The Interaction of Magnesium Nitrate with Organic Soil Amendments
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg(NO3)2 and Soil Amendments: Background and Objectives
The interaction between magnesium nitrate and organic soil amendments represents a critical area of study in agricultural and environmental sciences. This research topic has gained significant attention due to its potential impact on soil fertility, crop productivity, and environmental sustainability. The evolution of this field can be traced back to the early 20th century when scientists began to explore the effects of various chemical compounds on soil properties and plant growth.
Magnesium nitrate, Mg(NO3)2, is a highly soluble salt that serves as a source of both magnesium and nitrogen for plants. Its use in agriculture has been widespread due to its ability to address magnesium deficiencies while simultaneously providing readily available nitrogen. On the other hand, organic soil amendments, which include materials such as compost, manure, and crop residues, have been traditionally used to improve soil structure, increase organic matter content, and enhance microbial activity.
The convergence of these two soil management practices – the application of magnesium nitrate and the incorporation of organic amendments – has led to a complex interplay of chemical, physical, and biological processes in the soil. Understanding these interactions is crucial for optimizing nutrient management strategies and minimizing potential environmental impacts.
The primary objective of this technical research is to elucidate the mechanisms by which magnesium nitrate interacts with various organic soil amendments. This includes investigating how these interactions affect nutrient availability, soil pH, microbial communities, and overall soil health. Additionally, the research aims to explore the potential synergistic or antagonistic effects that may arise from the combined use of these soil inputs.
Another key goal is to assess the long-term implications of these interactions on soil fertility, crop yield, and environmental quality. This involves examining the fate of magnesium and nitrate ions in the presence of organic matter, their potential for leaching or retention in the soil profile, and their impact on the soil's cation exchange capacity.
Furthermore, this research seeks to develop guidelines for the optimal integration of magnesium nitrate with organic soil amendments in different agricultural systems. This includes determining ideal application rates, timing, and methods that maximize the benefits while minimizing potential drawbacks such as nutrient imbalances or environmental pollution.
By addressing these objectives, the study aims to contribute to the development of more sustainable and efficient soil management practices. The findings are expected to have significant implications for precision agriculture, organic farming, and the broader field of soil science, ultimately leading to improved crop production systems and reduced environmental footprint in agriculture.
Magnesium nitrate, Mg(NO3)2, is a highly soluble salt that serves as a source of both magnesium and nitrogen for plants. Its use in agriculture has been widespread due to its ability to address magnesium deficiencies while simultaneously providing readily available nitrogen. On the other hand, organic soil amendments, which include materials such as compost, manure, and crop residues, have been traditionally used to improve soil structure, increase organic matter content, and enhance microbial activity.
The convergence of these two soil management practices – the application of magnesium nitrate and the incorporation of organic amendments – has led to a complex interplay of chemical, physical, and biological processes in the soil. Understanding these interactions is crucial for optimizing nutrient management strategies and minimizing potential environmental impacts.
The primary objective of this technical research is to elucidate the mechanisms by which magnesium nitrate interacts with various organic soil amendments. This includes investigating how these interactions affect nutrient availability, soil pH, microbial communities, and overall soil health. Additionally, the research aims to explore the potential synergistic or antagonistic effects that may arise from the combined use of these soil inputs.
Another key goal is to assess the long-term implications of these interactions on soil fertility, crop yield, and environmental quality. This involves examining the fate of magnesium and nitrate ions in the presence of organic matter, their potential for leaching or retention in the soil profile, and their impact on the soil's cation exchange capacity.
Furthermore, this research seeks to develop guidelines for the optimal integration of magnesium nitrate with organic soil amendments in different agricultural systems. This includes determining ideal application rates, timing, and methods that maximize the benefits while minimizing potential drawbacks such as nutrient imbalances or environmental pollution.
By addressing these objectives, the study aims to contribute to the development of more sustainable and efficient soil management practices. The findings are expected to have significant implications for precision agriculture, organic farming, and the broader field of soil science, ultimately leading to improved crop production systems and reduced environmental footprint in agriculture.
