe where excess wind and solar power is converted to storable chemical energy.
Strengths: Extensive expertise in catalyst development and manufacturing; established global presence in industrial catalysis; strong intellectual property portfolio in electrochemical CO2 conversion. Weaknesses: Reliance on precious metals may impact cost-effectiveness at scale; technology primarily focused on CO as an intermediate rather than direct conversion to higher-value products.
Key Patents and Scientific Breakthroughs in Electrocatalysis
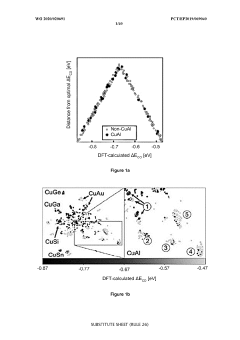
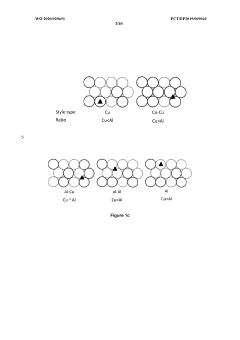
Copper catalysts for the electrochemical conversion of carbon dioxide or carbon monoxide to c2+ products
PatentWO2025040909A1
Innovation
- The development of copper catalysts with a modified surface layer incorporating specific metals (M) such as yttrium, lanthanum, or palladium, which improve the selectivity towards ethylene formation by maintaining a positive oxidation state and reducing the activation energy barrier for dimerization.
Catalysts for electrochemical co2 reduction and associated methods
PatentWO2020020691A1
Innovation
- Development of multi-metal electrocatalysts comprising copper (Cu) and at least one enhancer metal from Ge, Ga, Sn, Si, Ag, Au, Zn, or Al, with a preferred Cu-Al material, where Al is ion-implanted or evaporated into Cu and chemically etched to form a de-alloyed nanoporous structure, enhancing CO2 reduction activity and selectivity.
Techno-economic Assessment of Decentralized Implementation
The techno-economic assessment of decentralized implementation for CO2 conversion electrocatalysts reveals several critical factors affecting commercial viability. Initial capital expenditure represents a significant barrier, with specialized equipment costs ranging from $500,000 to $2 million for small-scale installations. This includes electrochemical reactors, separation systems, and renewable energy integration components. However, economies of scale are less pronounced compared to centralized systems, making the per-unit production costs relatively stable across different implementation sizes.
Operational expenses demonstrate interesting patterns in decentralized scenarios. Energy costs typically constitute 40-55% of operational expenses, with maintenance accounting for 15-25%. The levelized cost of products (LCOP) for decentralized CO2 conversion currently ranges between $0.85-2.30 per kilogram of product, depending on the target chemical and catalyst efficiency. This represents a 20-35% premium compared to centralized facilities but offers advantages in reduced transportation costs and grid independence.
Return on investment (ROI) calculations indicate payback periods of 5-8 years for most decentralized implementations, assuming current technology readiness levels and without substantial subsidies. This timeline could be reduced to 3-5 years with targeted improvements in catalyst performance, particularly in selectivity and stability under fluctuating power conditions typical of renewable energy sources.
Geographic factors significantly impact economic viability. Regions with high renewable energy potential but limited grid infrastructure demonstrate the most favorable economics, with potential cost reductions of 15-30% compared to grid-dependent locations. Additionally, areas with carbon pricing mechanisms or renewable energy incentives can improve ROI by 1-2 years.
Sensitivity analysis reveals that catalyst performance metrics—particularly energy efficiency, selectivity, and durability—represent the most influential variables in economic models. A 10% improvement in catalyst efficiency can translate to approximately 7-12% reduction in overall operational costs. This underscores the critical importance of continued research in catalyst development specifically tailored for decentralized applications.
Scale-appropriate technology development remains crucial, as current systems often represent scaled-down versions of industrial designs rather than purpose-built solutions for distributed implementation. Modular designs that can operate efficiently at 5-50 kW scales show the most promising economics, with potential for standardization to drive down manufacturing costs by 25-40% over the next decade.
Operational expenses demonstrate interesting patterns in decentralized scenarios. Energy costs typically constitute 40-55% of operational expenses, with maintenance accounting for 15-25%. The levelized cost of products (LCOP) for decentralized CO2 conversion currently ranges between $0.85-2.30 per kilogram of product, depending on the target chemical and catalyst efficiency. This represents a 20-35% premium compared to centralized facilities but offers advantages in reduced transportation costs and grid independence.
Return on investment (ROI) calculations indicate payback periods of 5-8 years for most decentralized implementations, assuming current technology readiness levels and without substantial subsidies. This timeline could be reduced to 3-5 years with targeted improvements in catalyst performance, particularly in selectivity and stability under fluctuating power conditions typical of renewable energy sources.
Geographic factors significantly impact economic viability. Regions with high renewable energy potential but limited grid infrastructure demonstrate the most favorable economics, with potential cost reductions of 15-30% compared to grid-dependent locations. Additionally, areas with carbon pricing mechanisms or renewable energy incentives can improve ROI by 1-2 years.
