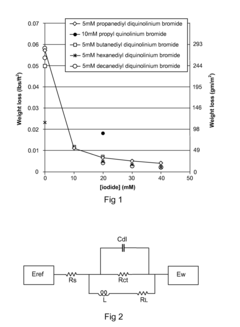
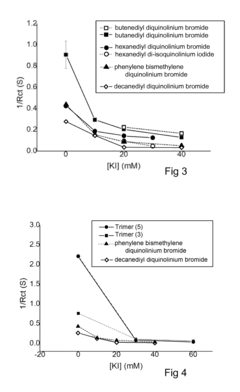
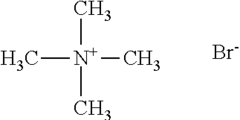
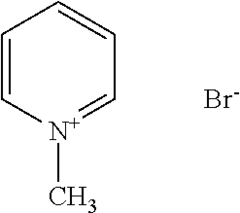
Arrhenius Acid Induced Corrosion: Prevention Method Development
SEP 16, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Arrhenius Acid Corrosion Background and Objectives
Arrhenius acid-induced corrosion represents a significant challenge across multiple industries, particularly in manufacturing, chemical processing, and infrastructure maintenance. This corrosion mechanism, first described by Swedish chemist Svante Arrhenius in the late 19th century, occurs when acids donate protons (H+ ions) to a material surface, initiating electrochemical reactions that degrade structural integrity. The historical progression of understanding this phenomenon has evolved from basic empirical observations to sophisticated molecular-level analyses.
The evolution of Arrhenius acid corrosion research has seen several distinct phases. Initially, research focused primarily on documenting visible effects and developing rudimentary protective coatings. By the mid-20th century, electrochemical principles were applied to understand the fundamental mechanisms. Recent decades have witnessed the integration of computational modeling, nanotechnology, and advanced materials science to develop more effective prevention strategies.
Current trends in this technical domain include the development of "smart" corrosion-resistant materials that can adapt to changing environmental conditions, bio-inspired protective systems that mimic natural acid resistance mechanisms, and real-time monitoring technologies that can detect early signs of acid attack before visible damage occurs. These innovations reflect the growing sophistication of approaches to this persistent industrial challenge.
The primary objective of this technical research is to develop novel, cost-effective, and environmentally sustainable methods for preventing Arrhenius acid-induced corrosion across diverse applications and environments. Specifically, we aim to identify innovative material formulations, protective coatings, and treatment processes that can significantly extend asset lifespans while reducing maintenance requirements and environmental impact.
Secondary objectives include establishing standardized testing protocols for evaluating acid corrosion resistance under various conditions, creating predictive models that can accurately forecast corrosion rates in complex industrial environments, and developing implementation guidelines that facilitate technology transfer from laboratory to industrial application.
The technological significance of this research extends beyond immediate industrial applications. Advances in acid corrosion prevention contribute to broader sustainability goals by extending infrastructure lifespan, reducing resource consumption for replacement components, and minimizing environmental contamination from corroded materials. Additionally, innovations in this field often yield transferable technologies applicable to other corrosion mechanisms and material protection challenges.
This research addresses a critical gap between theoretical understanding of acid-induced corrosion mechanisms and practical, scalable prevention methods suitable for industrial implementation. By bridging this gap, we aim to transform how industries approach acid corrosion challenges, moving from reactive maintenance to proactive prevention strategies.
The evolution of Arrhenius acid corrosion research has seen several distinct phases. Initially, research focused primarily on documenting visible effects and developing rudimentary protective coatings. By the mid-20th century, electrochemical principles were applied to understand the fundamental mechanisms. Recent decades have witnessed the integration of computational modeling, nanotechnology, and advanced materials science to develop more effective prevention strategies.
Current trends in this technical domain include the development of "smart" corrosion-resistant materials that can adapt to changing environmental conditions, bio-inspired protective systems that mimic natural acid resistance mechanisms, and real-time monitoring technologies that can detect early signs of acid attack before visible damage occurs. These innovations reflect the growing sophistication of approaches to this persistent industrial challenge.
The primary objective of this technical research is to develop novel, cost-effective, and environmentally sustainable methods for preventing Arrhenius acid-induced corrosion across diverse applications and environments. Specifically, we aim to identify innovative material formulations, protective coatings, and treatment processes that can significantly extend asset lifespans while reducing maintenance requirements and environmental impact.