Agricultural Market Demand Analysis
The agricultural market for magnesium nitrate and organic soil amendments has shown significant growth in recent years, driven by increasing demand for sustainable and efficient farming practices. Farmers are increasingly recognizing the benefits of combining inorganic fertilizers with organic soil amendments to improve soil health and crop yields. This trend is particularly evident in developed countries where precision agriculture and sustainable farming practices are gaining traction.
The global market for magnesium nitrate in agriculture is projected to expand steadily, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is primarily attributed to the rising adoption of fertigation techniques and the increasing awareness of magnesium's role in plant nutrition. Concurrently, the organic soil amendments market is experiencing robust growth, driven by the shift towards organic farming and the need for soil restoration in intensively cultivated areas.
Regionally, North America and Europe lead in the adoption of advanced soil management practices, including the use of magnesium nitrate in combination with organic amendments. These regions are characterized by stringent environmental regulations and a growing consumer preference for sustainably produced food. Asia-Pacific, particularly China and India, represents a rapidly growing market due to increasing agricultural intensification and government initiatives promoting balanced fertilizer use.
The demand for magnesium nitrate is closely tied to the horticultural sector, especially in greenhouse production and high-value crops. Its ability to provide readily available magnesium and nitrogen makes it an attractive option for farmers looking to optimize plant nutrition. The organic soil amendments market, on the other hand, is diverse, encompassing products such as compost, biochar, and humic substances. The interaction between magnesium nitrate and these organic amendments is of particular interest to farmers seeking to enhance nutrient uptake efficiency and soil structure.
Market analysis indicates that large-scale commercial farms are the primary consumers of magnesium nitrate, while organic soil amendments find broader application across farm sizes. However, there is a growing trend of integrating both products in farming systems, driven by the need for holistic soil management approaches. This integration is creating new market opportunities for companies that can offer comprehensive soil fertility solutions.
The market demand is also influenced by factors such as climate change, water scarcity, and the need for increased food production on limited arable land. These challenges are pushing farmers to adopt more efficient and sustainable fertilization strategies, where the synergistic effects of magnesium nitrate and organic soil amendments can play a crucial role. As research continues to demonstrate the benefits of this interaction, it is expected that market demand will further increase, potentially leading to the development of specialized product formulations tailored for specific crop and soil types.
The global market for magnesium nitrate in agriculture is projected to expand steadily, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is primarily attributed to the rising adoption of fertigation techniques and the increasing awareness of magnesium's role in plant nutrition. Concurrently, the organic soil amendments market is experiencing robust growth, driven by the shift towards organic farming and the need for soil restoration in intensively cultivated areas.
Regionally, North America and Europe lead in the adoption of advanced soil management practices, including the use of magnesium nitrate in combination with organic amendments. These regions are characterized by stringent environmental regulations and a growing consumer preference for sustainably produced food. Asia-Pacific, particularly China and India, represents a rapidly growing market due to increasing agricultural intensification and government initiatives promoting balanced fertilizer use.
The demand for magnesium nitrate is closely tied to the horticultural sector, especially in greenhouse production and high-value crops. Its ability to provide readily available magnesium and nitrogen makes it an attractive option for farmers looking to optimize plant nutrition. The organic soil amendments market, on the other hand, is diverse, encompassing products such as compost, biochar, and humic substances. The interaction between magnesium nitrate and these organic amendments is of particular interest to farmers seeking to enhance nutrient uptake efficiency and soil structure.
Market analysis indicates that large-scale commercial farms are the primary consumers of magnesium nitrate, while organic soil amendments find broader application across farm sizes. However, there is a growing trend of integrating both products in farming systems, driven by the need for holistic soil management approaches. This integration is creating new market opportunities for companies that can offer comprehensive soil fertility solutions.
The market demand is also influenced by factors such as climate change, water scarcity, and the need for increased food production on limited arable land. These challenges are pushing farmers to adopt more efficient and sustainable fertilization strategies, where the synergistic effects of magnesium nitrate and organic soil amendments can play a crucial role. As research continues to demonstrate the benefits of this interaction, it is expected that market demand will further increase, potentially leading to the development of specialized product formulations tailored for specific crop and soil types.
