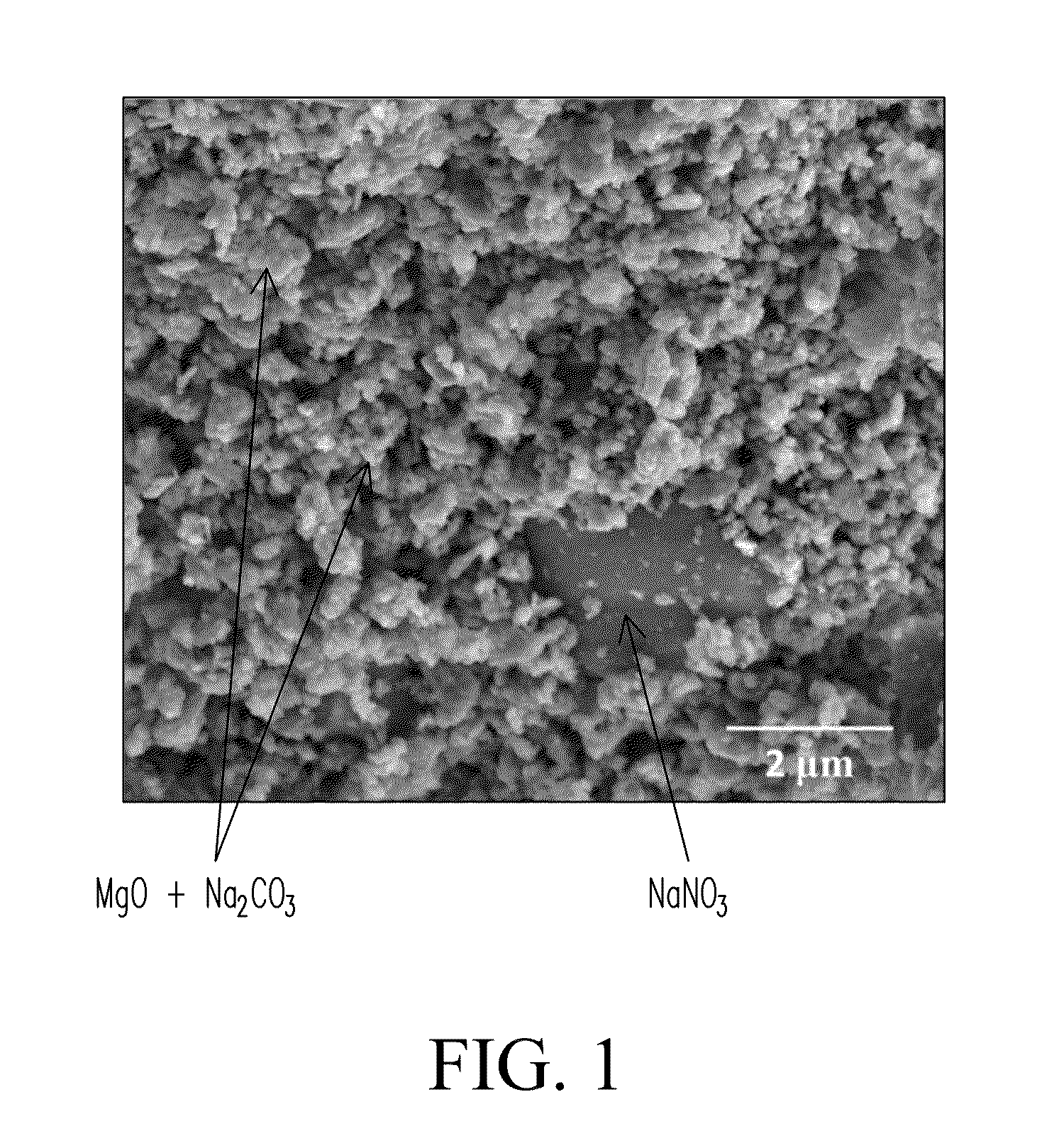
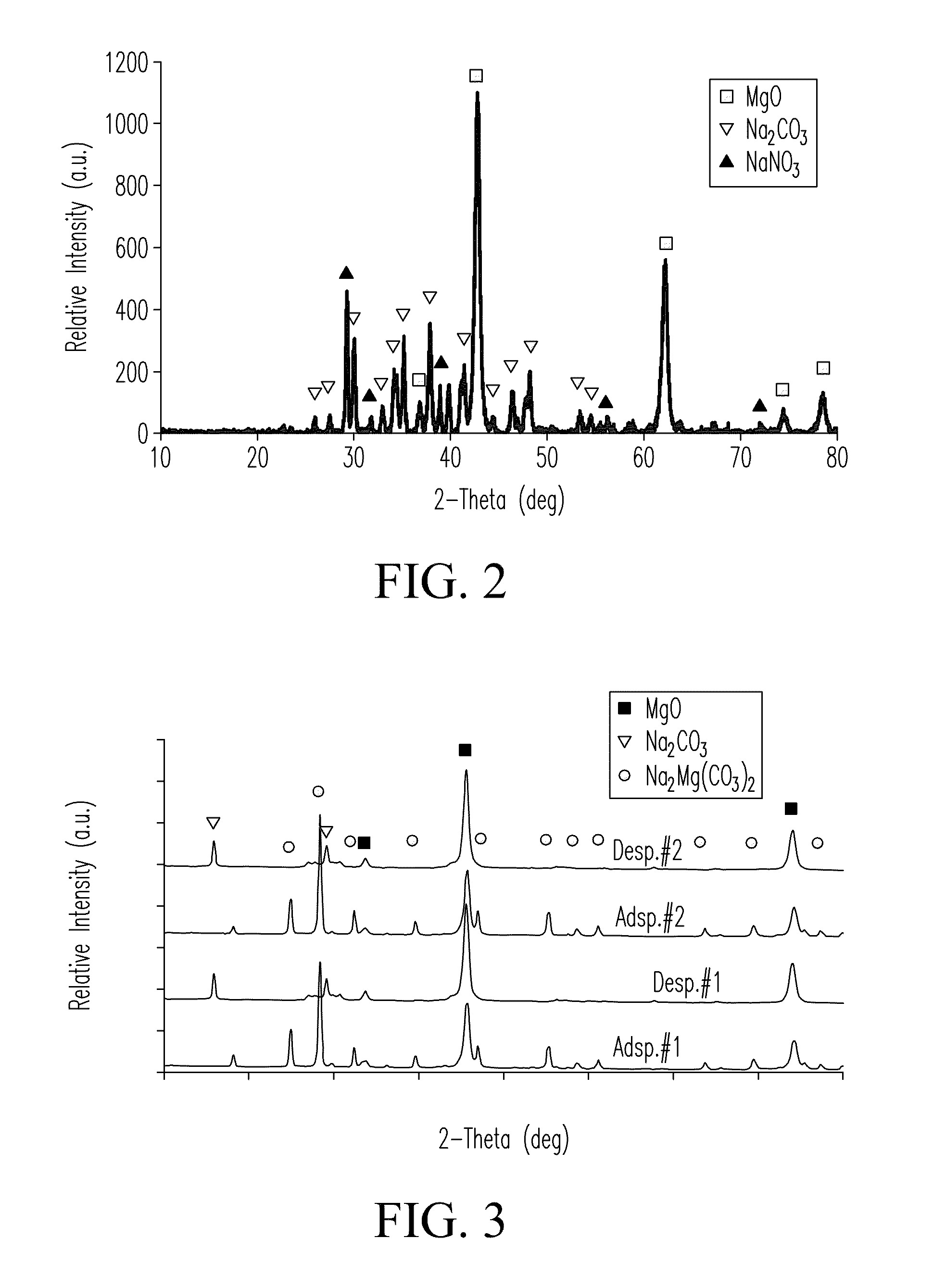
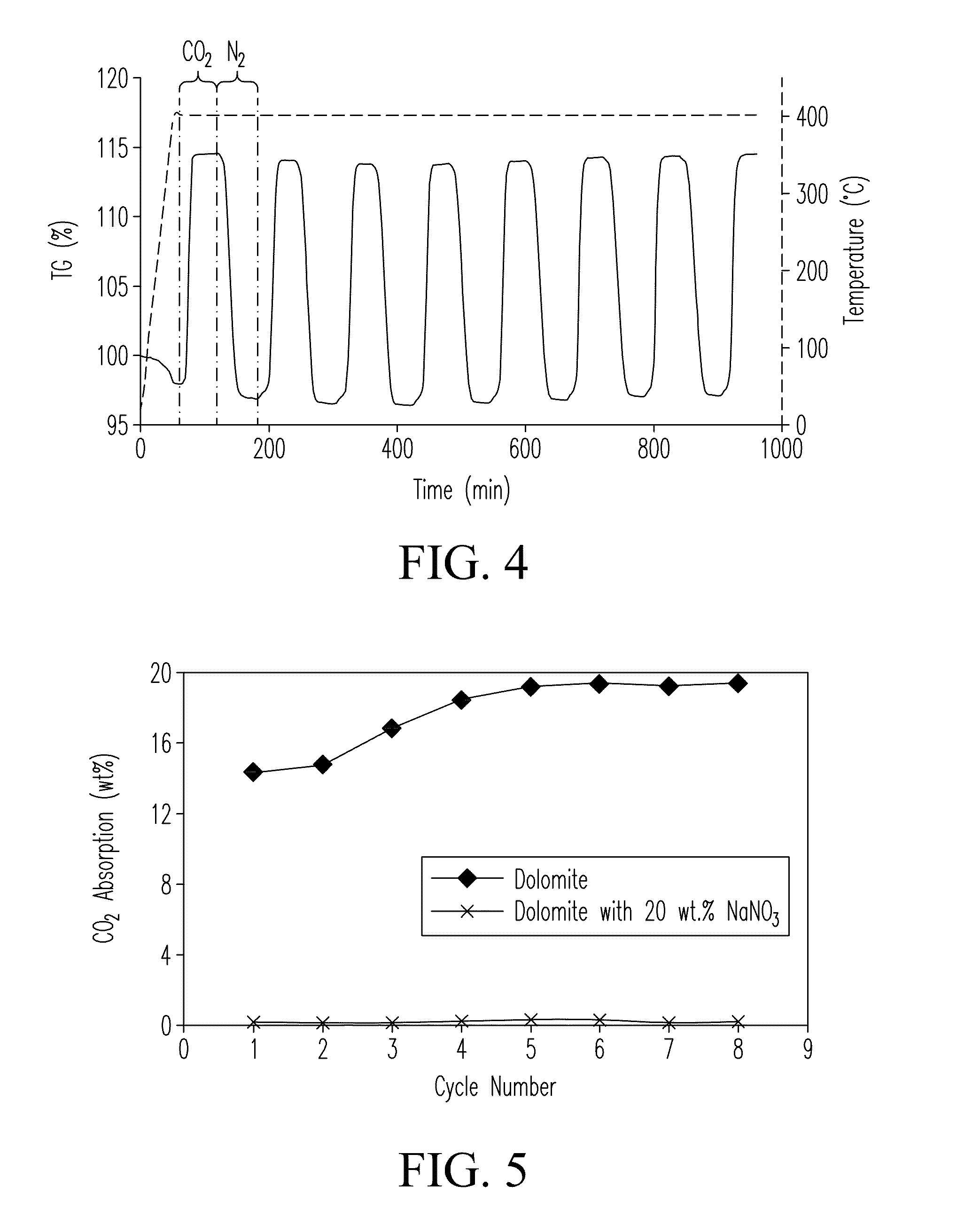
The Potential of Magnesium Nitrate in Carbon Capture Technologies
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate in Carbon Capture: Background and Objectives
Carbon capture and storage (CCS) has emerged as a critical technology in the global effort to mitigate climate change. As the world grapples with rising greenhouse gas emissions, innovative solutions are needed to effectively capture and sequester carbon dioxide from industrial processes and the atmosphere. In this context, magnesium nitrate has garnered attention as a potential game-changer in carbon capture technologies.
The exploration of magnesium nitrate in carbon capture is rooted in the broader field of chemical absorption methods for CO2 removal. Traditionally, amine-based solvents have been the go-to solution for carbon capture. However, these solvents face challenges such as high energy requirements for regeneration and susceptibility to degradation. This has spurred research into alternative materials that can offer improved efficiency and sustainability in carbon capture processes.
