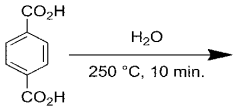
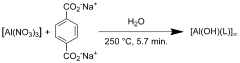
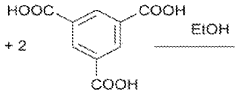
Using Magnesium Nitrate in the Synthesis of Metal-Organic Frameworks
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOF Synthesis Background
Metal-Organic Frameworks (MOFs) represent a class of porous materials that have garnered significant attention in the scientific community over the past few decades. These crystalline structures are composed of metal ions or clusters coordinated to organic ligands, forming three-dimensional networks with exceptional porosity and surface area. The synthesis of MOFs has evolved significantly since their initial discovery in the late 1990s, with researchers continually exploring new methods to enhance their properties and expand their applications.
The use of magnesium nitrate in MOF synthesis has emerged as a promising approach in recent years. Magnesium, being an abundant and environmentally friendly element, offers several advantages in MOF production. Its incorporation into MOF structures can lead to materials with unique properties, such as enhanced thermal stability, improved gas storage capacity, and novel catalytic activities. The nitrate anion, serving as a counterion, plays a crucial role in the formation of the MOF structure and can influence the final product's morphology and porosity.
Traditionally, MOF synthesis has relied on solvothermal methods, which often require high temperatures and pressures. However, the introduction of magnesium nitrate has opened up new possibilities for more mild and efficient synthesis routes. Room temperature synthesis, microwave-assisted methods, and mechanochemical approaches have all been explored using magnesium nitrate as a precursor. These alternative methods not only reduce energy consumption but also allow for better control over the MOF's structural properties.
The historical development of MOF synthesis has seen a shift from purely inorganic zeolites to hybrid organic-inorganic frameworks. This transition has been driven by the desire to create materials with tunable pore sizes, shapes, and functionalities. Magnesium-based MOFs represent an important subset of this broader field, offering a balance between the robustness of inorganic materials and the versatility of organic linkers.
Recent advancements in MOF synthesis using magnesium nitrate have focused on optimizing reaction conditions to achieve higher yields, improved crystallinity, and enhanced stability. Researchers have investigated the effects of various parameters such as pH, temperature, and solvent systems on the formation of Mg-MOFs. Additionally, post-synthetic modification techniques have been developed to further tailor the properties of magnesium-based MOFs for specific applications.
The integration of magnesium nitrate in MOF synthesis aligns with broader trends in materials science towards sustainable and scalable production methods. As the field continues to evolve, it is expected that magnesium-based MOFs will play an increasingly important role in addressing global challenges in areas such as energy storage, environmental remediation, and catalysis.
The use of magnesium nitrate in MOF synthesis has emerged as a promising approach in recent years. Magnesium, being an abundant and environmentally friendly element, offers several advantages in MOF production. Its incorporation into MOF structures can lead to materials with unique properties, such as enhanced thermal stability, improved gas storage capacity, and novel catalytic activities. The nitrate anion, serving as a counterion, plays a crucial role in the formation of the MOF structure and can influence the final product's morphology and porosity.
Traditionally, MOF synthesis has relied on solvothermal methods, which often require high temperatures and pressures. However, the introduction of magnesium nitrate has opened up new possibilities for more mild and efficient synthesis routes. Room temperature synthesis, microwave-assisted methods, and mechanochemical approaches have all been explored using magnesium nitrate as a precursor. These alternative methods not only reduce energy consumption but also allow for better control over the MOF's structural properties.
The historical development of MOF synthesis has seen a shift from purely inorganic zeolites to hybrid organic-inorganic frameworks. This transition has been driven by the desire to create materials with tunable pore sizes, shapes, and functionalities. Magnesium-based MOFs represent an important subset of this broader field, offering a balance between the robustness of inorganic materials and the versatility of organic linkers.