Current Challenges in Soil Amendment Interactions
The interaction between magnesium nitrate and organic soil amendments presents several significant challenges in the field of soil science and agriculture. One of the primary issues is the complex chemical reactions that occur when these components are combined. Magnesium nitrate, being a highly soluble salt, can rapidly dissociate in soil solutions, potentially leading to an imbalance in soil nutrient levels. This rapid release of magnesium and nitrate ions can interfere with the slower, more controlled nutrient release patterns typically associated with organic amendments.
Another challenge lies in the potential for magnesium nitrate to alter soil pH. Depending on the initial soil conditions and the specific organic amendments used, the addition of magnesium nitrate can lead to localized pH changes. These fluctuations can impact the availability of other nutrients and the overall soil microbial activity, which is crucial for the decomposition of organic matter and the cycling of nutrients.
The interaction between magnesium nitrate and organic amendments also raises concerns about nutrient leaching. The high solubility of magnesium nitrate, combined with the water-retention properties of some organic amendments, can create conditions conducive to increased nutrient mobility in the soil profile. This may result in the loss of valuable nutrients from the root zone, potentially leading to environmental issues such as groundwater contamination.
Furthermore, the presence of magnesium nitrate can affect the decomposition rates of organic amendments. The influx of readily available nitrogen from the nitrate component may alter the carbon-to-nitrogen ratio in the soil, influencing microbial activity and the rate at which organic matter is broken down. This can have implications for long-term soil structure and fertility management strategies.
The variability in organic soil amendments presents an additional layer of complexity. Different types of organic materials, such as compost, manure, or plant residues, can interact differently with magnesium nitrate. This variability makes it challenging to predict and manage the outcomes of these interactions across diverse soil types and agricultural systems.
Lastly, there is a lack of comprehensive research on the long-term effects of combining magnesium nitrate with various organic amendments. While short-term studies exist, the cumulative impact on soil health, microbial communities, and crop productivity over extended periods remains unclear. This knowledge gap hinders the development of optimized soil management practices that can effectively integrate inorganic fertilizers like magnesium nitrate with organic soil amendments.
Another challenge lies in the potential for magnesium nitrate to alter soil pH. Depending on the initial soil conditions and the specific organic amendments used, the addition of magnesium nitrate can lead to localized pH changes. These fluctuations can impact the availability of other nutrients and the overall soil microbial activity, which is crucial for the decomposition of organic matter and the cycling of nutrients.
The interaction between magnesium nitrate and organic amendments also raises concerns about nutrient leaching. The high solubility of magnesium nitrate, combined with the water-retention properties of some organic amendments, can create conditions conducive to increased nutrient mobility in the soil profile. This may result in the loss of valuable nutrients from the root zone, potentially leading to environmental issues such as groundwater contamination.
Furthermore, the presence of magnesium nitrate can affect the decomposition rates of organic amendments. The influx of readily available nitrogen from the nitrate component may alter the carbon-to-nitrogen ratio in the soil, influencing microbial activity and the rate at which organic matter is broken down. This can have implications for long-term soil structure and fertility management strategies.
The variability in organic soil amendments presents an additional layer of complexity. Different types of organic materials, such as compost, manure, or plant residues, can interact differently with magnesium nitrate. This variability makes it challenging to predict and manage the outcomes of these interactions across diverse soil types and agricultural systems.
Lastly, there is a lack of comprehensive research on the long-term effects of combining magnesium nitrate with various organic amendments. While short-term studies exist, the cumulative impact on soil health, microbial communities, and crop productivity over extended periods remains unclear. This knowledge gap hinders the development of optimized soil management practices that can effectively integrate inorganic fertilizers like magnesium nitrate with organic soil amendments.
