Comparing Magnesium Nitrate and Potassium Nitrate in Thermal Batteries
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermal Battery Evolution
Thermal batteries have undergone significant evolution since their inception in the 1940s. Initially developed for military applications, these power sources have seen continuous improvements in performance, reliability, and versatility over the decades.
The early thermal batteries utilized calcium/calcium chromate as the primary electrochemical couple. This combination provided a stable and reliable power source but was limited in its energy density and operational temperature range. As technology progressed, the focus shifted towards more efficient and higher energy density materials.
In the 1960s and 1970s, lithium-based chemistries emerged as a promising alternative. The introduction of lithium alloys as anodes, coupled with various metal sulfides as cathodes, marked a significant leap in thermal battery capabilities. These new chemistries offered higher voltage, improved energy density, and extended shelf life.
The 1980s and 1990s saw further refinements in lithium-based systems. The development of lithium-silicon alloys as anodes and iron disulfide cathodes became a standard configuration, offering a balance of performance and cost-effectiveness. This period also witnessed advancements in electrolyte compositions, with research focusing on optimizing the molten salt mixtures to enhance ionic conductivity and thermal stability.
The turn of the millennium brought about a renewed interest in thermal battery technology, driven by the increasing demand for high-power, long-shelf-life energy sources in both military and civilian applications. This era saw the exploration of novel cathode materials, including transition metal oxides and advanced composite structures, aimed at pushing the boundaries of power density and operational temperature ranges.
In recent years, the focus has shifted towards enhancing the versatility and environmental compatibility of thermal batteries. Research efforts have been directed at developing "green" thermal batteries with reduced environmental impact, exploring alternatives to traditional pyrotechnic heat sources, and improving the overall safety profile of these power systems.
The evolution of thermal batteries has also been marked by advancements in manufacturing techniques and quality control processes. Modern production methods have significantly improved the consistency and reliability of thermal batteries, making them suitable for an ever-expanding range of applications beyond their traditional military use.
As we look at the specific comparison between magnesium nitrate and potassium nitrate in thermal batteries, it's important to note that this represents a continuation of the ongoing quest for optimal electrolyte compositions. The exploration of these materials reflects the industry's commitment to fine-tuning thermal battery performance, seeking to balance factors such as ionic conductivity, melting point, and compatibility with other battery components.
The early thermal batteries utilized calcium/calcium chromate as the primary electrochemical couple. This combination provided a stable and reliable power source but was limited in its energy density and operational temperature range. As technology progressed, the focus shifted towards more efficient and higher energy density materials.
In the 1960s and 1970s, lithium-based chemistries emerged as a promising alternative. The introduction of lithium alloys as anodes, coupled with various metal sulfides as cathodes, marked a significant leap in thermal battery capabilities. These new chemistries offered higher voltage, improved energy density, and extended shelf life.
The 1980s and 1990s saw further refinements in lithium-based systems. The development of lithium-silicon alloys as anodes and iron disulfide cathodes became a standard configuration, offering a balance of performance and cost-effectiveness. This period also witnessed advancements in electrolyte compositions, with research focusing on optimizing the molten salt mixtures to enhance ionic conductivity and thermal stability.
The turn of the millennium brought about a renewed interest in thermal battery technology, driven by the increasing demand for high-power, long-shelf-life energy sources in both military and civilian applications. This era saw the exploration of novel cathode materials, including transition metal oxides and advanced composite structures, aimed at pushing the boundaries of power density and operational temperature ranges.
In recent years, the focus has shifted towards enhancing the versatility and environmental compatibility of thermal batteries. Research efforts have been directed at developing "green" thermal batteries with reduced environmental impact, exploring alternatives to traditional pyrotechnic heat sources, and improving the overall safety profile of these power systems.
The evolution of thermal batteries has also been marked by advancements in manufacturing techniques and quality control processes. Modern production methods have significantly improved the consistency and reliability of thermal batteries, making them suitable for an ever-expanding range of applications beyond their traditional military use.
As we look at the specific comparison between magnesium nitrate and potassium nitrate in thermal batteries, it's important to note that this represents a continuation of the ongoing quest for optimal electrolyte compositions. The exploration of these materials reflects the industry's commitment to fine-tuning thermal battery performance, seeking to balance factors such as ionic conductivity, melting point, and compatibility with other battery components.
Market Demand Analysis
The market demand for thermal batteries utilizing magnesium nitrate and potassium nitrate has been steadily growing, driven by increasing applications in defense, aerospace, and energy storage sectors. These batteries offer high energy density, long shelf life, and reliable performance under extreme conditions, making them crucial for various mission-critical applications.