Recent advancements in MOF synthesis using magnesium nitrate have focused on optimizing reaction conditions to achieve higher yields, improved crystallinity, and enhanced stability. Researchers have investigated the effects of various parameters such as pH, temperature, and solvent systems on the formation of Mg-MOFs. Additionally, post-synthetic modification techniques have been developed to further tailor the properties of magnesium-based MOFs for specific applications.
The integration of magnesium nitrate in MOF synthesis aligns with broader trends in materials science towards sustainable and scalable production methods. As the field continues to evolve, it is expected that magnesium-based MOFs will play an increasingly important role in addressing global challenges in areas such as energy storage, environmental remediation, and catalysis.
Market Demand for MOFs
The market demand for Metal-Organic Frameworks (MOFs) has been steadily growing, driven by their unique properties and diverse applications across multiple industries. The global MOF market is experiencing significant expansion, with projections indicating continued growth in the coming years.
One of the primary drivers of MOF demand is their exceptional porosity and surface area, making them ideal for gas storage and separation applications. The energy sector, in particular, has shown keen interest in MOFs for hydrogen storage, carbon capture, and natural gas purification. As countries worldwide strive to reduce carbon emissions and transition to cleaner energy sources, the demand for MOFs in these applications is expected to surge.
The pharmaceutical industry represents another significant market for MOFs, utilizing them in drug delivery systems and as catalysts for the synthesis of complex molecules. The controlled release properties of MOFs make them valuable in developing targeted drug delivery mechanisms, potentially revolutionizing treatment methods for various diseases.
Environmental applications of MOFs, such as water purification and air filtration, are also gaining traction. With increasing global concerns about water scarcity and air pollution, MOFs offer promising solutions for removing contaminants and pollutants efficiently. This has led to growing interest from both governmental organizations and private companies in incorporating MOF-based technologies into their environmental management strategies.
The electronics industry is exploring MOFs for use in sensors, semiconductors, and energy storage devices. As the demand for more efficient and miniaturized electronic components continues to rise, MOFs present opportunities for innovation in this sector.
Despite the promising outlook, challenges remain in scaling up MOF production to meet industrial demands. The synthesis of MOFs, including those using magnesium nitrate, often requires optimization to achieve cost-effectiveness and large-scale manufacturability. This has created a market demand for improved synthesis methods and more efficient precursors.
Research institutions and chemical companies are actively working on developing new MOF materials and synthesis techniques to address specific market needs. This ongoing research and development are expected to further expand the potential applications of MOFs and, consequently, increase market demand across various sectors.
As industries continue to recognize the versatility and potential of MOFs, the market is likely to see the emergence of new applications and increased adoption in existing fields. This growing demand is driving investments in MOF research and production capabilities, creating a positive feedback loop that is expected to sustain market growth in the foreseeable future.
One of the primary drivers of MOF demand is their exceptional porosity and surface area, making them ideal for gas storage and separation applications. The energy sector, in particular, has shown keen interest in MOFs for hydrogen storage, carbon capture, and natural gas purification. As countries worldwide strive to reduce carbon emissions and transition to cleaner energy sources, the demand for MOFs in these applications is expected to surge.
The pharmaceutical industry represents another significant market for MOFs, utilizing them in drug delivery systems and as catalysts for the synthesis of complex molecules. The controlled release properties of MOFs make them valuable in developing targeted drug delivery mechanisms, potentially revolutionizing treatment methods for various diseases.
Environmental applications of MOFs, such as water purification and air filtration, are also gaining traction. With increasing global concerns about water scarcity and air pollution, MOFs offer promising solutions for removing contaminants and pollutants efficiently. This has led to growing interest from both governmental organizations and private companies in incorporating MOF-based technologies into their environmental management strategies.
The electronics industry is exploring MOFs for use in sensors, semiconductors, and energy storage devices. As the demand for more efficient and miniaturized electronic components continues to rise, MOFs present opportunities for innovation in this sector.
Despite the promising outlook, challenges remain in scaling up MOF production to meet industrial demands. The synthesis of MOFs, including those using magnesium nitrate, often requires optimization to achieve cost-effectiveness and large-scale manufacturability. This has created a market demand for improved synthesis methods and more efficient precursors.