Magnesium nitrate, a inorganic compound with the chemical formula Mg(NO3)2, has shown promising characteristics for CO2 absorption. Its potential lies in its ability to form stable carbonate compounds when reacting with carbon dioxide, potentially offering a more energy-efficient and cost-effective capture method compared to conventional amine-based systems.
The primary objective of investigating magnesium nitrate in carbon capture technologies is to develop a more sustainable and efficient CO2 absorption process. Researchers aim to leverage the unique properties of magnesium nitrate to overcome the limitations of current carbon capture methods, particularly in terms of energy consumption and operational costs.
Key goals in this technological pursuit include enhancing the CO2 absorption capacity of magnesium nitrate-based systems, optimizing the regeneration process to minimize energy requirements, and ensuring long-term stability and recyclability of the absorbent material. Additionally, there is a focus on understanding the kinetics and thermodynamics of the absorption process to design more effective capture systems.
The development of magnesium nitrate-based carbon capture technologies aligns with the global trend towards more sustainable industrial processes. As countries and industries worldwide set ambitious targets for carbon neutrality, the demand for innovative and efficient carbon capture solutions continues to grow. This has created a fertile ground for research and development in alternative capture materials like magnesium nitrate.
Looking ahead, the potential applications of magnesium nitrate in carbon capture extend beyond traditional point-source emissions. There is growing interest in exploring its use in direct air capture (DAC) technologies, which aim to remove CO2 directly from the atmosphere. This could open up new avenues for large-scale carbon removal, complementing efforts to reduce emissions at their source.
The exploration of magnesium nitrate in carbon capture is rooted in the broader field of chemical absorption methods for CO2 removal. Traditionally, amine-based solvents have been the go-to solution for carbon capture. However, these solvents face challenges such as high energy requirements for regeneration and susceptibility to degradation. This has spurred research into alternative materials that can offer improved efficiency and sustainability in carbon capture processes.
Magnesium nitrate, a inorganic compound with the chemical formula Mg(NO3)2, has shown promising characteristics for CO2 absorption. Its potential lies in its ability to form stable carbonate compounds when reacting with carbon dioxide, potentially offering a more energy-efficient and cost-effective capture method compared to conventional amine-based systems.