In the defense sector, thermal batteries are extensively used in guided missiles, torpedoes, and smart munitions. The global defense market's expansion, particularly in regions like North America, Europe, and Asia-Pacific, has significantly boosted the demand for these batteries. The increasing focus on modernizing military equipment and enhancing missile systems has further accelerated this growth.
The aerospace industry represents another major market for thermal batteries. With the rise in satellite launches and space exploration missions, the demand for reliable power sources capable of withstanding harsh space environments has surged. Thermal batteries, with their ability to remain dormant for long periods and activate quickly when needed, are ideal for these applications.
The energy storage sector is emerging as a promising market for thermal batteries. As renewable energy sources become more prevalent, there's a growing need for efficient and reliable energy storage solutions. Thermal batteries, particularly those using magnesium nitrate and potassium nitrate, are being explored for grid-scale energy storage applications due to their high energy density and thermal stability.
Market analysis indicates that the global thermal battery market is expected to grow significantly in the coming years. The increasing adoption of electric vehicles and the development of smart grids are creating new opportunities for thermal battery applications, further driving market growth.
Comparing magnesium nitrate and potassium nitrate in thermal batteries, both materials have their unique advantages. Magnesium nitrate offers higher energy density and better performance at higher temperatures, making it suitable for applications requiring intense power output. Potassium nitrate, on the other hand, provides better stability and is more cost-effective, making it preferable for applications where long-term reliability is crucial.
The market demand for these specific materials in thermal batteries is influenced by factors such as performance requirements, cost considerations, and specific application needs. As research and development in this field continue, the market is likely to see innovations that optimize the use of both magnesium nitrate and potassium nitrate, potentially leading to hybrid solutions that leverage the strengths of both materials.
In the defense sector, thermal batteries are extensively used in guided missiles, torpedoes, and smart munitions. The global defense market's expansion, particularly in regions like North America, Europe, and Asia-Pacific, has significantly boosted the demand for these batteries. The increasing focus on modernizing military equipment and enhancing missile systems has further accelerated this growth.
The aerospace industry represents another major market for thermal batteries. With the rise in satellite launches and space exploration missions, the demand for reliable power sources capable of withstanding harsh space environments has surged. Thermal batteries, with their ability to remain dormant for long periods and activate quickly when needed, are ideal for these applications.
The energy storage sector is emerging as a promising market for thermal batteries. As renewable energy sources become more prevalent, there's a growing need for efficient and reliable energy storage solutions. Thermal batteries, particularly those using magnesium nitrate and potassium nitrate, are being explored for grid-scale energy storage applications due to their high energy density and thermal stability.
Market analysis indicates that the global thermal battery market is expected to grow significantly in the coming years. The increasing adoption of electric vehicles and the development of smart grids are creating new opportunities for thermal battery applications, further driving market growth.
Comparing magnesium nitrate and potassium nitrate in thermal batteries, both materials have their unique advantages. Magnesium nitrate offers higher energy density and better performance at higher temperatures, making it suitable for applications requiring intense power output. Potassium nitrate, on the other hand, provides better stability and is more cost-effective, making it preferable for applications where long-term reliability is crucial.
The market demand for these specific materials in thermal batteries is influenced by factors such as performance requirements, cost considerations, and specific application needs. As research and development in this field continue, the market is likely to see innovations that optimize the use of both magnesium nitrate and potassium nitrate, potentially leading to hybrid solutions that leverage the strengths of both materials.
Current Challenges
The current challenges in comparing magnesium nitrate and potassium nitrate in thermal batteries primarily revolve around performance optimization, cost-effectiveness, and environmental considerations. One of the main technical hurdles is achieving consistent and reliable performance across a wide range of operating temperatures. Magnesium nitrate and potassium nitrate exhibit different thermal properties, which can lead to variations in battery efficiency and output depending on the specific temperature conditions.
Another significant challenge lies in the scalability of production processes for these materials. While both compounds are relatively abundant, the purification and preparation methods required for use in thermal batteries can be complex and energy-intensive. This complexity often translates to higher production costs, which can limit the widespread adoption of these technologies in certain applications.
The long-term stability of these nitrate-based electrolytes presents another obstacle. Over time, thermal cycling and exposure to high temperatures can lead to degradation of the electrolyte materials, potentially compromising the battery's performance and lifespan. Researchers are actively working on developing more stable formulations and protective measures to mitigate these effects.
Environmental concerns also pose challenges in the use of these materials. While both magnesium nitrate and potassium nitrate are generally considered less harmful than some alternative battery components, there are still considerations regarding their disposal and potential environmental impact. Developing eco-friendly recycling processes for spent thermal batteries containing these compounds is an ongoing area of research and development.
The comparison between magnesium nitrate and potassium nitrate is further complicated by their different electrochemical properties. Each compound interacts uniquely with other battery components, such as the anode and cathode materials, which can affect overall battery performance. Optimizing these interactions to maximize energy density, power output, and cycle life remains a significant challenge for researchers and engineers in the field.