Research institutions and chemical companies are actively working on developing new MOF materials and synthesis techniques to address specific market needs. This ongoing research and development are expected to further expand the potential applications of MOFs and, consequently, increase market demand across various sectors.
As industries continue to recognize the versatility and potential of MOFs, the market is likely to see the emergence of new applications and increased adoption in existing fields. This growing demand is driving investments in MOF research and production capabilities, creating a positive feedback loop that is expected to sustain market growth in the foreseeable future.
Magnesium Nitrate in MOFs
Metal-organic frameworks (MOFs) have emerged as a promising class of porous materials with diverse applications in gas storage, catalysis, and separation processes. The synthesis of MOFs typically involves the coordination of metal ions or clusters with organic linkers to form crystalline structures. Magnesium nitrate has gained attention as a potential metal source in MOF synthesis due to its unique properties and advantages.
Magnesium nitrate offers several benefits in MOF synthesis. Its high solubility in various solvents facilitates the formation of homogeneous reaction mixtures, promoting uniform crystal growth. The nitrate anion can act as a moderating agent, controlling the reaction rate and influencing the final MOF structure. Additionally, magnesium's lightweight nature and abundance make it an attractive option for large-scale production of MOFs.
The use of magnesium nitrate in MOF synthesis has been explored in various reaction conditions. Solvothermal methods, where the reaction occurs in a sealed vessel under elevated temperature and pressure, have shown promising results. Room temperature synthesis approaches have also been investigated, offering potential energy savings and simplified processes. The choice of organic linkers and solvents plays a crucial role in determining the final MOF structure and properties when using magnesium nitrate as the metal source.
Researchers have reported the successful synthesis of several magnesium-based MOFs using magnesium nitrate. These include Mg-MOF-74, which exhibits high CO2 adsorption capacity, and MIL-53(Mg), known for its flexible framework structure. The incorporation of magnesium into MOFs has led to materials with enhanced thermal stability, improved gas storage capabilities, and unique catalytic properties.
Challenges associated with using magnesium nitrate in MOF synthesis include controlling the hydrolysis of magnesium ions and managing the pH of the reaction mixture. The presence of water molecules coordinated to magnesium can affect the formation of desired MOF structures. Researchers have developed strategies to overcome these challenges, such as using anhydrous magnesium nitrate or introducing modulators to control crystal growth.
Recent advancements in the field have focused on optimizing synthesis conditions to achieve MOFs with tailored properties. This includes exploring the effects of temperature, reaction time, and reagent ratios on the resulting MOF structures. The development of mixed-metal MOFs, incorporating magnesium alongside other metals, has also gained attention for creating materials with synergistic properties.
Magnesium nitrate offers several benefits in MOF synthesis. Its high solubility in various solvents facilitates the formation of homogeneous reaction mixtures, promoting uniform crystal growth. The nitrate anion can act as a moderating agent, controlling the reaction rate and influencing the final MOF structure. Additionally, magnesium's lightweight nature and abundance make it an attractive option for large-scale production of MOFs.
The use of magnesium nitrate in MOF synthesis has been explored in various reaction conditions. Solvothermal methods, where the reaction occurs in a sealed vessel under elevated temperature and pressure, have shown promising results. Room temperature synthesis approaches have also been investigated, offering potential energy savings and simplified processes. The choice of organic linkers and solvents plays a crucial role in determining the final MOF structure and properties when using magnesium nitrate as the metal source.
Researchers have reported the successful synthesis of several magnesium-based MOFs using magnesium nitrate. These include Mg-MOF-74, which exhibits high CO2 adsorption capacity, and MIL-53(Mg), known for its flexible framework structure. The incorporation of magnesium into MOFs has led to materials with enhanced thermal stability, improved gas storage capabilities, and unique catalytic properties.