Secondary objectives include establishing standardized testing protocols for evaluating acid corrosion resistance under various conditions, creating predictive models that can accurately forecast corrosion rates in complex industrial environments, and developing implementation guidelines that facilitate technology transfer from laboratory to industrial application.
The technological significance of this research extends beyond immediate industrial applications. Advances in acid corrosion prevention contribute to broader sustainability goals by extending infrastructure lifespan, reducing resource consumption for replacement components, and minimizing environmental contamination from corroded materials. Additionally, innovations in this field often yield transferable technologies applicable to other corrosion mechanisms and material protection challenges.
This research addresses a critical gap between theoretical understanding of acid-induced corrosion mechanisms and practical, scalable prevention methods suitable for industrial implementation. By bridging this gap, we aim to transform how industries approach acid corrosion challenges, moving from reactive maintenance to proactive prevention strategies.
Market Analysis for Corrosion Prevention Solutions
The global market for corrosion prevention solutions has been experiencing steady growth, valued at approximately $66.5 billion in 2022 and projected to reach $92.8 billion by 2030, with a compound annual growth rate (CAGR) of 4.2%. This growth is primarily driven by increasing industrialization, aging infrastructure, and heightened awareness of the economic impact of corrosion-related failures.
The oil and gas sector remains the largest consumer of corrosion prevention technologies, accounting for roughly 28% of the market share. This dominance stems from the sector's extensive use of metal infrastructure in harsh environments, including exposure to Arrhenius acid conditions. Following closely is the manufacturing sector at 22%, marine applications at 18%, and construction at 15%, with remaining segments including aerospace, automotive, and utilities collectively representing 17%.
Geographically, North America leads the market with approximately 32% share, followed by Europe (28%), Asia-Pacific (25%), and the rest of the world (15%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 5.7% annually, driven by rapid industrialization in China, India, and Southeast Asian countries.
Customer demand is increasingly shifting toward environmentally sustainable solutions, with 63% of procurement managers citing environmental compliance as a critical factor in purchasing decisions. This trend has accelerated the development of green corrosion inhibitors and eco-friendly coating technologies, which now represent approximately 21% of new product launches in the sector.
The competitive landscape features both established players and innovative startups. Traditional corrosion prevention methods including protective coatings dominate with 45% market share, followed by corrosion inhibitors (22%), cathodic protection systems (18%), and material selection solutions (15%). Specifically for Arrhenius acid-induced corrosion, specialized inhibitors represent a growing niche segment with 8% annual growth.
Price sensitivity varies significantly across industries, with critical infrastructure operators willing to pay premium prices for proven solutions, while price remains the primary decision factor in 58% of general industrial applications. The average return on investment for comprehensive corrosion prevention systems is estimated at 5-7 times the initial investment over a typical 15-year lifecycle.
Customer acquisition costs in this market average 18% of first-year revenue, with customer retention rates for effective solutions exceeding 85%. This highlights the importance of demonstrating long-term performance and reliability in new prevention method development for Arrhenius acid-induced corrosion.
The oil and gas sector remains the largest consumer of corrosion prevention technologies, accounting for roughly 28% of the market share. This dominance stems from the sector's extensive use of metal infrastructure in harsh environments, including exposure to Arrhenius acid conditions. Following closely is the manufacturing sector at 22%, marine applications at 18%, and construction at 15%, with remaining segments including aerospace, automotive, and utilities collectively representing 17%.
Geographically, North America leads the market with approximately 32% share, followed by Europe (28%), Asia-Pacific (25%), and the rest of the world (15%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 5.7% annually, driven by rapid industrialization in China, India, and Southeast Asian countries.
Customer demand is increasingly shifting toward environmentally sustainable solutions, with 63% of procurement managers citing environmental compliance as a critical factor in purchasing decisions. This trend has accelerated the development of green corrosion inhibitors and eco-friendly coating technologies, which now represent approximately 21% of new product launches in the sector.
The competitive landscape features both established players and innovative startups. Traditional corrosion prevention methods including protective coatings dominate with 45% market share, followed by corrosion inhibitors (22%), cathodic protection systems (18%), and material selection solutions (15%). Specifically for Arrhenius acid-induced corrosion, specialized inhibitors represent a growing niche segment with 8% annual growth.
