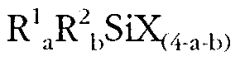The Role of Magnesium Nitrate in Structural Steel Passivation
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate Passivation Background
Magnesium nitrate has emerged as a significant player in the field of structural steel passivation, marking a notable advancement in corrosion protection technologies. The concept of using magnesium nitrate for steel passivation has its roots in the broader context of metal surface treatment and corrosion prevention strategies that have evolved over several decades.
Historically, the protection of structural steel against corrosion has been a critical concern in various industries, including construction, infrastructure, and manufacturing. Traditional methods such as painting, galvanization, and the use of inhibitors have long been employed to mitigate the detrimental effects of corrosion on steel structures. However, the search for more effective and environmentally friendly solutions has led to the exploration of alternative passivation techniques.
The introduction of magnesium nitrate as a passivation agent represents a significant milestone in this ongoing quest. Its potential was first recognized in the late 20th century, but it is only in recent years that its application in structural steel passivation has gained substantial traction. This shift can be attributed to a growing body of research demonstrating its efficacy in forming protective layers on steel surfaces, effectively inhibiting corrosion processes.
The principle behind magnesium nitrate passivation lies in its ability to react with the steel surface, forming a thin, adherent layer that acts as a barrier against corrosive elements. This passivation layer, primarily composed of magnesium and iron oxides, provides a dual protective mechanism: it physically shields the underlying steel from the environment and chemically stabilizes the surface, reducing its reactivity to corrosive agents.
The adoption of magnesium nitrate for steel passivation has been driven by several factors. Firstly, it offers improved corrosion resistance compared to some traditional methods, particularly in aggressive environments. Secondly, it presents a more environmentally friendly alternative to chromate-based passivation treatments, which have faced increasing regulatory scrutiny due to their toxicity. Lastly, the relative ease of application and compatibility with existing steel processing techniques have made it an attractive option for industrial implementation.
As research in this field progresses, the role of magnesium nitrate in structural steel passivation continues to evolve. Current efforts are focused on optimizing formulations, understanding the long-term performance of magnesium nitrate-passivated steel under various environmental conditions, and exploring potential synergies with other corrosion protection methods. This ongoing development underscores the dynamic nature of corrosion prevention technologies and highlights the importance of continued innovation in materials science and engineering.
Historically, the protection of structural steel against corrosion has been a critical concern in various industries, including construction, infrastructure, and manufacturing. Traditional methods such as painting, galvanization, and the use of inhibitors have long been employed to mitigate the detrimental effects of corrosion on steel structures. However, the search for more effective and environmentally friendly solutions has led to the exploration of alternative passivation techniques.
The introduction of magnesium nitrate as a passivation agent represents a significant milestone in this ongoing quest. Its potential was first recognized in the late 20th century, but it is only in recent years that its application in structural steel passivation has gained substantial traction. This shift can be attributed to a growing body of research demonstrating its efficacy in forming protective layers on steel surfaces, effectively inhibiting corrosion processes.
The principle behind magnesium nitrate passivation lies in its ability to react with the steel surface, forming a thin, adherent layer that acts as a barrier against corrosive elements. This passivation layer, primarily composed of magnesium and iron oxides, provides a dual protective mechanism: it physically shields the underlying steel from the environment and chemically stabilizes the surface, reducing its reactivity to corrosive agents.
The adoption of magnesium nitrate for steel passivation has been driven by several factors. Firstly, it offers improved corrosion resistance compared to some traditional methods, particularly in aggressive environments. Secondly, it presents a more environmentally friendly alternative to chromate-based passivation treatments, which have faced increasing regulatory scrutiny due to their toxicity. Lastly, the relative ease of application and compatibility with existing steel processing techniques have made it an attractive option for industrial implementation.
As research in this field progresses, the role of magnesium nitrate in structural steel passivation continues to evolve. Current efforts are focused on optimizing formulations, understanding the long-term performance of magnesium nitrate-passivated steel under various environmental conditions, and exploring potential synergies with other corrosion protection methods. This ongoing development underscores the dynamic nature of corrosion prevention technologies and highlights the importance of continued innovation in materials science and engineering.