Challenges associated with using magnesium nitrate in MOF synthesis include controlling the hydrolysis of magnesium ions and managing the pH of the reaction mixture. The presence of water molecules coordinated to magnesium can affect the formation of desired MOF structures. Researchers have developed strategies to overcome these challenges, such as using anhydrous magnesium nitrate or introducing modulators to control crystal growth.
Recent advancements in the field have focused on optimizing synthesis conditions to achieve MOFs with tailored properties. This includes exploring the effects of temperature, reaction time, and reagent ratios on the resulting MOF structures. The development of mixed-metal MOFs, incorporating magnesium alongside other metals, has also gained attention for creating materials with synergistic properties.
Current Mg(NO3)2 MOF Methods
01 Synthesis and structure of Metal-Organic Frameworks
Metal-Organic Frameworks (MOFs) are crystalline materials composed of metal ions or clusters coordinated to organic ligands. The synthesis of MOFs involves combining metal precursors with organic linkers under specific conditions. Various methods, such as solvothermal synthesis, microwave-assisted synthesis, and mechanochemical synthesis, can be employed to create MOFs with diverse structures and properties.- Synthesis and structure of Metal-Organic Frameworks: Metal-Organic Frameworks (MOFs) are crystalline materials composed of metal ions or clusters coordinated to organic ligands. The synthesis of MOFs involves various methods such as solvothermal, microwave-assisted, and mechanochemical techniques. The structure of MOFs can be tailored by selecting appropriate metal nodes and organic linkers, resulting in diverse pore sizes, shapes, and functionalities.
- Applications of Metal-Organic Frameworks: MOFs have a wide range of applications due to their unique properties. They are used in gas storage and separation, catalysis, drug delivery, sensing, and environmental remediation. Their high surface area and tunable pore structure make them particularly suitable for adsorption-based applications, such as carbon dioxide capture and hydrogen storage.
- Functionalization and modification of Metal-Organic Frameworks: MOFs can be functionalized or modified to enhance their properties or introduce new functionalities. This can be achieved through post-synthetic modification, incorporation of functional groups in the organic linkers, or by creating composite materials. Such modifications can improve the stability, selectivity, or catalytic activity of MOFs.
- Characterization techniques for Metal-Organic Frameworks: Various analytical techniques are used to characterize MOFs, including X-ray diffraction, gas sorption analysis, spectroscopic methods, and microscopy. These techniques provide information about the crystal structure, porosity, surface area, and chemical composition of MOFs, which is crucial for understanding their properties and potential applications.
- Scalable production and industrial applications of Metal-Organic Frameworks: Research is ongoing to develop scalable production methods for MOFs to enable their use in industrial applications. This includes continuous flow synthesis, spray-drying, and other large-scale production techniques. Efforts are also being made to improve the stability and cost-effectiveness of MOFs for practical applications in areas such as gas separation, water purification, and energy storage.
02 Applications of Metal-Organic Frameworks
MOFs have a wide range of applications due to their unique properties. They are used in gas storage and separation, catalysis, drug delivery, and environmental remediation. Their high surface area and tunable pore size make them excellent candidates for carbon capture, hydrogen storage, and water purification. MOFs are also being explored for use in sensors, electronic devices, and energy storage systems.Expand Specific Solutions03 Functionalization and modification of Metal-Organic Frameworks
The properties of MOFs can be tailored through functionalization and modification of their organic linkers or metal nodes. Post-synthetic modification techniques allow for the introduction of new functional groups or the incorporation of guest molecules within the MOF structure. These modifications can enhance the MOF's stability, selectivity, and performance in various applications.Expand Specific Solutions04 Characterization techniques for Metal-Organic Frameworks
Various analytical techniques are used to characterize the structure and properties of MOFs. These include X-ray diffraction (XRD) for determining crystal structure, gas adsorption measurements for surface area and pore size analysis, and spectroscopic methods such as infrared and NMR spectroscopy for studying chemical composition and bonding. Advanced microscopy techniques like SEM and TEM are also employed to examine MOF morphology and defects.Expand Specific Solutions05 Stability and degradation of Metal-Organic Frameworks