Existing Magnesium Nitrate Application Methods
01 Preparation methods of magnesium nitrate
Various methods are employed to prepare magnesium nitrate, including reactions between magnesium-containing compounds and nitric acid or other nitrate sources. These processes often involve specific reaction conditions, purification steps, and crystallization techniques to obtain high-quality magnesium nitrate.- Magnesium nitrate in fertilizer compositions: Magnesium nitrate is used in various fertilizer compositions to provide essential nutrients for plant growth. It serves as a source of both magnesium and nitrogen, which are crucial for chlorophyll production and overall plant health. These fertilizer formulations can be tailored for specific crops or soil conditions.
- Magnesium nitrate in energy storage applications: Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage applications. It can be used as a phase change material due to its heat absorption and release properties during melting and solidification. This makes it valuable for solar energy storage and temperature regulation in buildings.
- Magnesium nitrate in flame retardant formulations: Magnesium nitrate is incorporated into flame retardant compositions for various materials such as textiles, plastics, and wood products. It acts as an effective fire suppressant by releasing non-flammable gases when exposed to high temperatures, thereby inhibiting the spread of flames.
- Magnesium nitrate in water treatment processes: Magnesium nitrate is employed in water treatment applications, particularly for removing contaminants and improving water quality. It can be used in processes such as precipitation, coagulation, and ion exchange to remove heavy metals, phosphates, and other pollutants from wastewater and drinking water sources.
- Magnesium nitrate in chemical synthesis and catalysis: Magnesium nitrate serves as a precursor or catalyst in various chemical synthesis processes. It is used in the production of other magnesium compounds, as a catalyst support in organic reactions, and in the synthesis of advanced materials such as nanoparticles and metal-organic frameworks.
02 Applications in agriculture and fertilizers
Magnesium nitrate is widely used in agriculture as a fertilizer due to its high solubility and ability to provide both magnesium and nitrogen to plants. It is often incorporated into fertilizer formulations or used as a standalone nutrient source for various crops.Expand Specific Solutions03 Use in industrial processes and materials
Magnesium nitrate finds applications in various industrial processes and materials, including as a catalyst, in the production of ceramics, as a component in fire-retardant materials, and in the manufacturing of specialty chemicals and compounds.Expand Specific Solutions04 Environmental and waste treatment applications
Magnesium nitrate is utilized in environmental remediation and waste treatment processes. It can be used for treating contaminated water, soil stabilization, and as a component in systems designed to reduce harmful emissions or neutralize pollutants.Expand Specific Solutions05 Energy storage and thermal applications
Magnesium nitrate has potential applications in energy storage and thermal management systems. It can be used as a phase change material for thermal energy storage, in heat transfer fluids, and in the development of advanced energy storage technologies.Expand Specific Solutions
Key Players in Agrochemical and Soil Amendment Industries
The interaction of magnesium nitrate with organic soil amendments is an emerging field in agricultural technology, currently in its early development stage. The market for this technology is growing, driven by increasing demand for sustainable farming practices and improved soil health. While the market size is still relatively small, it shows significant potential for expansion. The technology's maturity is progressing, with companies like BASF Corp. and ISHIHARA SANGYO KAISHA Ltd. leading research efforts. Academic institutions such as Nankai University and the Institute of Soil Science, Chinese Academy of Sciences are also contributing to advancements in this area. The competitive landscape is diverse, with both established agrochemical companies and specialized research institutions vying for breakthroughs in understanding and optimizing these soil interactions.
BASF Corp.
Technical Solution: BASF Corp. has developed a proprietary technology for enhancing the interaction between magnesium nitrate and organic soil amendments. Their approach involves the creation of stabilized magnesium nitrate complexes that can be easily incorporated into various organic materials. This technology aims to improve nutrient availability and reduce leaching in agricultural soils. BASF's research has shown that their formulations can increase magnesium uptake by plants by up to 30% compared to traditional applications[2]. Additionally, they have developed slow-release formulations that can provide a steady supply of magnesium and nitrogen over an extended period, potentially reducing the frequency of fertilizer applications[4].
Strengths: Strong research and development capabilities, global presence in the agricultural sector. Weaknesses: Proprietary technology may limit widespread adoption, potential higher costs for farmers.