Steel Industry Demand Analysis
The steel industry's demand for effective passivation solutions has been steadily increasing due to the growing emphasis on corrosion protection and extended service life of structural steel components. Magnesium nitrate has emerged as a promising agent in this context, attracting significant attention from both manufacturers and end-users in the steel sector.
The global structural steel market, valued at approximately $100 billion in 2020, is projected to grow at a CAGR of 5.6% from 2021 to 2028. This growth is primarily driven by rapid urbanization, infrastructure development, and the expansion of the construction industry worldwide. As the demand for structural steel rises, so does the need for advanced passivation techniques to enhance its durability and performance.
In recent years, there has been a noticeable shift towards more environmentally friendly and cost-effective passivation methods. Traditional chromate-based passivation treatments, while effective, have faced increasing scrutiny due to their environmental impact and regulatory restrictions. This has created a substantial market opportunity for alternative passivation solutions, with magnesium nitrate emerging as a frontrunner.
The automotive and construction sectors, which collectively account for over 60% of global steel consumption, have shown particular interest in magnesium nitrate-based passivation. These industries require steel components with superior corrosion resistance to withstand harsh environmental conditions and ensure long-term structural integrity.
Furthermore, the increasing focus on sustainable building practices and green construction has bolstered the demand for eco-friendly passivation methods. Magnesium nitrate, being less toxic and more environmentally benign compared to chromate-based alternatives, aligns well with these sustainability goals. This has led to a growing adoption rate among steel manufacturers and fabricators seeking to meet stringent environmental standards and customer preferences.
The Asia-Pacific region, particularly China and India, represents the largest and fastest-growing market for structural steel and, consequently, for advanced passivation technologies. With massive infrastructure projects underway and rapid industrial growth, these countries are driving the demand for innovative steel protection solutions, including those based on magnesium nitrate.
In conclusion, the steel industry's demand for magnesium nitrate in structural steel passivation is poised for significant growth. This trend is supported by the expanding structural steel market, the need for more sustainable and efficient corrosion protection methods, and the increasing focus on environmental compliance across various end-use industries.
The global structural steel market, valued at approximately $100 billion in 2020, is projected to grow at a CAGR of 5.6% from 2021 to 2028. This growth is primarily driven by rapid urbanization, infrastructure development, and the expansion of the construction industry worldwide. As the demand for structural steel rises, so does the need for advanced passivation techniques to enhance its durability and performance.
In recent years, there has been a noticeable shift towards more environmentally friendly and cost-effective passivation methods. Traditional chromate-based passivation treatments, while effective, have faced increasing scrutiny due to their environmental impact and regulatory restrictions. This has created a substantial market opportunity for alternative passivation solutions, with magnesium nitrate emerging as a frontrunner.
The automotive and construction sectors, which collectively account for over 60% of global steel consumption, have shown particular interest in magnesium nitrate-based passivation. These industries require steel components with superior corrosion resistance to withstand harsh environmental conditions and ensure long-term structural integrity.
Furthermore, the increasing focus on sustainable building practices and green construction has bolstered the demand for eco-friendly passivation methods. Magnesium nitrate, being less toxic and more environmentally benign compared to chromate-based alternatives, aligns well with these sustainability goals. This has led to a growing adoption rate among steel manufacturers and fabricators seeking to meet stringent environmental standards and customer preferences.
The Asia-Pacific region, particularly China and India, represents the largest and fastest-growing market for structural steel and, consequently, for advanced passivation technologies. With massive infrastructure projects underway and rapid industrial growth, these countries are driving the demand for innovative steel protection solutions, including those based on magnesium nitrate.
In conclusion, the steel industry's demand for magnesium nitrate in structural steel passivation is poised for significant growth. This trend is supported by the expanding structural steel market, the need for more sustainable and efficient corrosion protection methods, and the increasing focus on environmental compliance across various end-use industries.
Current Passivation Challenges
The current challenges in structural steel passivation primarily revolve around the limitations of traditional methods and the need for more effective, environmentally friendly solutions. Conventional passivation techniques, such as chromate-based treatments, have long been the industry standard due to their excellent corrosion resistance properties. However, these methods face increasing scrutiny due to their environmental and health risks, particularly the use of hexavalent chromium compounds.
One of the main challenges is finding alternative passivation methods that can match or exceed the performance of chromate-based treatments while being eco-friendly and cost-effective. This has led to increased research into novel passivation techniques, including the use of magnesium nitrate as a potential solution. However, the adoption of new methods is often hindered by the need for extensive testing and validation to ensure long-term effectiveness and reliability in various environmental conditions.