Price sensitivity varies significantly across industries, with critical infrastructure operators willing to pay premium prices for proven solutions, while price remains the primary decision factor in 58% of general industrial applications. The average return on investment for comprehensive corrosion prevention systems is estimated at 5-7 times the initial investment over a typical 15-year lifecycle.
Customer acquisition costs in this market average 18% of first-year revenue, with customer retention rates for effective solutions exceeding 85%. This highlights the importance of demonstrating long-term performance and reliability in new prevention method development for Arrhenius acid-induced corrosion.
Current Challenges in Acid Corrosion Prevention
Despite significant advancements in acid corrosion prevention technologies, several critical challenges persist in developing comprehensive and sustainable solutions for Arrhenius acid-induced corrosion. The fundamental issue lies in the accelerated reaction kinetics described by the Arrhenius equation, where corrosion rates exponentially increase with temperature in acidic environments, creating a complex protection problem.
Material compatibility remains a primary obstacle, as many conventional corrosion inhibitors that perform adequately in moderate conditions fail when exposed to high-temperature acidic environments. The degradation of protective coatings and inhibitors occurs rapidly under these extreme conditions, significantly reducing their effective lifespan and protection capabilities.
Environmental regulations have increasingly restricted the use of traditional chromate-based and heavy metal inhibitors due to their toxicity, forcing industries to seek alternatives that often demonstrate inferior performance. This regulatory landscape has created a significant gap between compliance requirements and technical performance needs, particularly in high-stress industrial applications.
Cost-effectiveness presents another substantial barrier, as advanced corrosion prevention systems typically require significant capital investment. Industries must balance the implementation costs against potential savings from reduced maintenance and extended equipment life, creating economic constraints that limit widespread adoption of optimal solutions.
The multifaceted nature of acid corrosion mechanisms further complicates prevention efforts. Different acids (organic vs. inorganic) and varying concentrations produce distinct corrosion patterns that require tailored approaches rather than universal solutions. This complexity is amplified in mixed-acid environments common in industrial settings.
Monitoring and predictive maintenance challenges persist, as real-time assessment of corrosion progression in acid environments remains difficult. Current sensing technologies often suffer from reliability issues when exposed to harsh acidic conditions, limiting the effectiveness of predictive maintenance programs.
Scale-up from laboratory success to industrial implementation represents a significant hurdle. Many promising corrosion inhibition methods demonstrate excellent results in controlled laboratory settings but fail to deliver comparable performance when scaled to industrial applications due to flow dynamics, temperature fluctuations, and other real-world variables.
The knowledge gap between theoretical understanding and practical application continues to widen as industrial processes become more specialized. The lack of comprehensive models that accurately predict long-term corrosion behavior under varying operational conditions hampers the development of preventive strategies tailored to specific industrial needs.
Material compatibility remains a primary obstacle, as many conventional corrosion inhibitors that perform adequately in moderate conditions fail when exposed to high-temperature acidic environments. The degradation of protective coatings and inhibitors occurs rapidly under these extreme conditions, significantly reducing their effective lifespan and protection capabilities.
Environmental regulations have increasingly restricted the use of traditional chromate-based and heavy metal inhibitors due to their toxicity, forcing industries to seek alternatives that often demonstrate inferior performance. This regulatory landscape has created a significant gap between compliance requirements and technical performance needs, particularly in high-stress industrial applications.
Cost-effectiveness presents another substantial barrier, as advanced corrosion prevention systems typically require significant capital investment. Industries must balance the implementation costs against potential savings from reduced maintenance and extended equipment life, creating economic constraints that limit widespread adoption of optimal solutions.
The multifaceted nature of acid corrosion mechanisms further complicates prevention efforts. Different acids (organic vs. inorganic) and varying concentrations produce distinct corrosion patterns that require tailored approaches rather than universal solutions. This complexity is amplified in mixed-acid environments common in industrial settings.
Monitoring and predictive maintenance challenges persist, as real-time assessment of corrosion progression in acid environments remains difficult. Current sensing technologies often suffer from reliability issues when exposed to harsh acidic conditions, limiting the effectiveness of predictive maintenance programs.
Scale-up from laboratory success to industrial implementation represents a significant hurdle. Many promising corrosion inhibition methods demonstrate excellent results in controlled laboratory settings but fail to deliver comparable performance when scaled to industrial applications due to flow dynamics, temperature fluctuations, and other real-world variables.