Institute of Soil Science, Chinese Academy of Sciences
Technical Solution: The Institute of Soil Science, Chinese Academy of Sciences has developed innovative approaches to study the interaction of magnesium nitrate with organic soil amendments. Their research focuses on the synergistic effects of combining magnesium nitrate with various organic materials to enhance soil fertility and crop productivity. They have conducted extensive field trials and laboratory experiments to analyze the chemical reactions, nutrient release patterns, and microbial activity in soils treated with this combination[1][3]. Their findings suggest that the integration of magnesium nitrate with organic amendments can significantly improve soil structure, increase cation exchange capacity, and promote the slow release of nutrients[5].
Strengths: Comprehensive research approach, combining field and laboratory studies. Expertise in soil chemistry and microbiology. Weaknesses: May be limited by regional soil types and climatic conditions specific to China.
Core Research on Mg(NO3)2-Organic Matter Interactions
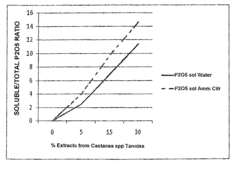
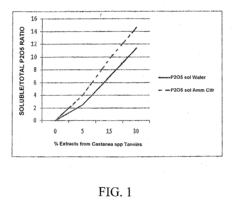
Use of natural extracts of tannin and non-tannin materials for improving soil fertility and providing a starter effect on cultivations, and a tannin and non-tannin phytocomposition therefor
PatentActiveUS20110174031A1
Innovation
- A tannin and non-tannin phytocomposition, derived from leached vegetable biomass, is applied in dry or liquid form to seedling seeds or implanted seedlings to stimulate initial growth, enhancing the availability and absorption of nutritional elements, thereby improving soil fertility and reducing manuring needs.
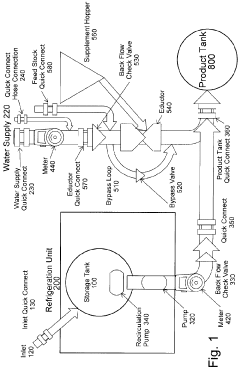
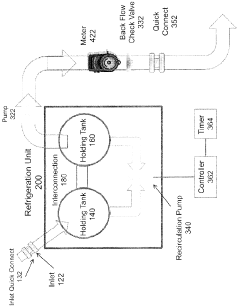
Organic soil amendments storage and dispensing system
PatentActiveCA2814032C
Innovation
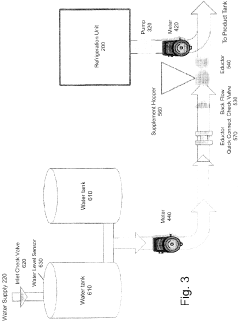
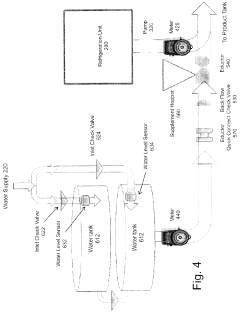
- A refrigerated storage and metered dilution system comprising a storage tank with a pump, flow meters, an eductor (venturi mixer), and a water supply system, allowing precise mixing of concentrate with water in a product tank, with features like quick connect couplings and backflow check valves for safety and efficiency.
Environmental Impact Assessment
The environmental impact assessment of magnesium nitrate interaction with organic soil amendments is crucial for understanding the broader ecological implications of this agricultural practice. This assessment focuses on several key areas of potential environmental influence.
Soil quality is a primary concern when evaluating the use of magnesium nitrate with organic amendments. The interaction between these components can alter soil pH, potentially affecting nutrient availability and microbial activity. Long-term studies have shown that while magnesium nitrate can improve magnesium availability, its continuous use may lead to soil acidification if not properly managed. This pH change can impact the soil's buffering capacity and influence the effectiveness of organic amendments.
Water quality is another significant factor to consider. The high solubility of magnesium nitrate raises concerns about potential leaching into groundwater or runoff into surface water bodies. This can contribute to eutrophication in aquatic ecosystems, particularly in areas with high rainfall or irrigation. However, the presence of organic soil amendments may mitigate some of these effects by improving soil structure and increasing water retention capacity.