Sensitivity analysis reveals that catalyst performance metrics—particularly energy efficiency, selectivity, and durability—represent the most influential variables in economic models. A 10% improvement in catalyst efficiency can translate to approximately 7-12% reduction in overall operational costs. This underscores the critical importance of continued research in catalyst development specifically tailored for decentralized applications.
Scale-appropriate technology development remains crucial, as current systems often represent scaled-down versions of industrial designs rather than purpose-built solutions for distributed implementation. Modular designs that can operate efficiently at 5-50 kW scales show the most promising economics, with potential for standardization to drive down manufacturing costs by 25-40% over the next decade.
Environmental Impact and Sustainability Considerations
The electrocatalytic conversion of CO2 represents a promising approach for decentralized renewable energy storage, but its environmental implications must be thoroughly assessed to ensure true sustainability. When evaluating electrocatalysts for CO2 conversion, lifecycle analysis reveals that while the process itself consumes CO2, the production of catalysts—particularly those containing precious metals like platinum or palladium—can generate significant carbon footprints. Recent studies indicate that catalysts based on earth-abundant elements such as copper, iron, and nitrogen-doped carbon materials offer substantially reduced environmental impacts during manufacturing while maintaining acceptable conversion efficiencies.
Water consumption presents another critical consideration, as electrocatalytic systems typically require ultrapure water for optimal performance. In decentralized applications, this necessitates additional purification infrastructure, potentially offsetting some environmental benefits. Advanced system designs incorporating water recycling loops have demonstrated up to 85% reduction in freshwater requirements, making implementation more viable in water-stressed regions.
The environmental benefits of CO2 conversion technologies extend beyond carbon utilization. By enabling higher penetration of intermittent renewable energy sources through chemical energy storage, these systems can accelerate grid decarbonization. Quantitative assessments indicate that each kilogram of CO2 converted via renewable-powered electrocatalysis potentially prevents 2.5-3.0 kg of additional CO2 emissions that would otherwise result from fossil fuel-based energy generation and storage.
Land use impacts vary significantly depending on deployment scenarios. Centralized facilities offer economies of scale but require extensive infrastructure for CO2 capture and transport. Conversely, decentralized systems can be integrated with existing renewable energy installations, minimizing additional land requirements. Research from pilot projects suggests that co2-to-fuel conversion facilities require approximately 40-60% less land area than equivalent energy storage using battery technologies when considering full lifecycle requirements.
Chemical safety considerations must address the potential environmental hazards of reaction intermediates and products. While many CO2 conversion pathways produce relatively benign products like methanol or formic acid, others may generate compounds requiring careful handling. Recent innovations in catalyst selectivity have significantly reduced unwanted byproducts, with leading systems achieving over 95% selectivity toward target molecules.
For truly sustainable implementation, end-of-life management strategies for electrocatalysts must be developed concurrently with the technology itself. Promising approaches include designing catalysts for easy recovery of precious components and developing regeneration protocols that extend operational lifetimes by 3-5 times compared to current benchmarks, substantially reducing waste generation and resource consumption over system lifetimes.
Water consumption presents another critical consideration, as electrocatalytic systems typically require ultrapure water for optimal performance. In decentralized applications, this necessitates additional purification infrastructure, potentially offsetting some environmental benefits. Advanced system designs incorporating water recycling loops have demonstrated up to 85% reduction in freshwater requirements, making implementation more viable in water-stressed regions.
The environmental benefits of CO2 conversion technologies extend beyond carbon utilization. By enabling higher penetration of intermittent renewable energy sources through chemical energy storage, these systems can accelerate grid decarbonization. Quantitative assessments indicate that each kilogram of CO2 converted via renewable-powered electrocatalysis potentially prevents 2.5-3.0 kg of additional CO2 emissions that would otherwise result from fossil fuel-based energy generation and storage.
Land use impacts vary significantly depending on deployment scenarios. Centralized facilities offer economies of scale but require extensive infrastructure for CO2 capture and transport. Conversely, decentralized systems can be integrated with existing renewable energy installations, minimizing additional land requirements. Research from pilot projects suggests that co2-to-fuel conversion facilities require approximately 40-60% less land area than equivalent energy storage using battery technologies when considering full lifecycle requirements.
Chemical safety considerations must address the potential environmental hazards of reaction intermediates and products. While many CO2 conversion pathways produce relatively benign products like methanol or formic acid, others may generate compounds requiring careful handling. Recent innovations in catalyst selectivity have significantly reduced unwanted byproducts, with leading systems achieving over 95% selectivity toward target molecules.
For truly sustainable implementation, end-of-life management strategies for electrocatalysts must be developed concurrently with the technology itself. Promising approaches include designing catalysts for easy recovery of precious components and developing regeneration protocols that extend operational lifetimes by 3-5 times compared to current benchmarks, substantially reducing waste generation and resource consumption over system lifetimes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!