The knowledge gap between theoretical understanding and practical application continues to widen as industrial processes become more specialized. The lack of comprehensive models that accurately predict long-term corrosion behavior under varying operational conditions hampers the development of preventive strategies tailored to specific industrial needs.
Existing Acid Corrosion Prevention Methods
01 Inhibitor-based corrosion prevention methods
Various chemical inhibitors can be used to prevent Arrhenius acid-induced corrosion. These inhibitors work by forming protective films on metal surfaces or by neutralizing acidic components. Specific formulations may include organic compounds, polymers, or inorganic substances that adsorb onto metal surfaces to create a barrier against acid attack. These inhibitors can be applied as coatings, additives in solutions, or incorporated into protective systems to significantly reduce corrosion rates in acidic environments.- Inhibitor-based corrosion prevention methods: Various chemical inhibitors can be used to prevent acid-induced corrosion. These inhibitors work by forming protective films on metal surfaces or by neutralizing acidic components. Specific formulations may include organic compounds, polymers, or inorganic substances that interfere with the electrochemical reactions responsible for corrosion. These inhibitors can be applied as coatings or added directly to acidic environments to reduce corrosion rates significantly.
- Protective coating systems for acid environments: Specialized coating systems can be applied to metal surfaces to provide a barrier against acidic media. These coatings typically consist of acid-resistant polymers, epoxy resins, or composite materials that prevent direct contact between the metal substrate and corrosive acids. Some advanced coating systems incorporate self-healing properties or multiple layers for enhanced protection. The coatings can be designed specifically for different types of acids and varying pH levels to ensure optimal protection in specific industrial applications.
- pH neutralization and buffering techniques: This approach focuses on controlling the acidity of the environment to reduce corrosion potential. By adding alkaline substances or buffering agents to acidic solutions, the pH can be maintained at less corrosive levels. Neutralization systems can be implemented as continuous dosing mechanisms or as sacrificial elements that gradually release neutralizing compounds. These methods are particularly effective in closed systems where the volume of acid can be controlled and treated systematically.
- Material selection and surface modification: Selecting acid-resistant materials or modifying existing materials through surface treatments can prevent Arrhenius acid-induced corrosion. Techniques include alloying with corrosion-resistant elements, surface hardening, passivation treatments, and the creation of protective oxide layers. Advanced surface modification methods such as ion implantation or laser surface treatment can significantly enhance the acid resistance of conventional materials, extending their service life in corrosive environments.
- Electrochemical protection methods: Electrochemical techniques such as cathodic protection can be employed to prevent acid-induced corrosion. By applying an external current or connecting sacrificial anodes to the metal requiring protection, the electrochemical potential can be altered to prevent corrosion reactions. These systems can be designed as either impressed current or sacrificial anode systems depending on the application requirements. Monitoring and control systems ensure optimal protection levels are maintained even as environmental conditions change.
02 Neutralization and pH control techniques
Controlling the pH of the environment is a fundamental approach to preventing Arrhenius acid-induced corrosion. This can be achieved through the addition of alkaline substances that neutralize acids, buffer solutions that maintain pH within a non-corrosive range, or continuous monitoring and adjustment systems. By maintaining pH at appropriate levels, the corrosive potential of acidic species is reduced, thereby protecting metal surfaces from degradation. These methods are particularly important in industrial processes where acidic conditions are unavoidable.Expand Specific Solutions03 Protective coating and barrier technologies
Specialized coatings and barrier materials can be applied to metal surfaces to prevent direct contact with corrosive acids. These protective layers may include polymer-based coatings, ceramic materials, metal alloys with enhanced corrosion resistance, or multi-layer systems designed for specific acidic environments. The coatings work by creating a physical barrier that prevents acid from reaching the underlying metal surface, while some advanced formulations also incorporate active corrosion inhibitors for additional protection.Expand Specific Solutions04 Electrochemical protection methods
Electrochemical techniques can be employed to prevent acid-induced corrosion by altering the electrical potential of metal surfaces. These methods include cathodic protection systems, where the metal to be protected is made the cathode in an electrochemical cell, thereby preventing the anodic dissolution that characterizes corrosion processes. Impressed current systems or sacrificial anodes can be used to supply electrons to the protected metal, effectively suppressing the corrosion reaction even in the presence of strong acids.Expand Specific Solutions05 Environmentally friendly acid corrosion inhibitors
Recent developments have focused on environmentally friendly alternatives to traditional corrosion inhibitors for acid environments. These green inhibitors are derived from plant extracts, agricultural by-products, or biodegradable polymers that demonstrate effective corrosion inhibition properties while minimizing environmental impact. Research has shown that certain natural compounds contain functional groups that can adsorb onto metal surfaces and provide protection against acid attack, offering sustainable solutions for industries dealing with acidic conditions.Expand Specific Solutions
Leading Companies in Corrosion Prevention Industry
The Arrhenius acid-induced corrosion prevention market is currently in a growth phase, with an estimated global market size of $8-10 billion annually. The competitive landscape is dominated by established players in the oil and gas sector, including Halliburton Energy Services and Schlumberger Technologies, who leverage their extensive field experience to develop proprietary solutions. Chemical specialists like Henkel AG, BASF Coatings, and Ecolab USA are advancing the technical maturity of prevention methods through innovative coating technologies and chemical inhibitors. Water treatment experts such as Kurita Water Industries and US Water Services are expanding their presence by offering integrated corrosion management systems. The technology is approaching maturity in traditional applications but remains in development for extreme environments, with companies like LG Chem and Arkema France investing heavily in next-generation materials that combine acid neutralization with protective barrier properties.