The primary objective of investigating magnesium nitrate in carbon capture technologies is to develop a more sustainable and efficient CO2 absorption process. Researchers aim to leverage the unique properties of magnesium nitrate to overcome the limitations of current carbon capture methods, particularly in terms of energy consumption and operational costs.
Key goals in this technological pursuit include enhancing the CO2 absorption capacity of magnesium nitrate-based systems, optimizing the regeneration process to minimize energy requirements, and ensuring long-term stability and recyclability of the absorbent material. Additionally, there is a focus on understanding the kinetics and thermodynamics of the absorption process to design more effective capture systems.
The development of magnesium nitrate-based carbon capture technologies aligns with the global trend towards more sustainable industrial processes. As countries and industries worldwide set ambitious targets for carbon neutrality, the demand for innovative and efficient carbon capture solutions continues to grow. This has created a fertile ground for research and development in alternative capture materials like magnesium nitrate.
Looking ahead, the potential applications of magnesium nitrate in carbon capture extend beyond traditional point-source emissions. There is growing interest in exploring its use in direct air capture (DAC) technologies, which aim to remove CO2 directly from the atmosphere. This could open up new avenues for large-scale carbon removal, complementing efforts to reduce emissions at their source.
Market Analysis for Carbon Capture Technologies
The carbon capture technology market has been experiencing significant growth in recent years, driven by increasing global concerns about climate change and the urgent need to reduce greenhouse gas emissions. As governments worldwide implement stricter environmental regulations and set ambitious carbon reduction targets, the demand for effective carbon capture solutions has surged.
The global carbon capture and storage (CCS) market was valued at approximately $3.5 billion in 2020 and is projected to reach $7.0 billion by 2026, growing at a CAGR of 13.2% during the forecast period. This growth is primarily attributed to the rising adoption of CCS technologies across various industries, including power generation, oil and gas, cement, and chemical manufacturing.
The market for carbon capture technologies can be segmented based on technology type, application, and geography. Post-combustion capture, pre-combustion capture, and oxy-fuel combustion are the primary technology types, with post-combustion capture currently dominating the market due to its compatibility with existing infrastructure. In terms of application, the power generation sector holds the largest market share, followed by industrial processes and natural gas processing.
Geographically, North America leads the carbon capture market, accounting for over 40% of the global market share. This dominance is largely due to favorable government policies, substantial investments in research and development, and the presence of major industry players. Europe follows closely, with countries like Norway and the United Kingdom at the forefront of CCS deployment. The Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing industrialization and stringent emission regulations in countries like China and Japan.
The potential of magnesium nitrate in carbon capture technologies represents an emerging segment within this market. While traditional carbon capture methods primarily rely on amine-based solvents, the use of magnesium nitrate offers several advantages, including higher CO2 absorption capacity, lower regeneration energy requirements, and reduced equipment corrosion. These benefits could potentially lead to more cost-effective and efficient carbon capture processes, thereby expanding the overall market for CCS technologies.
However, the adoption of magnesium nitrate-based carbon capture solutions is still in its early stages, and significant research and development efforts are required to fully realize its potential. As the technology matures and demonstrates its effectiveness at scale, it is expected to carve out a growing share of the carbon capture market, particularly in applications where traditional methods face limitations or high operational costs.
The global carbon capture and storage (CCS) market was valued at approximately $3.5 billion in 2020 and is projected to reach $7.0 billion by 2026, growing at a CAGR of 13.2% during the forecast period. This growth is primarily attributed to the rising adoption of CCS technologies across various industries, including power generation, oil and gas, cement, and chemical manufacturing.
The market for carbon capture technologies can be segmented based on technology type, application, and geography. Post-combustion capture, pre-combustion capture, and oxy-fuel combustion are the primary technology types, with post-combustion capture currently dominating the market due to its compatibility with existing infrastructure. In terms of application, the power generation sector holds the largest market share, followed by industrial processes and natural gas processing.
Geographically, North America leads the carbon capture market, accounting for over 40% of the global market share. This dominance is largely due to favorable government policies, substantial investments in research and development, and the presence of major industry players. Europe follows closely, with countries like Norway and the United Kingdom at the forefront of CCS deployment. The Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing industrialization and stringent emission regulations in countries like China and Japan.
The potential of magnesium nitrate in carbon capture technologies represents an emerging segment within this market. While traditional carbon capture methods primarily rely on amine-based solvents, the use of magnesium nitrate offers several advantages, including higher CO2 absorption capacity, lower regeneration energy requirements, and reduced equipment corrosion. These benefits could potentially lead to more cost-effective and efficient carbon capture processes, thereby expanding the overall market for CCS technologies.
However, the adoption of magnesium nitrate-based carbon capture solutions is still in its early stages, and significant research and development efforts are required to fully realize its potential. As the technology matures and demonstrates its effectiveness at scale, it is expected to carve out a growing share of the carbon capture market, particularly in applications where traditional methods face limitations or high operational costs.