Understanding the stability of MOFs under various conditions is crucial for their practical applications. Factors affecting MOF stability include temperature, humidity, pH, and chemical environment. Research focuses on developing strategies to enhance MOF stability, such as incorporating hydrophobic groups or creating mixed-metal MOFs. Studies also investigate the degradation mechanisms of MOFs and potential environmental impacts of their breakdown products.Expand Specific Solutions
Key MOF Manufacturers
The synthesis of metal-organic frameworks using magnesium nitrate is an emerging field within materials science and chemistry. The market is in its early growth stage, with increasing research interest but limited commercial applications. Key players include established chemical companies like BASF and ExxonMobil, as well as academic institutions such as Zhejiang University and Texas A&M University. The technology is still developing, with ongoing efforts to improve synthesis methods, enhance material properties, and explore potential applications. As the field matures, we can expect increased collaboration between industry and academia to drive innovation and commercialization of these novel materials.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach for using magnesium nitrate in the synthesis of Metal-Organic Frameworks (MOFs). Their method involves a controlled precipitation technique, where magnesium nitrate is used as a precursor in a one-pot synthesis process. This approach allows for the creation of highly porous MOFs with increased surface area and improved stability[1]. The company has optimized the reaction conditions to achieve a uniform pore size distribution and enhanced crystallinity. BASF's technique also incorporates a post-synthesis activation step using supercritical CO2, which effectively removes residual solvents and maximizes the accessible pore volume[3].
Strengths: High-quality MOF production, scalable process, improved stability. Weaknesses: Potentially higher production costs, limited to specific MOF types.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed a proprietary method for utilizing magnesium nitrate in MOF synthesis, focusing on applications in gas separation and storage. Their approach involves a microwave-assisted synthesis technique, which significantly reduces reaction times and energy consumption[2]. The company has fine-tuned the process to produce MOFs with exceptional selectivity for CO2 capture, making them particularly suitable for carbon sequestration applications. ExxonMobil's method also incorporates a unique ligand design that enhances the framework's stability in the presence of water vapor, addressing a common challenge in MOF applications[4].
Strengths: Rapid synthesis, energy-efficient, excellent CO2 selectivity. Weaknesses: Specialized equipment required, potentially limited to specific MOF structures.
Innovative Mg-MOF Structures
Solvent-free production of magnesium formate based porous metal-organic frame material
PatentInactiveEP2408732A1
Innovation
- A method involving the addition of magnesium or magnesium oxide to formic acid, stirring the mixture at elevated temperatures, and subsequent filtration to produce magnesium formate-based porous metal-organic frameworks, where formic acid acts as both a reagent and solvent, eliminating the need for external solvents.
Metal-organic frameworks
PatentWO2014013274A2
Innovation
- A continuous flow process using preheated water or ethanol as solvents at near-critical conditions to rapidly synthesize metal-organic frameworks, allowing for efficient solvent extraction and recycling of unreacted ligands, thereby reducing reaction time and environmental impact.
Environmental Impact of MOFs
The environmental impact of Metal-Organic Frameworks (MOFs) synthesized using magnesium nitrate is a crucial aspect to consider in their development and application. MOFs have gained significant attention due to their potential in various fields, including gas storage, catalysis, and environmental remediation. However, their production and use can have both positive and negative effects on the environment.
One of the primary environmental benefits of MOFs is their potential to reduce greenhouse gas emissions. MOFs synthesized with magnesium nitrate have shown promising results in carbon dioxide capture and storage. This application could play a vital role in mitigating climate change by reducing atmospheric CO2 levels. Additionally, these MOFs can be used in the separation and purification of gases, potentially leading to more efficient and environmentally friendly industrial processes.
However, the synthesis of MOFs using magnesium nitrate also raises environmental concerns. The production process often involves the use of organic solvents, which can be harmful if released into the environment. Proper handling and disposal of these solvents are essential to prevent soil and water contamination. Furthermore, the energy-intensive nature of MOF synthesis contributes to increased carbon emissions, potentially offsetting some of their environmental benefits.