Lastly, the integration of these materials into existing battery manufacturing processes presents technical challenges. Adapting production lines and quality control measures to accommodate the specific requirements of magnesium nitrate or potassium nitrate-based thermal batteries can be costly and time-consuming. This integration challenge often acts as a barrier to the rapid commercialization and widespread adoption of new thermal battery technologies utilizing these compounds.
Another significant challenge lies in the scalability of production processes for these materials. While both compounds are relatively abundant, the purification and preparation methods required for use in thermal batteries can be complex and energy-intensive. This complexity often translates to higher production costs, which can limit the widespread adoption of these technologies in certain applications.
The long-term stability of these nitrate-based electrolytes presents another obstacle. Over time, thermal cycling and exposure to high temperatures can lead to degradation of the electrolyte materials, potentially compromising the battery's performance and lifespan. Researchers are actively working on developing more stable formulations and protective measures to mitigate these effects.
Environmental concerns also pose challenges in the use of these materials. While both magnesium nitrate and potassium nitrate are generally considered less harmful than some alternative battery components, there are still considerations regarding their disposal and potential environmental impact. Developing eco-friendly recycling processes for spent thermal batteries containing these compounds is an ongoing area of research and development.
The comparison between magnesium nitrate and potassium nitrate is further complicated by their different electrochemical properties. Each compound interacts uniquely with other battery components, such as the anode and cathode materials, which can affect overall battery performance. Optimizing these interactions to maximize energy density, power output, and cycle life remains a significant challenge for researchers and engineers in the field.
Lastly, the integration of these materials into existing battery manufacturing processes presents technical challenges. Adapting production lines and quality control measures to accommodate the specific requirements of