Current Status and Challenges in Magnesium-Based Carbon Capture
Magnesium-based carbon capture technologies have gained significant attention in recent years as a promising approach to mitigate greenhouse gas emissions. The current status of these technologies reveals both progress and challenges in their development and implementation.
At present, magnesium-based carbon capture methods primarily focus on the use of magnesium oxide (MgO) and magnesium hydroxide (Mg(OH)2) as sorbents. These materials have shown potential due to their high CO2 absorption capacity and relatively low regeneration energy requirements. Research has demonstrated that MgO can theoretically capture up to 1.09 kg of CO2 per kg of sorbent, making it an attractive option for large-scale carbon capture applications.
However, the practical implementation of magnesium-based carbon capture faces several challenges. One of the primary issues is the slow kinetics of the carbonation reaction, which limits the overall efficiency of the process. This is particularly problematic in flue gas conditions where CO2 concentrations are relatively low. Researchers are actively working on enhancing the reaction rates through various methods, including the development of novel catalyst systems and the optimization of sorbent particle size and morphology.
Another significant challenge is the degradation of magnesium-based sorbents over multiple capture-regeneration cycles. The repeated exposure to high temperatures during regeneration can lead to sintering and loss of surface area, reducing the sorbent's effectiveness over time. This necessitates the development of more stable sorbent formulations that can maintain their performance over extended periods.
The energy intensity of the regeneration process also presents a hurdle for widespread adoption. While magnesium-based sorbents generally require less energy for regeneration compared to some alternatives, further improvements are needed to make the technology economically viable on a large scale. Researchers are exploring various approaches to reduce the energy demand, including the use of renewable energy sources and the integration of waste heat recovery systems.
From a geographical perspective, the development of magnesium-based carbon capture technologies is not evenly distributed. Countries with significant magnesium resources, such as China, Russia, and the United States, are at the forefront of research and development in this field. However, international collaboration is growing, with research institutions and companies from various countries contributing to the advancement of these technologies.
The current regulatory landscape also plays a crucial role in shaping the development of magnesium-based carbon capture. While there is increasing support for carbon capture technologies in many countries, the lack of a unified global framework for carbon pricing and emissions reduction targets creates uncertainty for long-term investments in this area.
In conclusion, while magnesium-based carbon capture technologies show promise, they are still in the early stages of development and face several technical and economic challenges. Overcoming these hurdles will require continued research, innovation, and supportive policy frameworks to realize the full potential of these technologies in addressing global climate change.
At present, magnesium-based carbon capture methods primarily focus on the use of magnesium oxide (MgO) and magnesium hydroxide (Mg(OH)2) as sorbents. These materials have shown potential due to their high CO2 absorption capacity and relatively low regeneration energy requirements. Research has demonstrated that MgO can theoretically capture up to 1.09 kg of CO2 per kg of sorbent, making it an attractive option for large-scale carbon capture applications.
However, the practical implementation of magnesium-based carbon capture faces several challenges. One of the primary issues is the slow kinetics of the carbonation reaction, which limits the overall efficiency of the process. This is particularly problematic in flue gas conditions where CO2 concentrations are relatively low. Researchers are actively working on enhancing the reaction rates through various methods, including the development of novel catalyst systems and the optimization of sorbent particle size and morphology.
Another significant challenge is the degradation of magnesium-based sorbents over multiple capture-regeneration cycles. The repeated exposure to high temperatures during regeneration can lead to sintering and loss of surface area, reducing the sorbent's effectiveness over time. This necessitates the development of more stable sorbent formulations that can maintain their performance over extended periods.
The energy intensity of the regeneration process also presents a hurdle for widespread adoption. While magnesium-based sorbents generally require less energy for regeneration compared to some alternatives, further improvements are needed to make the technology economically viable on a large scale. Researchers are exploring various approaches to reduce the energy demand, including the use of renewable energy sources and the integration of waste heat recovery systems.
From a geographical perspective, the development of magnesium-based carbon capture technologies is not evenly distributed. Countries with significant magnesium resources, such as China, Russia, and the United States, are at the forefront of research and development in this field. However, international collaboration is growing, with research institutions and companies from various countries contributing to the advancement of these technologies.
The current regulatory landscape also plays a crucial role in shaping the development of magnesium-based carbon capture. While there is increasing support for carbon capture technologies in many countries, the lack of a unified global framework for carbon pricing and emissions reduction targets creates uncertainty for long-term investments in this area.
In conclusion, while magnesium-based carbon capture technologies show promise, they are still in the early stages of development and face several technical and economic challenges. Overcoming these hurdles will require continued research, innovation, and supportive policy frameworks to realize the full potential of these technologies in addressing global climate change.