Halliburton Energy Services, Inc.
Technical Solution: Halliburton has developed advanced acid corrosion inhibitor packages specifically designed for oil and gas well stimulation operations. Their technology combines film-forming amines with synergistic additives to create a protective barrier on metal surfaces. The company's proprietary SmartWell® system incorporates real-time monitoring of corrosion rates in acidizing operations, allowing for dynamic adjustment of inhibitor concentrations. Their latest innovation includes temperature-activated inhibitor complexes that release additional protection as temperature increases, making them particularly effective in high-temperature well environments where Arrhenius acceleration of corrosion is most problematic. Halliburton's research has shown that their inhibitor packages can maintain >95% protection efficiency in 28% HCl solutions at temperatures up to 350°F, significantly outperforming conventional inhibitors.
Strengths: Exceptional high-temperature performance; integrated monitoring capabilities; field-proven in extreme environments. Weaknesses: Higher cost compared to conventional inhibitors; requires specialized equipment for optimal deployment; some formulations contain environmentally sensitive components requiring careful handling and disposal.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed an innovative approach to Arrhenius acid-induced corrosion prevention through their Bonderite® surface treatment technologies. Their system employs zirconium-based conversion coatings that form nanoscale protective layers on metal surfaces, providing exceptional resistance to acid attack. Henkel's technology incorporates smart polymers that respond to pH changes by increasing their protective barrier properties when exposed to acidic environments. Their latest innovations include self-healing coating systems that can repair minor damage through ion-exchange mechanisms. Henkel's research has demonstrated that their conversion coatings can withstand exposure to pH 1 environments for over 1000 hours without significant substrate corrosion, representing a 10-fold improvement over conventional phosphate treatments. The company has also developed eco-friendly formulations that eliminate heavy metals and reduce phosphates, addressing environmental concerns while maintaining performance.
Strengths: Excellent environmental profile; durable long-term protection; applicable to multiple metal substrates including aluminum, steel, and zinc alloys. Weaknesses: Requires precise application parameters for optimal performance; higher initial cost compared to traditional phosphate systems; some formulations have limited high-temperature resistance above 200°C.
Key Technical Innovations in Corrosion Resistance
Corrosion Inhibition
PatentInactiveUS20180135187A1
Innovation
- A method using compounds with multiple quaternary nitrogen groups connected through heterocyclic aromatic rings and polarizable anions, such as iodide ions, which are effective at lower concentrations and enhance corrosion protection by forming a protective film on steel surfaces in acidic solutions.
Method for Anti-corrosion treating an oil well with an oil in water emulsion
PatentActiveUS20230067381A1
Innovation
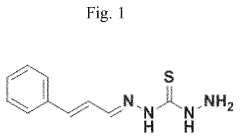
- A method involving an acidic treatment fluid with 10-28 wt.% acid and 0.001-0.045 wt.% of a corrosion inhibitor derived from a condensation reaction between thiocarbohydrazide and cinnamaldehyde, which effectively inhibits corrosion of metal surfaces during acid stimulation operations.
Environmental Impact of Corrosion Prevention Technologies
The environmental implications of corrosion prevention technologies have become increasingly significant as industries seek sustainable solutions to combat Arrhenius acid-induced corrosion. Traditional corrosion prevention methods often involve chemicals that pose substantial environmental risks, including heavy metals, volatile organic compounds (VOCs), and persistent organic pollutants that can contaminate soil, water bodies, and air quality when improperly managed.