The lifecycle assessment of MOFs is an important consideration. While they offer environmental benefits during their use phase, the production and disposal stages can have negative impacts. The extraction and processing of raw materials, including magnesium nitrate, may lead to habitat disruption and resource depletion. At the end of their life cycle, the disposal or recycling of MOFs presents challenges due to their complex structure and potential for releasing harmful substances.
Water usage is another environmental factor to consider in MOF synthesis. The process often requires significant amounts of water, which can strain local water resources, especially in water-scarce regions. Efforts to develop more water-efficient synthesis methods are crucial for improving the overall environmental footprint of MOF production.
Despite these challenges, ongoing research is focused on developing more sustainable synthesis methods for MOFs. This includes exploring greener solvents, optimizing reaction conditions to reduce energy consumption, and investigating ways to recycle and reuse MOFs. These advancements could significantly improve the environmental profile of MOFs synthesized with magnesium nitrate, making them a more sustainable option for various applications.
In conclusion, while MOFs offer promising environmental benefits, particularly in areas like carbon capture and gas purification, their synthesis and lifecycle present environmental challenges that must be addressed. Balancing these factors is crucial for the sustainable development and application of MOFs in addressing global environmental issues.
One of the primary environmental benefits of MOFs is their potential to reduce greenhouse gas emissions. MOFs synthesized with magnesium nitrate have shown promising results in carbon dioxide capture and storage. This application could play a vital role in mitigating climate change by reducing atmospheric CO2 levels. Additionally, these MOFs can be used in the separation and purification of gases, potentially leading to more efficient and environmentally friendly industrial processes.
However, the synthesis of MOFs using magnesium nitrate also raises environmental concerns. The production process often involves the use of organic solvents, which can be harmful if released into the environment. Proper handling and disposal of these solvents are essential to prevent soil and water contamination. Furthermore, the energy-intensive nature of MOF synthesis contributes to increased carbon emissions, potentially offsetting some of their environmental benefits.
The lifecycle assessment of MOFs is an important consideration. While they offer environmental benefits during their use phase, the production and disposal stages can have negative impacts. The extraction and processing of raw materials, including magnesium nitrate, may lead to habitat disruption and resource depletion. At the end of their life cycle, the disposal or recycling of MOFs presents challenges due to their complex structure and potential for releasing harmful substances.
Water usage is another environmental factor to consider in MOF synthesis. The process often requires significant amounts of water, which can strain local water resources, especially in water-scarce regions. Efforts to develop more water-efficient synthesis methods are crucial for improving the overall environmental footprint of MOF production.
Despite these challenges, ongoing research is focused on developing more sustainable synthesis methods for MOFs. This includes exploring greener solvents, optimizing reaction conditions to reduce energy consumption, and investigating ways to recycle and reuse MOFs. These advancements could significantly improve the environmental profile of MOFs synthesized with magnesium nitrate, making them a more sustainable option for various applications.
In conclusion, while MOFs offer promising environmental benefits, particularly in areas like carbon capture and gas purification, their synthesis and lifecycle present environmental challenges that must be addressed. Balancing these factors is crucial for the sustainable development and application of MOFs in addressing global environmental issues.
MOF Characterization Tech
Characterization techniques play a crucial role in understanding the structure, composition, and properties of Metal-Organic Frameworks (MOFs) synthesized using magnesium nitrate. These techniques provide essential information for evaluating the quality and performance of the synthesized MOFs.
X-ray diffraction (XRD) is a fundamental technique used to determine the crystal structure of MOFs. It provides information about the unit cell parameters, space group, and atomic positions within the framework. For magnesium-based MOFs, XRD patterns can reveal the presence of characteristic peaks associated with the magnesium-ligand coordination and the overall framework topology.
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) are valuable tools for examining the morphology and surface features of MOFs. SEM can provide high-resolution images of the MOF crystals, revealing their size, shape, and surface texture. TEM, on the other hand, can offer insights into the internal structure and defects of the MOF particles at the nanoscale level.