magnesium nitrate or potassium nitrate-based thermal batteries can be costly and time-consuming. This integration challenge often acts as a barrier to the rapid commercialization and widespread adoption of new thermal battery technologies utilizing these compounds.
Existing Solutions
01 Electrolyte composition and design
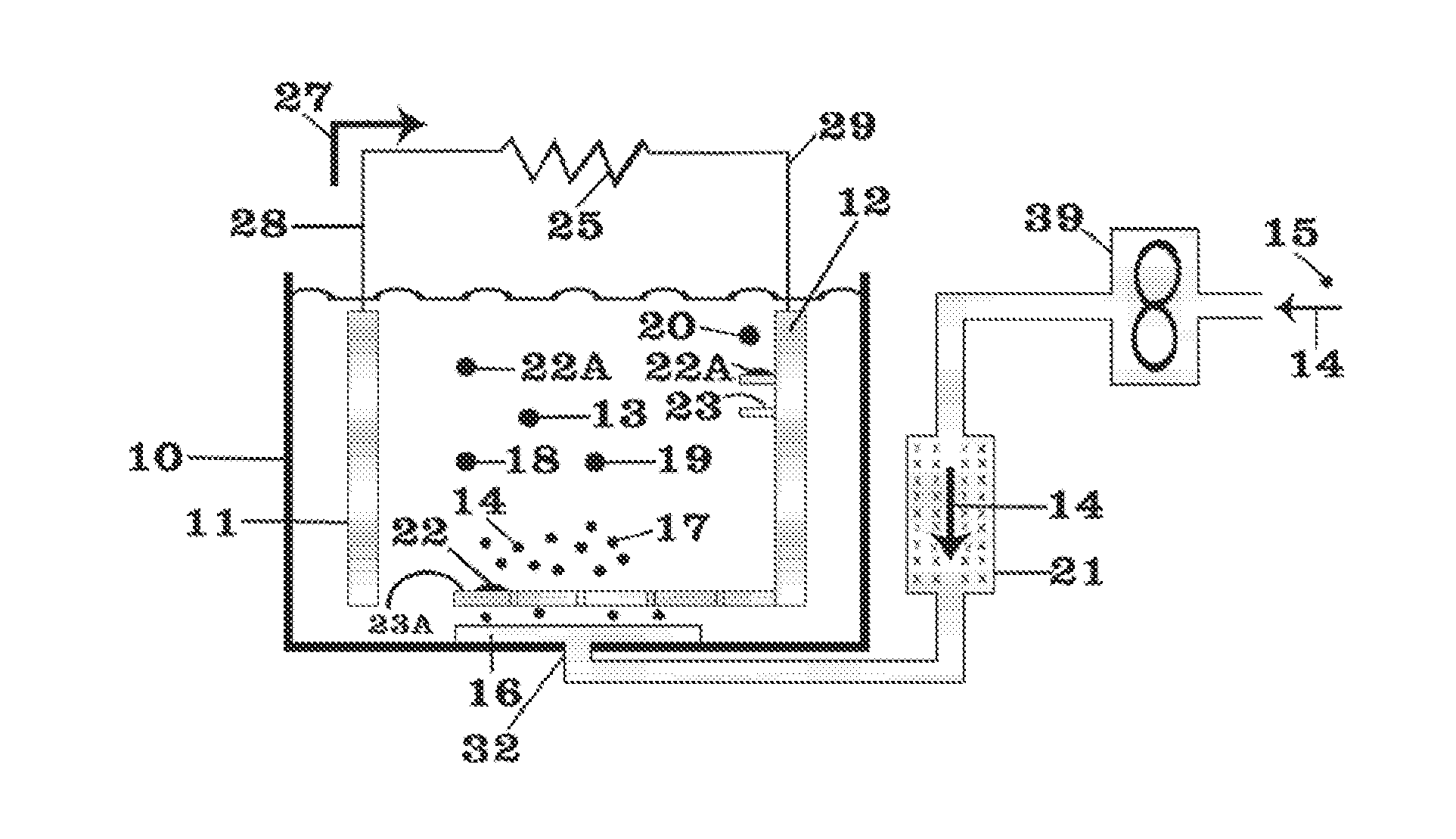
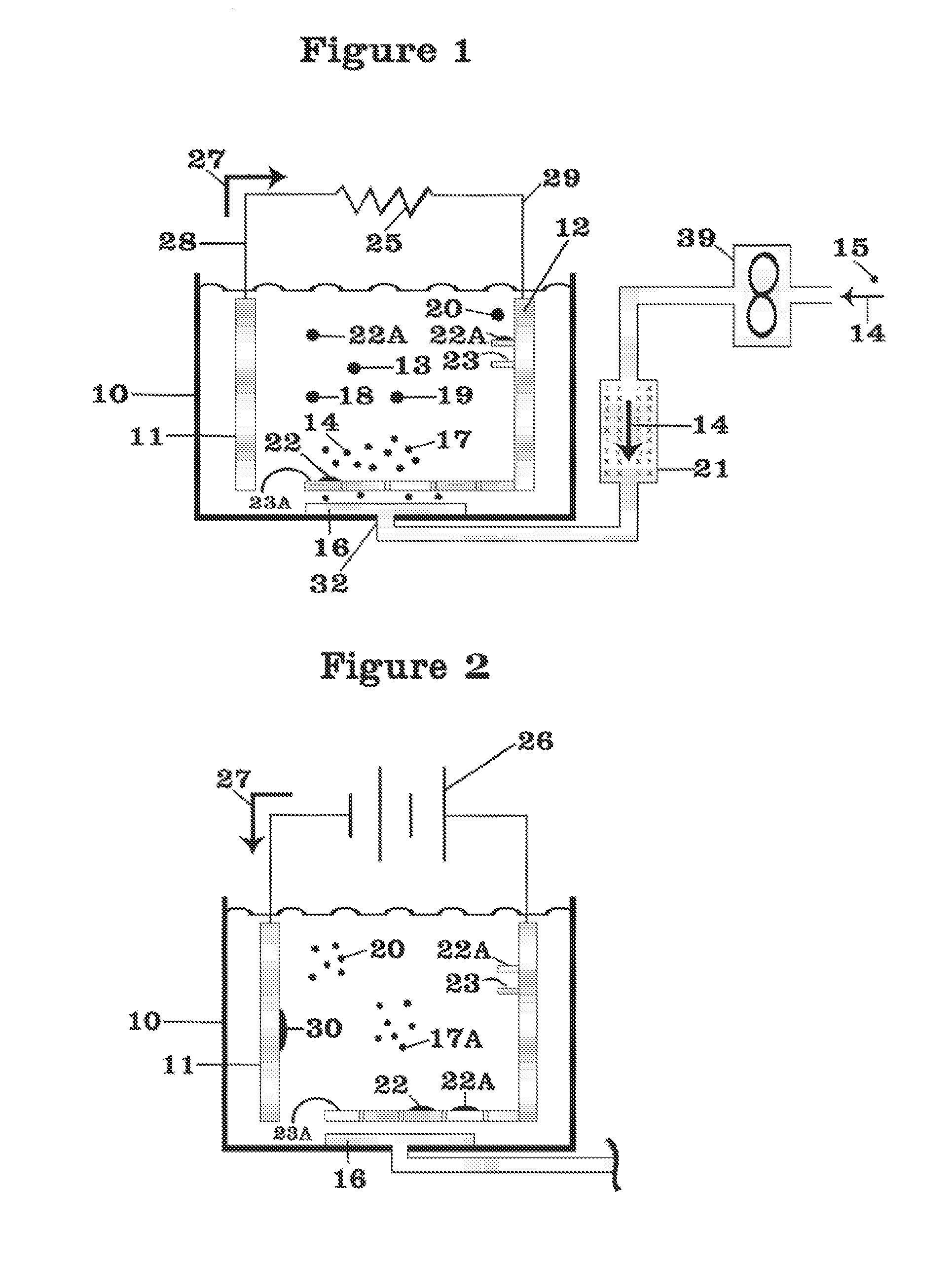
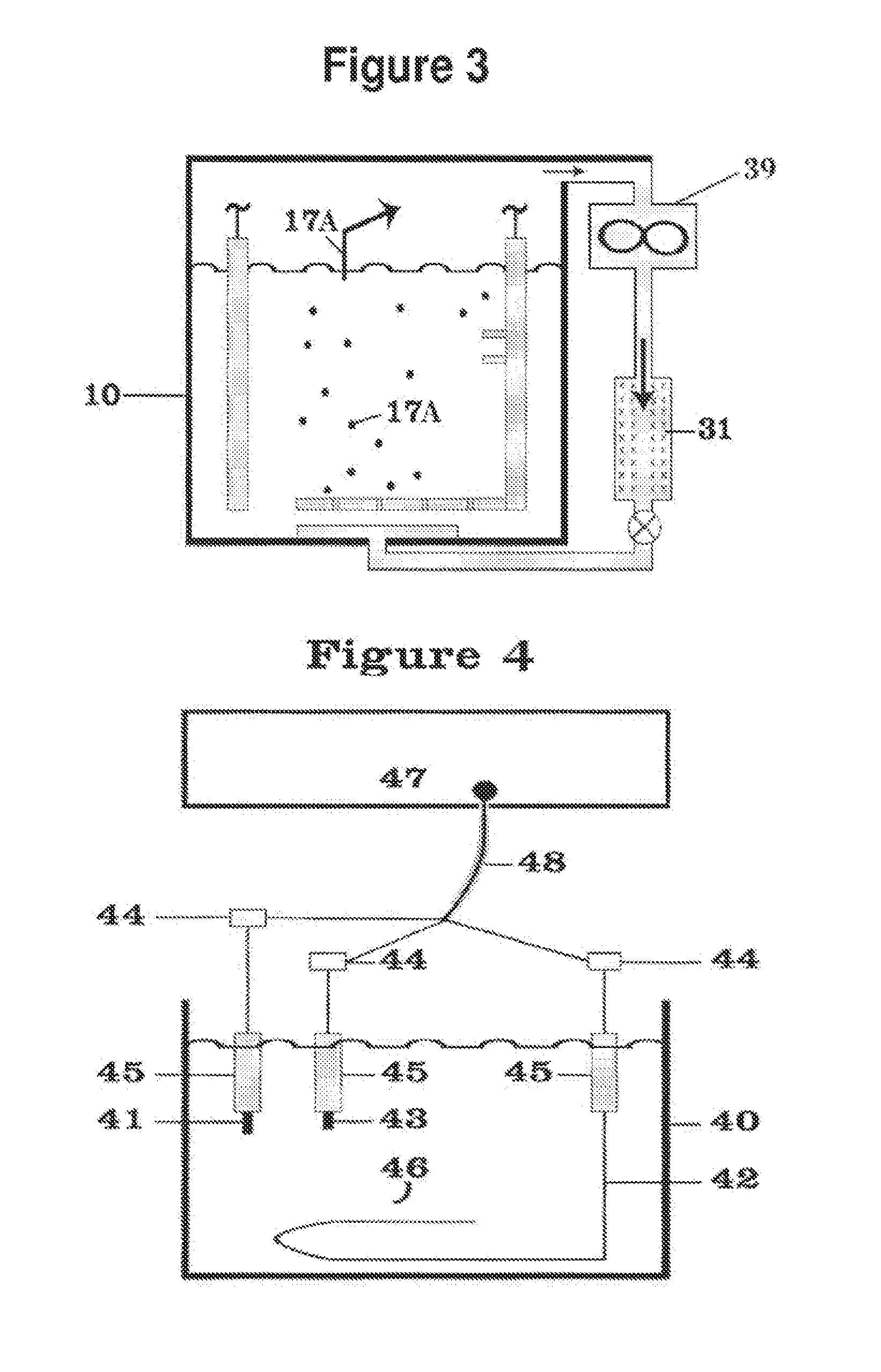
Thermal batteries utilize specialized electrolyte compositions to enhance performance and safety. These electrolytes are often molten salts or solid-state materials that become conductive at high temperatures. The design of the electrolyte system is crucial for maintaining stable operation and improving energy density in thermal batteries.- Thermal battery composition and structure: Thermal batteries are composed of specific materials and structures to optimize heat storage and release. Key components include electrolytes, anodes, cathodes, and separators. The design focuses on maximizing energy density, thermal conductivity, and operational temperature range. Advanced materials and configurations are employed to enhance performance and longevity.