Existing Magnesium Nitrate Carbon Capture Solutions
01 Magnesium-based sorbents for CO2 capture
Magnesium-based materials, particularly magnesium nitrate, are used as effective sorbents for carbon dioxide capture. These sorbents can be synthesized and modified to enhance their CO2 adsorption capacity and selectivity. The process often involves the preparation of porous structures or nanoparticles to increase the surface area for CO2 capture.- Magnesium-based sorbents for CO2 capture: Magnesium-based materials, particularly magnesium nitrate, are used as effective sorbents for carbon dioxide capture. These sorbents can be synthesized and modified to enhance their CO2 adsorption capacity and selectivity. The use of magnesium nitrate in carbon capture processes offers advantages such as high CO2 uptake, good regeneration properties, and relatively low cost.
- Carbon capture systems using magnesium nitrate solutions: Carbon capture systems utilizing magnesium nitrate solutions have been developed for efficient CO2 removal from industrial emissions. These systems often involve absorption columns or reactors where the magnesium nitrate solution comes into contact with the CO2-rich gas stream. The process can be optimized by controlling parameters such as temperature, pressure, and solution concentration.
- Regeneration of magnesium nitrate-based CO2 sorbents: Methods for regenerating magnesium nitrate-based CO2 sorbents have been developed to ensure their long-term effectiveness in carbon capture processes. These regeneration techniques may involve thermal treatment, pressure swing, or chemical processes to release the captured CO2 and restore the sorbent's adsorption capacity. Efficient regeneration is crucial for the economic viability of the carbon capture system.
- Integration of magnesium nitrate carbon capture in industrial processes: Magnesium nitrate-based carbon capture technologies have been integrated into various industrial processes, such as power plants, cement production, and chemical manufacturing. These integrated systems aim to reduce overall CO2 emissions while minimizing the impact on the primary industrial process. The integration often involves heat recovery and process optimization to enhance overall efficiency.
- Novel composite materials incorporating magnesium nitrate for enhanced CO2 capture: Research has focused on developing novel composite materials that incorporate magnesium nitrate for improved CO2 capture performance. These composites may combine magnesium nitrate with other materials such as porous supports, nanoparticles, or polymers to enhance adsorption capacity, kinetics, and stability. The synergistic effects of these composite materials can lead to more efficient and cost-effective carbon capture solutions.
02 Carbon capture systems using magnesium compounds
Carbon capture systems incorporating magnesium compounds, including magnesium nitrate, are designed for efficient CO2 removal from industrial emissions or ambient air. These systems may involve various processes such as absorption, adsorption, or chemical looping, utilizing the reactivity of magnesium compounds with CO2.Expand Specific Solutions03 Regeneration of magnesium-based CO2 sorbents
Methods for regenerating magnesium-based carbon dioxide sorbents, including those containing magnesium nitrate, are developed to ensure the long-term efficiency of the capture process. These techniques may involve thermal treatment, pressure swing, or chemical processes to release captured CO2 and restore the sorbent's capacity for repeated use.Expand Specific Solutions04 Enhancement of CO2 capture using magnesium nitrate composites
Composite materials incorporating magnesium nitrate with other compounds or supports are developed to improve carbon dioxide capture performance. These composites may include combinations with other metal oxides, polymers, or porous materials to enhance adsorption capacity, kinetics, and stability of the sorbent.Expand Specific Solutions05 Process integration for magnesium nitrate-based carbon capture
Integration of magnesium nitrate-based carbon capture technologies into existing industrial processes or power plants is explored. This involves designing efficient heat integration schemes, optimizing process conditions, and developing novel reactor configurations to maximize CO2 capture while minimizing energy penalties and operational costs.Expand Specific Solutions
Key Players in Carbon Capture Industry
The carbon capture technology market is in a growth phase, with increasing interest due to global climate change concerns. The market size is expanding, driven by government initiatives and corporate sustainability goals. Technological maturity varies, with some established players like Carbonfree Chemicals Holdings LLC and SABIC Global Technologies BV leading in commercial applications. Emerging companies such as Negative Emissions Materials, Inc. and Planetary Technologies, Inc. are focusing on innovative approaches. Academic institutions like MIT and King Fahd University of Petroleum & Minerals are contributing to research advancements. The involvement of major energy companies like Shell Oil Co. and INPEX Corp. indicates the industry's potential for significant growth and technological development in the coming years.
Carbonfree Chemicals Holdings LLC
Technical Solution: Carbonfree Chemicals has developed a proprietary Skymine® process that utilizes magnesium-based compounds, including magnesium nitrate, for carbon capture. The process involves reacting CO2 with a magnesium-rich solution to form stable carbonate minerals. This technology can capture up to 90% of CO2 emissions from industrial flue gases[1]. The process also produces valuable by-products such as hydrochloric acid and sodium bicarbonate, which can offset operational costs. Carbonfree's approach is particularly effective for high-volume, stationary CO2 sources like power plants and cement factories[2].