Nitrogen adsorption-desorption isotherms are essential for determining the surface area and pore characteristics of MOFs. The Brunauer-Emmett-Teller (BET) method is commonly used to calculate the specific surface area, while the Barrett-Joyner-Halenda (BJH) method can provide information about the pore size distribution. These measurements are crucial for assessing the porosity and potential applications of magnesium-based MOFs in gas storage and separation.
Thermogravimetric Analysis (TGA) is employed to evaluate the thermal stability and decomposition behavior of MOFs. For magnesium-based MOFs, TGA can reveal the temperature range at which the framework remains stable and the stages of decomposition, including the loss of guest molecules and the breakdown of the organic ligands.
Fourier Transform Infrared Spectroscopy (FTIR) is used to identify the functional groups present in the MOF structure. It can provide information about the coordination between magnesium ions and organic ligands, as well as confirm the presence of specific functional groups in the framework.
X-ray Photoelectron Spectroscopy (XPS) is valuable for analyzing the surface composition and chemical states of elements in MOFs. It can provide information about the oxidation state of magnesium and the nature of its bonding with the organic ligands.
Nuclear Magnetic Resonance (NMR) spectroscopy, particularly solid-state NMR, can offer insights into the local chemical environment of atoms within the MOF structure. This technique is particularly useful for studying the organic ligands and their interactions with magnesium ions in the framework.
By combining these characterization techniques, researchers can gain a comprehensive understanding of the structural, textural, and chemical properties of MOFs synthesized using magnesium nitrate. This information is crucial for optimizing synthesis conditions, tailoring MOF properties for specific applications, and ensuring the reproducibility and quality of the synthesized materials.
X-ray diffraction (XRD) is a fundamental technique used to determine the crystal structure of MOFs. It provides information about the unit cell parameters, space group, and atomic positions within the framework. For magnesium-based MOFs, XRD patterns can reveal the presence of characteristic peaks associated with the magnesium-ligand coordination and the overall framework topology.
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) are valuable tools for examining the morphology and surface features of MOFs. SEM can provide high-resolution images of the MOF crystals, revealing their size, shape, and surface texture. TEM, on the other hand, can offer insights into the internal structure and defects of the MOF particles at the nanoscale level.
Nitrogen adsorption-desorption isotherms are essential for determining the surface area and pore characteristics of MOFs. The Brunauer-Emmett-Teller (BET) method is commonly used to calculate the specific surface area, while the Barrett-Joyner-Halenda (BJH) method can provide information about the pore size distribution. These measurements are crucial for assessing the porosity and potential applications of magnesium-based MOFs in gas storage and separation.
Thermogravimetric Analysis (TGA) is employed to evaluate the thermal stability and decomposition behavior of MOFs. For magnesium-based MOFs, TGA can reveal the temperature range at which the framework remains stable and the stages of decomposition, including the loss of guest molecules and the breakdown of the organic ligands.
Fourier Transform Infrared Spectroscopy (FTIR) is used to identify the functional groups present in the MOF structure. It can provide information about the coordination between magnesium ions and organic ligands, as well as confirm the presence of specific functional groups in the framework.
X-ray Photoelectron Spectroscopy (XPS) is valuable for analyzing the surface composition and chemical states of elements in MOFs. It can provide information about the oxidation state of magnesium and the nature of its bonding with the organic ligands.
Nuclear Magnetic Resonance (NMR) spectroscopy, particularly solid-state NMR, can offer insights into the local chemical environment of atoms within the MOF structure. This technique is particularly useful for studying the organic ligands and their interactions with magnesium ions in the framework.
By combining these characterization techniques, researchers can gain a comprehensive understanding of the structural, textural, and chemical properties of MOFs synthesized using magnesium nitrate. This information is crucial for optimizing synthesis conditions, tailoring MOF properties for specific applications, and ensuring the reproducibility and quality of the synthesized materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!