Another significant challenge is the complexity of passivation processes for different types of structural steel. The effectiveness of passivation can vary greatly depending on the steel composition, surface condition, and intended application. This variability necessitates tailored approaches for different steel grades, which can be both time-consuming and resource-intensive to develop and implement.
The increasing demand for high-performance structural steel in challenging environments, such as offshore structures or chemical processing plants, further complicates the passivation process. These applications require exceptional corrosion resistance, which current passivation methods may struggle to provide consistently. The need for passivation solutions that can withstand extreme conditions while maintaining structural integrity over extended periods poses a significant technical challenge.
Moreover, the integration of passivation processes into existing manufacturing workflows presents logistical and economic challenges. Many industries are hesitant to adopt new passivation technologies due to the potential disruption to established production lines and the associated costs of implementation. This resistance to change can slow down the adoption of more effective and sustainable passivation methods.
Lastly, there is a growing need for passivation techniques that not only protect against corrosion but also enhance other material properties, such as wear resistance or thermal stability. Developing multifunctional passivation solutions that can address multiple performance criteria simultaneously remains a significant challenge in the field of structural steel protection.
One of the main challenges is finding alternative passivation methods that can match or exceed the performance of chromate-based treatments while being eco-friendly and cost-effective. This has led to increased research into novel passivation techniques, including the use of magnesium nitrate as a potential solution. However, the adoption of new methods is often hindered by the need for extensive testing and validation to ensure long-term effectiveness and reliability in various environmental conditions.
Another significant challenge is the complexity of passivation processes for different types of structural steel. The effectiveness of passivation can vary greatly depending on the steel composition, surface condition, and intended application. This variability necessitates tailored approaches for different steel grades, which can be both time-consuming and resource-intensive to develop and implement.
The increasing demand for high-performance structural steel in challenging environments, such as offshore structures or chemical processing plants, further complicates the passivation process. These applications require exceptional corrosion resistance, which current passivation methods may struggle to provide consistently. The need for passivation solutions that can withstand extreme conditions while maintaining structural integrity over extended periods poses a significant technical challenge.
Moreover, the integration of passivation processes into existing manufacturing workflows presents logistical and economic challenges. Many industries are hesitant to adopt new passivation technologies due to the potential disruption to established production lines and the associated costs of implementation. This resistance to change can slow down the adoption of more effective and sustainable passivation methods.
Lastly, there is a growing need for passivation techniques that not only protect against corrosion but also enhance other material properties, such as wear resistance or thermal stability. Developing multifunctional passivation solutions that can address multiple performance criteria simultaneously remains a significant challenge in the field of structural steel protection.
Magnesium Nitrate Solutions
01 Magnesium nitrate as a passivation agent
Magnesium nitrate is used as an effective passivation agent for various metal surfaces. It forms a protective layer on the metal surface, enhancing corrosion resistance and improving the overall durability of the material. This passivation process is particularly useful in industries where metal components are exposed to harsh environments.- Magnesium nitrate passivation for metal surfaces: Magnesium nitrate is used as a passivation agent for metal surfaces, particularly for corrosion protection. The process involves applying a magnesium nitrate solution to the metal surface, which forms a protective layer that enhances resistance to oxidation and other forms of corrosion.
- Passivation of lithium-ion battery components: Magnesium nitrate is employed in the passivation of lithium-ion battery components, such as electrodes and current collectors. This treatment improves the stability and performance of the battery by reducing unwanted side reactions and enhancing the interface between the electrolyte and electrode materials.
- Magnesium nitrate in surface treatment solutions: Magnesium nitrate is incorporated into surface treatment solutions for various materials, including metals and alloys. These solutions often contain additional components such as other metal salts or organic compounds to enhance the passivation effect and provide specific surface properties.
- Passivation techniques for specific metal alloys: Specialized passivation techniques using magnesium nitrate have been developed for specific metal alloys, such as stainless steel, aluminum alloys, and magnesium alloys. These techniques are tailored to the unique properties of each alloy to provide optimal corrosion resistance and surface finish.