Air quality impacts, while less direct, are also noteworthy. The application of magnesium nitrate and organic amendments can influence soil microbial activity, potentially affecting greenhouse gas emissions from agricultural soils. Some studies suggest that the combination may lead to reduced nitrous oxide emissions compared to conventional fertilizers, but this can vary depending on soil type and environmental conditions.
Biodiversity effects must be carefully evaluated. The alteration of soil chemistry through magnesium nitrate and organic amendment interactions can influence plant communities and soil fauna. While improved nutrient availability may enhance crop growth, it could also affect the composition of native plant species in adjacent ecosystems. The impact on soil microorganisms is particularly important, as these play a crucial role in nutrient cycling and soil health.
Long-term ecosystem changes are a critical aspect of the environmental impact assessment. The cumulative effects of repeated applications of magnesium nitrate with organic amendments can lead to shifts in soil ecology over time. This may include changes in soil organic matter content, microbial community structure, and overall soil fertility. Understanding these long-term impacts is essential for sustainable agricultural practices and ecosystem management.
In conclusion, the environmental impact assessment of magnesium nitrate interaction with organic soil amendments reveals a complex interplay of effects on soil, water, air, and biodiversity. While there are potential benefits in terms of improved nutrient management and soil health, careful consideration must be given to the long-term ecological consequences. Ongoing monitoring and research are necessary to fully understand and mitigate any negative environmental impacts while maximizing the agricultural benefits of this practice.
Soil quality is a primary concern when evaluating the use of magnesium nitrate with organic amendments. The interaction between these components can alter soil pH, potentially affecting nutrient availability and microbial activity. Long-term studies have shown that while magnesium nitrate can improve magnesium availability, its continuous use may lead to soil acidification if not properly managed. This pH change can impact the soil's buffering capacity and influence the effectiveness of organic amendments.
Water quality is another significant factor to consider. The high solubility of magnesium nitrate raises concerns about potential leaching into groundwater or runoff into surface water bodies. This can contribute to eutrophication in aquatic ecosystems, particularly in areas with high rainfall or irrigation. However, the presence of organic soil amendments may mitigate some of these effects by improving soil structure and increasing water retention capacity.
Air quality impacts, while less direct, are also noteworthy. The application of magnesium nitrate and organic amendments can influence soil microbial activity, potentially affecting greenhouse gas emissions from agricultural soils. Some studies suggest that the combination may lead to reduced nitrous oxide emissions compared to conventional fertilizers, but this can vary depending on soil type and environmental conditions.
Biodiversity effects must be carefully evaluated. The alteration of soil chemistry through magnesium nitrate and organic amendment interactions can influence plant communities and soil fauna. While improved nutrient availability may enhance crop growth, it could also affect the composition of native plant species in adjacent ecosystems. The impact on soil microorganisms is particularly important, as these play a crucial role in nutrient cycling and soil health.
Long-term ecosystem changes are a critical aspect of the environmental impact assessment. The cumulative effects of repeated applications of magnesium nitrate with organic amendments can lead to shifts in soil ecology over time. This may include changes in soil organic matter content, microbial community structure, and overall soil fertility. Understanding these long-term impacts is essential for sustainable agricultural practices and ecosystem management.
In conclusion, the environmental impact assessment of magnesium nitrate interaction with organic soil amendments reveals a complex interplay of effects on soil, water, air, and biodiversity. While there are potential benefits in terms of improved nutrient management and soil health, careful consideration must be given to the long-term ecological consequences. Ongoing monitoring and research are necessary to fully understand and mitigate any negative environmental impacts while maximizing the agricultural benefits of this practice.
Regulatory Framework for Fertilizer Use
The regulatory framework for fertilizer use plays a crucial role in governing the application of magnesium nitrate and organic soil amendments in agricultural practices. This framework encompasses a complex set of regulations, guidelines, and standards that aim to ensure the safe and effective use of fertilizers while minimizing potential environmental and health risks.