Strengths: High capture efficiency, valuable by-products, applicable to large-scale industrial emissions. Weaknesses: Requires significant amounts of magnesium-rich materials, potential environmental impacts of mineral extraction.
SABIC Global Technologies BV
Technical Solution: SABIC has been exploring the use of magnesium nitrate in carbon capture technologies as part of its broader sustainability initiatives. Their approach involves incorporating magnesium nitrate into advanced sorbent materials for CO2 capture. These materials are designed to have high CO2 adsorption capacity and selectivity, as well as good regeneration properties. SABIC's research focuses on optimizing the sorbent composition and structure to enhance CO2 capture performance in various industrial settings, including petrochemical plants and refineries[3]. The company is also investigating the potential of magnesium nitrate-based materials in direct air capture (DAC) applications.
Strengths: Leverages existing chemical expertise, potential for integration into SABIC's own operations. Weaknesses: Still in research phase, may face challenges in scaling up to industrial levels.
Innovative Approaches in Magnesium-Based Carbon Sequestration
System, sorbents, and processes for capture and release of co2
PatentInactiveUS20130287663A1
Innovation
- A sorbent composition comprising magnesium oxide (MgO) and alkali-metal nitrates, with optional alkali-metal carbonates, that operates at warm temperatures to capture and release CO2, forming reversible solid metal carbonate salts, enhancing CO2 sorption capacity and efficiency.
Advanced capacitive electrode materials innovative synthesis of mgo-carbon nanocomposites
PatentPendingIN202441039158A
Innovation
- The development of magnesium oxide (MgO)-carbon nanocomposites with precise synthesis methods like sol-gel, hydrothermal synthesis, and chemical vapor deposition to create a homogeneous composite with well-dispersed MgO nanoparticles within a carbon matrix, leveraging the high specific capacitance of MgO and excellent conductivity of carbon materials.
Environmental Impact Assessment of Magnesium Nitrate Usage
The environmental impact assessment of magnesium nitrate usage in carbon capture technologies is a critical aspect that requires thorough evaluation. Magnesium nitrate, while promising for its potential in carbon capture, presents both benefits and challenges from an environmental perspective.
One of the primary environmental advantages of using magnesium nitrate in carbon capture is its potential to significantly reduce greenhouse gas emissions. By effectively capturing and storing carbon dioxide, this technology could play a crucial role in mitigating climate change. The process could lead to a substantial decrease in atmospheric CO2 levels, contributing to global efforts to limit temperature rise and associated environmental impacts.
However, the production and use of magnesium nitrate also raise environmental concerns. The mining and processing of magnesium and nitrogen compounds for magnesium nitrate production can have significant environmental impacts, including habitat destruction, water pollution, and energy consumption. These processes often involve the use of fossil fuels, potentially offsetting some of the carbon capture benefits.
Water usage is another important consideration in the environmental assessment of magnesium nitrate-based carbon capture. The technology may require substantial amounts of water, which could strain local water resources, particularly in water-scarce regions. This could lead to competition with other essential water uses, such as agriculture and domestic consumption.
The disposal or storage of captured carbon dioxide also presents environmental challenges. While the capture process itself may be effective, the long-term storage of CO2 must be carefully managed to prevent leakage back into the atmosphere. Potential storage sites, such as underground geological formations, must be thoroughly assessed for their stability and containment capabilities to ensure environmental safety.
Furthermore, the use of magnesium nitrate in large-scale carbon capture operations could potentially impact local ecosystems. The release of nitrates into the environment, if not properly controlled, could lead to eutrophication of water bodies, affecting aquatic life and water quality. Careful monitoring and management of the entire carbon capture process would be necessary to minimize these risks.
On the positive side, the development of magnesium nitrate-based carbon capture technologies could drive innovation in cleaner industrial processes. This could lead to the development of more environmentally friendly manufacturing techniques for magnesium nitrate and related compounds, potentially reducing the overall environmental footprint of the technology over time.
In conclusion, while magnesium nitrate shows promise in carbon capture technologies, its environmental impact is complex and multifaceted. A comprehensive life cycle assessment would be crucial to fully understand and quantify its net environmental effect, balancing its potential for reducing atmospheric CO2 against the environmental costs of its production, use, and the management of captured carbon.
One of the primary environmental advantages of using magnesium nitrate in carbon capture is its potential to significantly reduce greenhouse gas emissions. By effectively capturing and storing carbon dioxide, this technology could play a crucial role in mitigating climate change. The process could lead to a substantial decrease in atmospheric CO2 levels, contributing to global efforts to limit temperature rise and associated environmental impacts.
However, the production and use of magnesium nitrate also raise environmental concerns. The mining and processing of magnesium and nitrogen compounds for magnesium nitrate production can have significant environmental impacts, including habitat destruction, water pollution, and energy consumption. These processes often involve the use of fossil fuels, potentially offsetting some of the carbon capture benefits.