- Temperature control and management systems: Efficient temperature control and management systems are crucial for thermal battery operation. These systems regulate heat distribution, prevent overheating, and maintain optimal operating conditions. They may include sensors, cooling mechanisms, and intelligent control algorithms to ensure safe and effective thermal energy storage and release.
- Integration with renewable energy sources: Thermal batteries are increasingly integrated with renewable energy sources such as solar and wind power. This integration allows for efficient energy storage during peak production periods and subsequent release during high demand or low production times. The system design focuses on optimizing energy capture, storage, and distribution to enhance overall renewable energy utilization.
- Phase change materials in thermal batteries: Phase change materials (PCMs) are utilized in thermal batteries to enhance heat storage capacity and efficiency. These materials absorb or release large amounts of latent heat during phase transitions, allowing for high-density thermal energy storage. The selection and integration of PCMs are critical for optimizing the performance and energy density of thermal batteries.
- Applications in electric vehicles and grid storage: Thermal batteries find significant applications in electric vehicles and grid-scale energy storage. In electric vehicles, they can be used for efficient cabin heating and battery temperature management. For grid storage, thermal batteries offer a cost-effective solution for large-scale energy storage, helping to balance supply and demand in renewable energy systems.
02 Thermal management systems
Effective thermal management is essential for the operation and longevity of thermal batteries. This includes heat generation, distribution, and dissipation mechanisms. Advanced thermal management systems can improve battery efficiency, extend operational life, and enhance safety by maintaining optimal temperature ranges during discharge.Expand Specific Solutions03 Electrode materials and structures
The choice and design of electrode materials significantly impact thermal battery performance. Innovations in electrode structures, such as using advanced composites or nanostructured materials, can improve energy density, power output, and cycle life. Electrode design also focuses on enhancing the interface between electrodes and electrolytes for better ionic conductivity.Expand Specific Solutions04 Battery activation and control systems
Thermal batteries require precise activation and control mechanisms to initiate and maintain their high-temperature operation. This includes pyrotechnic ignition systems, temperature sensors, and electronic control units. Advanced activation systems aim to reduce response time and improve reliability in various operational conditions.Expand Specific Solutions05 Integration with energy storage systems
Thermal batteries are increasingly being integrated with other energy storage technologies to create hybrid systems. These combinations can leverage the high power density of thermal batteries with the long-term storage capabilities of other technologies. Such integrations aim to provide more versatile and efficient energy solutions for various applications, including grid stabilization and renewable energy storage.Expand Specific Solutions
Key Industry Players
The thermal battery market, focusing on the comparison of magnesium nitrate and potassium nitrate, is in a growth phase with increasing demand for reliable energy storage solutions. The market size is expanding due to applications in aerospace, defense, and renewable energy sectors. Technologically, both compounds are well-established, but ongoing research aims to enhance their performance and efficiency. Companies like Toyota Motor Corp., Siemens AG, and Murata Manufacturing Co. Ltd. are actively involved in advancing thermal battery technologies, leveraging their expertise in materials science and energy systems to develop more efficient and sustainable solutions.
Shanghai Institute of Applied Physics, Chinese Academy of Sci
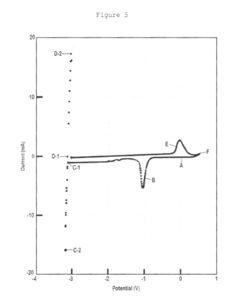
Technical Solution: The Shanghai Institute of Applied Physics has conducted extensive research on thermal batteries, focusing on the comparison of magnesium nitrate and potassium nitrate as electrolytes. Their studies have shown that magnesium nitrate-based thermal batteries exhibit higher specific energy and power density compared to potassium nitrate-based ones[1]. They have developed a novel cathode material using a composite of Fe2O3 and MgO, which enhances the performance of magnesium nitrate-based thermal batteries[2]. Their research also indicates that magnesium nitrate electrolytes have lower melting points and better ionic conductivity, leading to improved battery activation times and overall efficiency[3].
Strengths: Higher energy density, improved activation time, and better ionic conductivity. Weaknesses: Potential for increased corrosion of battery components and more complex manufacturing processes.
Central South University
Technical Solution: Central South University has made significant advancements in thermal battery technology, particularly in comparing magnesium nitrate and potassium nitrate electrolytes. Their research team has developed a novel electrolyte mixture combining magnesium nitrate with other salts to optimize performance[1]. They have also investigated the use of nano-sized cathode materials to enhance the electrochemical properties of both magnesium and potassium nitrate-based thermal batteries[2]. Their studies have shown that magnesium nitrate-based electrolytes offer improved thermal stability and higher operating voltages compared to potassium nitrate[3]. Additionally, they have explored the use of advanced separator materials to mitigate some of the corrosion issues associated with magnesium nitrate electrolytes[4].