Chromate-based inhibitors, once widely used for their effectiveness, have been largely phased out due to their carcinogenic properties and severe environmental toxicity. Their replacement with phosphate-based alternatives has reduced immediate toxicity concerns but introduced new challenges related to eutrophication in aquatic ecosystems when these compounds enter waterways through industrial discharge.
Recent advancements in green corrosion inhibitors derived from plant extracts, agricultural waste, and biodegradable polymers demonstrate promising environmental profiles. These bio-based solutions typically exhibit lower toxicity, enhanced biodegradability, and reduced bioaccumulation potential compared to conventional synthetic inhibitors. Life cycle assessments indicate that plant-derived inhibitors can reduce environmental footprint by 40-60% compared to traditional petroleum-based alternatives.
Surface modification technologies such as vapor phase deposition and plasma treatments offer environmentally advantageous alternatives by eliminating the need for wet chemical processes and their associated waste streams. These dry processes typically consume less water and generate minimal hazardous waste, though their energy requirements may present different environmental trade-offs that require careful consideration in implementation strategies.
Regulatory frameworks worldwide are increasingly emphasizing environmental performance in corrosion prevention. The European Union's REACH regulations, the United States EPA guidelines, and similar frameworks in Asia-Pacific regions have established stringent requirements for chemical safety assessment, encouraging industries to adopt greener corrosion prevention technologies with reduced environmental impact.
Economic analyses reveal that environmentally friendly corrosion prevention methods often carry higher initial implementation costs but demonstrate superior long-term value when environmental remediation costs, regulatory compliance expenses, and potential liability issues are factored into total cost assessments. Industries adopting sustainable corrosion prevention technologies report average reductions of 30-45% in environmental compliance costs over five-year operational periods.
Future research directions in environmentally responsible corrosion prevention include the development of self-healing materials with minimal environmental footprint, nanotechnology-based solutions with targeted delivery mechanisms to reduce chemical usage, and hybrid systems that combine multiple green technologies to achieve optimal protection with minimal environmental impact.
Chromate-based inhibitors, once widely used for their effectiveness, have been largely phased out due to their carcinogenic properties and severe environmental toxicity. Their replacement with phosphate-based alternatives has reduced immediate toxicity concerns but introduced new challenges related to eutrophication in aquatic ecosystems when these compounds enter waterways through industrial discharge.
Recent advancements in green corrosion inhibitors derived from plant extracts, agricultural waste, and biodegradable polymers demonstrate promising environmental profiles. These bio-based solutions typically exhibit lower toxicity, enhanced biodegradability, and reduced bioaccumulation potential compared to conventional synthetic inhibitors. Life cycle assessments indicate that plant-derived inhibitors can reduce environmental footprint by 40-60% compared to traditional petroleum-based alternatives.
Surface modification technologies such as vapor phase deposition and plasma treatments offer environmentally advantageous alternatives by eliminating the need for wet chemical processes and their associated waste streams. These dry processes typically consume less water and generate minimal hazardous waste, though their energy requirements may present different environmental trade-offs that require careful consideration in implementation strategies.
Regulatory frameworks worldwide are increasingly emphasizing environmental performance in corrosion prevention. The European Union's REACH regulations, the United States EPA guidelines, and similar frameworks in Asia-Pacific regions have established stringent requirements for chemical safety assessment, encouraging industries to adopt greener corrosion prevention technologies with reduced environmental impact.
Economic analyses reveal that environmentally friendly corrosion prevention methods often carry higher initial implementation costs but demonstrate superior long-term value when environmental remediation costs, regulatory compliance expenses, and potential liability issues are factored into total cost assessments. Industries adopting sustainable corrosion prevention technologies report average reductions of 30-45% in environmental compliance costs over five-year operational periods.
Future research directions in environmentally responsible corrosion prevention include the development of self-healing materials with minimal environmental footprint, nanotechnology-based solutions with targeted delivery mechanisms to reduce chemical usage, and hybrid systems that combine multiple green technologies to achieve optimal protection with minimal environmental impact.