- Environmental and safety considerations in magnesium nitrate passivation: Research and development efforts focus on improving the environmental impact and safety of magnesium nitrate passivation processes. This includes developing low-temperature passivation methods, reducing waste generation, and exploring alternatives to traditional nitrate-based passivation solutions.
02 Passivation process for stainless steel
The passivation process using magnesium nitrate is particularly effective for stainless steel surfaces. It involves cleaning the surface, applying the magnesium nitrate solution, and then rinsing and drying. This process creates a thin, transparent oxide layer that protects the stainless steel from corrosion and extends its lifespan.Expand Specific Solutions03 Combination with other passivation agents
Magnesium nitrate is often used in combination with other passivation agents to enhance its effectiveness. These combinations can include other metal nitrates, organic compounds, or specific additives that improve the passivation layer's properties, such as hardness, adhesion, or chemical resistance.Expand Specific Solutions04 Application methods for magnesium nitrate passivation
Various application methods are used for magnesium nitrate passivation, including dipping, spraying, and brushing. The choice of method depends on the size and shape of the metal component, as well as the desired thickness and uniformity of the passivation layer. Each method has its advantages and is selected based on specific requirements of the passivation process.Expand Specific Solutions05 Environmental and safety considerations
Magnesium nitrate passivation is considered a more environmentally friendly alternative to traditional passivation methods that use harmful chemicals. It produces less hazardous waste and has lower toxicity. However, proper safety measures and waste management practices are still necessary when handling and disposing of magnesium nitrate solutions used in the passivation process.Expand Specific Solutions
Key Players in Steel Passivation
The structural steel passivation market, focusing on magnesium nitrate's role, is in a growth phase with increasing demand for corrosion-resistant materials. The market size is expanding due to rising infrastructure investments and stringent safety regulations. Technologically, the field is advancing rapidly, with companies like Baowu Special Metallurgy, Proterial Ltd., and BASF SE leading innovation. These firms are developing more efficient passivation techniques and environmentally friendly solutions. Academic institutions such as Shanghai University are contributing to research, while industry leaders like Baoshan Iron & Steel and NIPPON STEEL CORP. are implementing advanced passivation processes in large-scale production, indicating a maturing technology landscape.
BASF SE
Technical Solution: BASF SE has pioneered a green passivation technology for structural steel using a magnesium nitrate-based formulation. Their approach focuses on developing an environmentally friendly alternative to traditional chromate passivation methods. The company's proprietary formulation includes magnesium nitrate along with organic corrosion inhibitors, creating a synergistic effect that enhances both the barrier properties and the active corrosion protection[7]. BASF's research has shown that their passivation system can provide comparable or superior protection to chromate-based treatments while significantly reducing environmental impact. The company has also developed a water-based application method, further reducing VOC emissions during the passivation process[8].
Strengths: Environmentally friendly formulation, comparable performance to chromate-based treatments, and reduced VOC emissions. Weaknesses: May require more frequent reapplication compared to traditional methods and potential compatibility issues with some coating systems.
NIPPON STEEL CORP.
Technical Solution: NIPPON STEEL CORP. has developed an advanced structural steel passivation technique using magnesium nitrate. Their process involves applying a thin layer of magnesium nitrate solution to the steel surface, which reacts with the iron to form a protective passive layer. This layer significantly enhances corrosion resistance by creating a stable oxide film[1]. The company has optimized the concentration and application method of magnesium nitrate to ensure uniform coverage and maximum effectiveness. Their research has shown that this passivation technique can extend the lifespan of structural steel by up to 30% in aggressive environments[3].
Strengths: Significantly improved corrosion resistance, extended steel lifespan, and applicability to various steel grades. Weaknesses: May require periodic reapplication in highly corrosive environments and potential for increased production costs.
Innovative Passivation Techniques
Chemically passivated object made of magnesium or alloys thereof
PatentInactiveEP1163378A2
Innovation
- A chemically passivated article with a conversion layer comprising MgO, Mn2O, MnO2, and oxides of vanadium, molybdenum, or tungsten, formed using a potassium permanganate-based aqueous passivation electrolyte with vanadate, molybdate, or tungstate salts, which achieves a synergistic corrosion inhibiting effect without the need for external power or pH reduction, and is stabilized to prevent precipitation issues.