Water usage is another important consideration in the environmental assessment of magnesium nitrate-based carbon capture. The technology may require substantial amounts of water, which could strain local water resources, particularly in water-scarce regions. This could lead to competition with other essential water uses, such as agriculture and domestic consumption.
The disposal or storage of captured carbon dioxide also presents environmental challenges. While the capture process itself may be effective, the long-term storage of CO2 must be carefully managed to prevent leakage back into the atmosphere. Potential storage sites, such as underground geological formations, must be thoroughly assessed for their stability and containment capabilities to ensure environmental safety.
Furthermore, the use of magnesium nitrate in large-scale carbon capture operations could potentially impact local ecosystems. The release of nitrates into the environment, if not properly controlled, could lead to eutrophication of water bodies, affecting aquatic life and water quality. Careful monitoring and management of the entire carbon capture process would be necessary to minimize these risks.
On the positive side, the development of magnesium nitrate-based carbon capture technologies could drive innovation in cleaner industrial processes. This could lead to the development of more environmentally friendly manufacturing techniques for magnesium nitrate and related compounds, potentially reducing the overall environmental footprint of the technology over time.
In conclusion, while magnesium nitrate shows promise in carbon capture technologies, its environmental impact is complex and multifaceted. A comprehensive life cycle assessment would be crucial to fully understand and quantify its net environmental effect, balancing its potential for reducing atmospheric CO2 against the environmental costs of its production, use, and the management of captured carbon.
Economic Viability of Magnesium Nitrate Carbon Capture Systems
The economic viability of magnesium nitrate carbon capture systems hinges on several key factors that must be carefully evaluated. Initial capital investment for implementing these systems can be substantial, encompassing equipment costs, installation, and necessary infrastructure modifications. However, the long-term operational expenses may prove more favorable compared to traditional carbon capture methods. Magnesium nitrate's potential for regeneration and reuse in multiple capture cycles could significantly reduce ongoing material costs, a crucial aspect in determining overall economic feasibility.
Energy consumption is another critical consideration. The energy required for the capture process, including any heating or cooling needs, must be weighed against the system's carbon reduction efficiency. If the energy input is too high, it may offset the environmental benefits and increase operational costs. However, if magnesium nitrate systems can operate at lower temperatures or with less energy-intensive processes than conventional methods, they could offer a competitive advantage in terms of both economics and sustainability.
The scalability of magnesium nitrate carbon capture technology is essential for its economic viability. Large-scale implementation would likely benefit from economies of scale, potentially reducing per-unit costs as the technology matures and becomes more widely adopted. This scalability factor is crucial for industries seeking cost-effective carbon capture solutions that can be integrated into existing operations without prohibitive expenses.
Market dynamics also play a significant role in the economic assessment. The value of captured carbon, either for storage or utilization in other industrial processes, can greatly impact the return on investment for these systems. As carbon pricing mechanisms and regulatory frameworks evolve, the economic incentives for implementing carbon capture technologies may increase, potentially improving the financial attractiveness of magnesium nitrate-based systems.
Lastly, the lifecycle costs and environmental impact of magnesium nitrate production and disposal must be factored into the economic equation. If the material can be sourced sustainably and recycled efficiently, it could contribute to a more favorable economic profile compared to alternatives that require frequent replacement or have higher environmental footprints. The potential for integrating magnesium nitrate carbon capture into circular economy models could further enhance its economic viability and appeal to industries seeking comprehensive sustainability solutions.
Energy consumption is another critical consideration. The energy required for the capture process, including any heating or cooling needs, must be weighed against the system's carbon reduction efficiency. If the energy input is too high, it may offset the environmental benefits and increase operational costs. However, if magnesium nitrate systems can operate at lower temperatures or with less energy-intensive processes than conventional methods, they could offer a competitive advantage in terms of both economics and sustainability.
The scalability of magnesium nitrate carbon capture technology is essential for its economic viability. Large-scale implementation would likely benefit from economies of scale, potentially reducing per-unit costs as the technology matures and becomes more widely adopted. This scalability factor is crucial for industries seeking cost-effective carbon capture solutions that can be integrated into existing operations without prohibitive expenses.
Market dynamics also play a significant role in the economic assessment. The value of captured carbon, either for storage or utilization in other industrial processes, can greatly impact the return on investment for these systems. As carbon pricing mechanisms and regulatory frameworks evolve, the economic incentives for implementing carbon capture technologies may increase, potentially improving the financial attractiveness of magnesium nitrate-based systems.
Lastly, the lifecycle costs and environmental impact of magnesium nitrate production and disposal must be factored into the economic equation. If the material can be sourced sustainably and recycled efficiently, it could contribute to a more favorable economic profile compared to alternatives that require frequent replacement or have higher environmental footprints. The potential for integrating magnesium nitrate carbon capture into circular economy models could further enhance its economic viability and appeal to industries seeking comprehensive sustainability solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!