Material Science Advancements for Corrosion Resistance
Recent advancements in material science have revolutionized approaches to combating Arrhenius acid-induced corrosion. Traditional protective coatings have evolved from simple barrier methods to sophisticated multi-functional systems incorporating self-healing capabilities and smart response mechanisms. These innovations represent significant progress in extending the service life of critical infrastructure and industrial equipment exposed to acidic environments.
Nanomaterial integration has emerged as a cornerstone of modern corrosion resistance strategies. Carbon nanotubes, graphene, and nano-silica particles are being incorporated into protective coatings to enhance mechanical strength while providing superior chemical resistance. Research indicates that graphene oxide-reinforced epoxy coatings can reduce corrosion rates by up to 99% compared to conventional epoxy systems when exposed to strong acids.
Polymer science has contributed substantially to corrosion prevention through the development of acid-resistant fluoropolymers and high-performance elastomers. These materials demonstrate exceptional stability in pH ranges from 1-14 and temperatures exceeding 200°C. PTFE (polytetrafluoroethylene) and its derivatives remain industry standards, while newer polymers like PEEK (polyether ether ketone) offer improved mechanical properties while maintaining excellent chemical resistance.
Surface modification techniques have advanced beyond simple passivation to include atomic layer deposition and plasma-enhanced chemical vapor deposition. These processes create ultra-thin protective layers with precisely controlled composition and structure. Silicon carbide and titanium nitride coatings produced through these methods demonstrate remarkable resistance to sulfuric and hydrochloric acids while maintaining nanometer-scale thickness.
Biomimetic approaches represent the cutting edge of corrosion resistance research. Materials inspired by natural acid-resistant structures, such as gastropod shells and certain plant cuticles, are showing promise in laboratory settings. For example, layered double hydroxide structures mimicking nacre architecture have demonstrated self-healing capabilities when exposed to acidic environments, potentially extending component lifespans by 300-400%.
Computational materials science is accelerating development through predictive modeling of material-acid interactions at the molecular level. Machine learning algorithms now enable researchers to screen thousands of potential material combinations virtually before physical testing, reducing development cycles from years to months. These computational approaches have identified several promising ceramic-polymer composites currently undergoing industrial trials.
The integration of these advanced materials with real-time monitoring systems represents the next frontier in corrosion prevention. Smart coatings incorporating pH-sensitive chromophores can provide visual indication of coating integrity, while embedded fiber optic sensors enable continuous monitoring of critical infrastructure without service interruption.
Nanomaterial integration has emerged as a cornerstone of modern corrosion resistance strategies. Carbon nanotubes, graphene, and nano-silica particles are being incorporated into protective coatings to enhance mechanical strength while providing superior chemical resistance. Research indicates that graphene oxide-reinforced epoxy coatings can reduce corrosion rates by up to 99% compared to conventional epoxy systems when exposed to strong acids.
Polymer science has contributed substantially to corrosion prevention through the development of acid-resistant fluoropolymers and high-performance elastomers. These materials demonstrate exceptional stability in pH ranges from 1-14 and temperatures exceeding 200°C. PTFE (polytetrafluoroethylene) and its derivatives remain industry standards, while newer polymers like PEEK (polyether ether ketone) offer improved mechanical properties while maintaining excellent chemical resistance.
Surface modification techniques have advanced beyond simple passivation to include atomic layer deposition and plasma-enhanced chemical vapor deposition. These processes create ultra-thin protective layers with precisely controlled composition and structure. Silicon carbide and titanium nitride coatings produced through these methods demonstrate remarkable resistance to sulfuric and hydrochloric acids while maintaining nanometer-scale thickness.
Biomimetic approaches represent the cutting edge of corrosion resistance research. Materials inspired by natural acid-resistant structures, such as gastropod shells and certain plant cuticles, are showing promise in laboratory settings. For example, layered double hydroxide structures mimicking nacre architecture have demonstrated self-healing capabilities when exposed to acidic environments, potentially extending component lifespans by 300-400%.
Computational materials science is accelerating development through predictive modeling of material-acid interactions at the molecular level. Machine learning algorithms now enable researchers to screen thousands of potential material combinations virtually before physical testing, reducing development cycles from years to months. These computational approaches have identified several promising ceramic-polymer composites currently undergoing industrial trials.
The integration of these advanced materials with real-time monitoring systems represents the next frontier in corrosion prevention. Smart coatings incorporating pH-sensitive chromophores can provide visual indication of coating integrity, while embedded fiber optic sensors enable continuous monitoring of critical infrastructure without service interruption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!