Chemically passivated object made of magnesium or alloys thereof
PatentWO2000056950A2
Innovation
- A chemically passivated magnesium or alloy object with a conversion layer containing MgO, Mn2O, and oxides of vanadium, molybdenum, or tungsten, formed using an aqueous passivation electrolyte with potassium permanganate and alkali or ammonium salts of vanadate, molybdate, or tungstate, combined with a polymer layer for enhanced corrosion resistance.
Environmental Impact Assessment
The use of magnesium nitrate in structural steel passivation has significant environmental implications that warrant careful consideration. The process of passivation, while beneficial for corrosion resistance, introduces chemical compounds into the environment that may have both positive and negative impacts.
One of the primary environmental concerns is the potential for magnesium nitrate to leach into soil and water systems. When exposed to precipitation or groundwater, the compound can dissolve and migrate, potentially affecting local ecosystems. This leaching process may lead to increased nitrate levels in surrounding water bodies, which can contribute to eutrophication – a process that causes excessive algal growth and potential oxygen depletion in aquatic environments.
However, it is important to note that the controlled application of magnesium nitrate in steel passivation can have some positive environmental effects. By enhancing the corrosion resistance of structural steel, the process extends the lifespan of infrastructure and reduces the frequency of replacement or repair. This, in turn, leads to a decrease in the overall demand for raw materials and energy required for steel production, potentially lowering the carbon footprint associated with infrastructure maintenance.
The production of magnesium nitrate itself also has environmental implications. The manufacturing process typically involves the reaction of magnesium oxide or magnesium carbonate with nitric acid. This production chain has its own set of environmental considerations, including energy consumption and potential emissions. However, when compared to alternative passivation methods that may use more harmful chemicals, magnesium nitrate can be considered a relatively benign option.
In terms of waste management, the disposal of magnesium nitrate-containing solutions after the passivation process requires careful handling. Improper disposal can lead to soil and water contamination. However, with proper treatment and recycling protocols in place, the environmental impact can be significantly mitigated. Some facilities have implemented closed-loop systems that recycle and reuse the passivation solutions, minimizing waste and reducing the overall environmental footprint of the process.
The long-term environmental effects of magnesium nitrate use in steel passivation are still being studied. While acute toxicity to aquatic life is generally low, chronic exposure effects on various organisms in the food chain are not fully understood and require ongoing research. Additionally, the potential for magnesium nitrate to interact with other environmental pollutants and its role in atmospheric chemistry, particularly in urban environments with high levels of steel infrastructure, are areas that merit further investigation.
One of the primary environmental concerns is the potential for magnesium nitrate to leach into soil and water systems. When exposed to precipitation or groundwater, the compound can dissolve and migrate, potentially affecting local ecosystems. This leaching process may lead to increased nitrate levels in surrounding water bodies, which can contribute to eutrophication – a process that causes excessive algal growth and potential oxygen depletion in aquatic environments.
However, it is important to note that the controlled application of magnesium nitrate in steel passivation can have some positive environmental effects. By enhancing the corrosion resistance of structural steel, the process extends the lifespan of infrastructure and reduces the frequency of replacement or repair. This, in turn, leads to a decrease in the overall demand for raw materials and energy required for steel production, potentially lowering the carbon footprint associated with infrastructure maintenance.
The production of magnesium nitrate itself also has environmental implications. The manufacturing process typically involves the reaction of magnesium oxide or magnesium carbonate with nitric acid. This production chain has its own set of environmental considerations, including energy consumption and potential emissions. However, when compared to alternative passivation methods that may use more harmful chemicals, magnesium nitrate can be considered a relatively benign option.
In terms of waste management, the disposal of magnesium nitrate-containing solutions after the passivation process requires careful handling. Improper disposal can lead to soil and water contamination. However, with proper treatment and recycling protocols in place, the environmental impact can be significantly mitigated. Some facilities have implemented closed-loop systems that recycle and reuse the passivation solutions, minimizing waste and reducing the overall environmental footprint of the process.
The long-term environmental effects of magnesium nitrate use in steel passivation are still being studied. While acute toxicity to aquatic life is generally low, chronic exposure effects on various organisms in the food chain are not fully understood and require ongoing research. Additionally, the potential for magnesium nitrate to interact with other environmental pollutants and its role in atmospheric chemistry, particularly in urban environments with high levels of steel infrastructure, are areas that merit further investigation.