Strengths: Improved thermal stability, higher operating voltages, and innovative electrolyte mixtures. Weaknesses: Potential for increased production costs due to the use of nano-materials and advanced separators.
Core Innovations
Lithium-air battery for electric vehicles and other applications using molten nitrate electrolytes
PatentInactiveUS20160028133A1
Innovation
- The use of a molten nitrate electrolyte system with lithium nitrate or its mixtures, which provides stability for the lithium anode, supports oxygen reduction through nitrate ions, and operates at higher temperatures to enhance kinetics, reducing the need for expensive catalysts and minimizing the risk of flammability.
Heat storage means
PatentWO2004007635A1
Innovation
- Development of ternary mixtures composed of water and two salts from the group of lithium nitrate, sodium nitrate, magnesium nitrate, potassium nitrate, calcium nitrate, and zinc nitrate, which are formulated to have melting points within the desired range, allowing for stable heat storage and buffering.
Environmental Impact
The environmental impact of thermal batteries using magnesium nitrate and potassium nitrate as electrolytes is a crucial consideration in their development and application. Both compounds have distinct environmental implications that must be carefully evaluated.
Magnesium nitrate, when used in thermal batteries, presents several environmental concerns. Its production process involves the extraction of magnesium from natural sources, which can lead to habitat disruption and soil degradation. The mining and refining of magnesium also contribute to greenhouse gas emissions and energy consumption. However, magnesium is abundant in the Earth's crust, making it a relatively sustainable resource.
In contrast, potassium nitrate production primarily relies on the Haber-Bosch process, which is energy-intensive and contributes significantly to global carbon emissions. The extraction of potassium from mineral deposits can also lead to land disturbance and potential groundwater contamination. Nevertheless, potassium nitrate is biodegradable and can be used as a fertilizer, potentially offsetting some of its environmental impacts.
The disposal of thermal batteries containing these compounds poses additional environmental challenges. Improper disposal can lead to soil and water contamination, as both magnesium and potassium nitrates are highly soluble in water. This solubility increases the risk of nutrient pollution in aquatic ecosystems, potentially causing eutrophication and harmful algal blooms.
Recycling and proper end-of-life management of thermal batteries are essential to mitigate these environmental risks. Advanced recycling technologies can recover valuable materials from spent batteries, reducing the need for raw material extraction and minimizing waste. However, the recycling processes themselves may have environmental impacts that need to be considered.
The production and use of thermal batteries also have implications for air quality. The manufacturing processes for both magnesium and potassium nitrates can release particulate matter and nitrogen oxides into the atmosphere, contributing to air pollution and potentially affecting human health in surrounding areas.
When comparing the two compounds, magnesium nitrate generally has a lower environmental footprint in terms of production and resource availability. However, potassium nitrate's potential for reuse as a fertilizer gives it an advantage in terms of circular economy principles. The choice between the two should consider the entire life cycle of the thermal battery, from raw material extraction to disposal or recycling.
In conclusion, while both magnesium nitrate and potassium nitrate have environmental impacts when used in thermal batteries, their effects differ in nature and intensity. Ongoing research and development efforts should focus on minimizing these impacts through improved production methods, enhanced battery efficiency, and advanced recycling technologies. Additionally, comprehensive life cycle assessments are necessary to fully understand and compare the environmental implications of using these compounds in thermal battery applications.
Magnesium nitrate, when used in thermal batteries, presents several environmental concerns. Its production process involves the extraction of magnesium from natural sources, which can lead to habitat disruption and soil degradation. The mining and refining of magnesium also contribute to greenhouse gas emissions and energy consumption. However, magnesium is abundant in the Earth's crust, making it a relatively sustainable resource.
In contrast, potassium nitrate production primarily relies on the Haber-Bosch process, which is energy-intensive and contributes significantly to global carbon emissions. The extraction of potassium from mineral deposits can also lead to land disturbance and potential groundwater contamination. Nevertheless, potassium nitrate is biodegradable and can be used as a fertilizer, potentially offsetting some of its environmental impacts.
The disposal of thermal batteries containing these compounds poses additional environmental challenges. Improper disposal can lead to soil and water contamination, as both magnesium and potassium nitrates are highly soluble in water. This solubility increases the risk of nutrient pollution in aquatic ecosystems, potentially causing eutrophication and harmful algal blooms.
Recycling and proper end-of-life management of thermal batteries are essential to mitigate these environmental risks. Advanced recycling technologies can recover valuable materials from spent batteries, reducing the need for raw material extraction and minimizing waste. However, the recycling processes themselves may have environmental impacts that need to be considered.