At the federal level in the United States, the Environmental Protection Agency (EPA) oversees the regulation of fertilizers under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). This act requires manufacturers to register their products and provide detailed information about their composition, intended use, and potential environmental impacts. The EPA also sets standards for labeling and packaging of fertilizers, including magnesium nitrate and organic soil amendments.
The U.S. Department of Agriculture (USDA) also plays a significant role in regulating fertilizer use, particularly through its National Organic Program (NOP). The NOP establishes standards for organic production, including the use of organic soil amendments and restrictions on synthetic fertilizers like magnesium nitrate in organic farming systems.
At the state level, regulations can vary significantly. Many states have their own fertilizer laws that may be more stringent than federal regulations. These state-level regulations often focus on specific aspects such as nutrient management plans, application rates, and timing of fertilizer use. Some states require soil testing before fertilizer application to ensure appropriate use and prevent over-application.
International regulations also impact the use of magnesium nitrate and organic soil amendments. The European Union, for instance, has established the Fertilizing Products Regulation (FPR) to harmonize rules across member states. This regulation sets strict criteria for the composition, safety, and efficacy of fertilizers, including both inorganic fertilizers like magnesium nitrate and organic soil amendments.
The interaction between magnesium nitrate and organic soil amendments is subject to specific regulatory considerations. Many jurisdictions require detailed documentation of nutrient management practices, including the combined use of synthetic and organic fertilizers. Farmers and agricultural professionals must adhere to best management practices that take into account the potential synergistic or antagonistic effects of these interactions.
Regulatory bodies also focus on the environmental impact of fertilizer use. This includes regulations aimed at preventing nutrient runoff and leaching, which can lead to water pollution. The Clean Water Act in the United States, for example, empowers the EPA to regulate fertilizer use in watersheds to protect water quality.
As research continues to reveal more about the complex interactions between different types of fertilizers and soil amendments, regulatory frameworks are likely to evolve. This may include more specific guidelines on the combined use of magnesium nitrate and organic soil amendments, as well as updated recommendations for optimal application rates and timing to maximize benefits while minimizing environmental risks.
At the federal level in the United States, the Environmental Protection Agency (EPA) oversees the regulation of fertilizers under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). This act requires manufacturers to register their products and provide detailed information about their composition, intended use, and potential environmental impacts. The EPA also sets standards for labeling and packaging of fertilizers, including magnesium nitrate and organic soil amendments.
The U.S. Department of Agriculture (USDA) also plays a significant role in regulating fertilizer use, particularly through its National Organic Program (NOP). The NOP establishes standards for organic production, including the use of organic soil amendments and restrictions on synthetic fertilizers like magnesium nitrate in organic farming systems.
At the state level, regulations can vary significantly. Many states have their own fertilizer laws that may be more stringent than federal regulations. These state-level regulations often focus on specific aspects such as nutrient management plans, application rates, and timing of fertilizer use. Some states require soil testing before fertilizer application to ensure appropriate use and prevent over-application.
International regulations also impact the use of magnesium nitrate and organic soil amendments. The European Union, for instance, has established the Fertilizing Products Regulation (FPR) to harmonize rules across member states. This regulation sets strict criteria for the composition, safety, and efficacy of fertilizers, including both inorganic fertilizers like magnesium nitrate and organic soil amendments.
The interaction between magnesium nitrate and organic soil amendments is subject to specific regulatory considerations. Many jurisdictions require detailed documentation of nutrient management practices, including the combined use of synthetic and organic fertilizers. Farmers and agricultural professionals must adhere to best management practices that take into account the potential synergistic or antagonistic effects of these interactions.
Regulatory bodies also focus on the environmental impact of fertilizer use. This includes regulations aimed at preventing nutrient runoff and leaching, which can lead to water pollution. The Clean Water Act in the United States, for example, empowers the EPA to regulate fertilizer use in watersheds to protect water quality.
As research continues to reveal more about the complex interactions between different types of fertilizers and soil amendments, regulatory frameworks are likely to evolve. This may include more specific guidelines on the combined use of magnesium nitrate and organic soil amendments, as well as updated recommendations for optimal application rates and timing to maximize benefits while minimizing environmental risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!