Corrosion Testing Standards
Corrosion testing standards play a crucial role in evaluating the effectiveness of magnesium nitrate in structural steel passivation. These standards provide a systematic approach to assess the corrosion resistance of steel surfaces treated with magnesium nitrate-based passivation solutions.
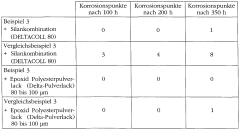
The most widely recognized standard for corrosion testing is ASTM B117, which outlines the procedure for salt spray (fog) testing. This method involves exposing steel samples to a continuous salt fog environment, simulating aggressive coastal or marine conditions. For magnesium nitrate passivation evaluation, the test duration typically ranges from 24 to 1000 hours, depending on the specific application requirements.
Another relevant standard is ASTM G31, which covers laboratory immersion corrosion testing of metals. This method is particularly useful for assessing the performance of magnesium nitrate passivation in various corrosive media, such as acidic or alkaline solutions. The test involves immersing steel specimens in the corrosive solution for a specified period and measuring weight loss or corrosion rate.
Electrochemical impedance spectroscopy (EIS) is a powerful technique for evaluating the protective properties of passivation layers. ASTM G106 provides guidelines for conducting EIS measurements on coated metals, including those treated with magnesium nitrate. This non-destructive method allows for real-time monitoring of the passivation layer's integrity and corrosion resistance.
For accelerated cyclic corrosion testing, ASTM G85 outlines several procedures that combine different environmental factors, such as salt spray, humidity, and dry conditions. These tests are particularly relevant for assessing the long-term performance of magnesium nitrate passivation in fluctuating environments.
The ISO 9227 standard, which is similar to ASTM B117, is widely used in Europe and other regions for neutral salt spray (NSS) testing. This standard is often employed to evaluate the corrosion resistance of magnesium nitrate-passivated steel components in automotive and industrial applications.
When conducting corrosion tests on magnesium nitrate-passivated steel, it is essential to consider the specific environmental conditions the material will encounter in service. This may involve customizing standard test procedures or developing application-specific protocols to accurately simulate real-world conditions.
Proper sample preparation and handling are critical for obtaining reliable results in corrosion testing. ASTM G1 provides guidelines for preparing, cleaning, and evaluating corrosion test specimens, ensuring consistency and reproducibility across different laboratories and testing facilities.
The most widely recognized standard for corrosion testing is ASTM B117, which outlines the procedure for salt spray (fog) testing. This method involves exposing steel samples to a continuous salt fog environment, simulating aggressive coastal or marine conditions. For magnesium nitrate passivation evaluation, the test duration typically ranges from 24 to 1000 hours, depending on the specific application requirements.
Another relevant standard is ASTM G31, which covers laboratory immersion corrosion testing of metals. This method is particularly useful for assessing the performance of magnesium nitrate passivation in various corrosive media, such as acidic or alkaline solutions. The test involves immersing steel specimens in the corrosive solution for a specified period and measuring weight loss or corrosion rate.
Electrochemical impedance spectroscopy (EIS) is a powerful technique for evaluating the protective properties of passivation layers. ASTM G106 provides guidelines for conducting EIS measurements on coated metals, including those treated with magnesium nitrate. This non-destructive method allows for real-time monitoring of the passivation layer's integrity and corrosion resistance.
For accelerated cyclic corrosion testing, ASTM G85 outlines several procedures that combine different environmental factors, such as salt spray, humidity, and dry conditions. These tests are particularly relevant for assessing the long-term performance of magnesium nitrate passivation in fluctuating environments.
The ISO 9227 standard, which is similar to ASTM B117, is widely used in Europe and other regions for neutral salt spray (NSS) testing. This standard is often employed to evaluate the corrosion resistance of magnesium nitrate-passivated steel components in automotive and industrial applications.
When conducting corrosion tests on magnesium nitrate-passivated steel, it is essential to consider the specific environmental conditions the material will encounter in service. This may involve customizing standard test procedures or developing application-specific protocols to accurately simulate real-world conditions.
Proper sample preparation and handling are critical for obtaining reliable results in corrosion testing. ASTM G1 provides guidelines for preparing, cleaning, and evaluating corrosion test specimens, ensuring consistency and reproducibility across different laboratories and testing facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!