The production and use of thermal batteries also have implications for air quality. The manufacturing processes for both magnesium and potassium nitrates can release particulate matter and nitrogen oxides into the atmosphere, contributing to air pollution and potentially affecting human health in surrounding areas.
When comparing the two compounds, magnesium nitrate generally has a lower environmental footprint in terms of production and resource availability. However, potassium nitrate's potential for reuse as a fertilizer gives it an advantage in terms of circular economy principles. The choice between the two should consider the entire life cycle of the thermal battery, from raw material extraction to disposal or recycling.
In conclusion, while both magnesium nitrate and potassium nitrate have environmental impacts when used in thermal batteries, their effects differ in nature and intensity. Ongoing research and development efforts should focus on minimizing these impacts through improved production methods, enhanced battery efficiency, and advanced recycling technologies. Additionally, comprehensive life cycle assessments are necessary to fully understand and compare the environmental implications of using these compounds in thermal battery applications.
Safety Regulations
The safety regulations surrounding the use of magnesium nitrate and potassium nitrate in thermal batteries are crucial for ensuring the proper handling, storage, and disposal of these materials. Both compounds are classified as oxidizers, which means they can significantly increase the rate of combustion of other substances. As such, they are subject to strict regulatory oversight in many jurisdictions.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific guidelines for the handling of these materials in industrial settings. These regulations mandate the use of appropriate personal protective equipment (PPE), including respiratory protection, chemical-resistant gloves, and safety goggles. Additionally, OSHA requires proper labeling of containers and the availability of Safety Data Sheets (SDS) for all employees working with these substances.
The Department of Transportation (DOT) classifies both magnesium nitrate and potassium nitrate as Class 5.1 oxidizers, which impacts their transportation requirements. Specific packaging, labeling, and documentation are necessary when shipping these materials. The DOT also imposes quantity limitations and special provisions for air, rail, and road transport to minimize the risk of accidents during transit.
From an environmental perspective, the Environmental Protection Agency (EPA) regulates the disposal of these compounds. Improper disposal can lead to soil and water contamination, necessitating strict adherence to waste management protocols. Facilities handling these materials must comply with the Resource Conservation and Recovery Act (RCRA) guidelines for hazardous waste management.
Internationally, the United Nations' Globally Harmonized System of Classification and Labeling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. This system is widely adopted and ensures consistency in the classification and labeling of magnesium nitrate and potassium nitrate across different countries, facilitating international trade and safety compliance.
In the context of thermal battery manufacturing, additional industry-specific regulations may apply. For instance, the National Fire Protection Association (NFPA) provides standards for the storage and handling of oxidizers in industrial facilities. These standards outline specific requirements for fire protection systems, storage arrangements, and emergency response procedures.
It is important to note that regulations may vary between countries and even between different states or regions within a country. Therefore, manufacturers and users of thermal batteries must stay informed about local regulations and any updates to ensure continuous compliance. Regular safety audits and employee training programs are essential to maintain a safe working environment and meet regulatory requirements.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific guidelines for the handling of these materials in industrial settings. These regulations mandate the use of appropriate personal protective equipment (PPE), including respiratory protection, chemical-resistant gloves, and safety goggles. Additionally, OSHA requires proper labeling of containers and the availability of Safety Data Sheets (SDS) for all employees working with these substances.
The Department of Transportation (DOT) classifies both magnesium nitrate and potassium nitrate as Class 5.1 oxidizers, which impacts their transportation requirements. Specific packaging, labeling, and documentation are necessary when shipping these materials. The DOT also imposes quantity limitations and special provisions for air, rail, and road transport to minimize the risk of accidents during transit.
From an environmental perspective, the Environmental Protection Agency (EPA) regulates the disposal of these compounds. Improper disposal can lead to soil and water contamination, necessitating strict adherence to waste management protocols. Facilities handling these materials must comply with the Resource Conservation and Recovery Act (RCRA) guidelines for hazardous waste management.
Internationally, the United Nations' Globally Harmonized System of Classification and Labeling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. This system is widely adopted and ensures consistency in the classification and labeling of magnesium nitrate and potassium nitrate across different countries, facilitating international trade and safety compliance.
In the context of thermal battery manufacturing, additional industry-specific regulations may apply. For instance, the National Fire Protection Association (NFPA) provides standards for the storage and handling of oxidizers in industrial facilities. These standards outline specific requirements for fire protection systems, storage arrangements, and emergency response procedures.
It is important to note that regulations may vary between countries and even between different states or regions within a country. Therefore, manufacturers and users of thermal batteries must stay informed about local regulations and any updates to ensure continuous compliance. Regular safety audits and employee training programs are essential to maintain a safe working environment and meet regulatory requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!