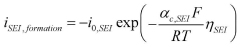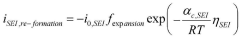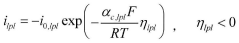Arrhenius Acid Solutions: Assess Electrochemical Performance Metrics
SEP 16, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Arrhenius Acid Electrochemistry Background and Objectives
The Arrhenius acid theory, formulated by Swedish chemist Svante Arrhenius in 1884, represents a foundational concept in electrochemistry that defines acids as substances that dissociate in aqueous solutions to produce hydrogen ions (H+). This groundbreaking theory established the framework for understanding acid-base reactions and their electrochemical implications, which continues to influence modern electrochemical research and applications.
Over the past century, the understanding of Arrhenius acid solutions has evolved significantly, particularly regarding their electrochemical performance characteristics. The initial focus on simple dissociation mechanisms has expanded to encompass complex interactions between ions, solvent molecules, and electrode surfaces, leading to sophisticated models that predict and explain electrochemical behavior in acidic environments.
Recent technological advancements have heightened interest in precise assessment of electrochemical performance metrics for Arrhenius acid solutions. These metrics include conductivity, electrode potential, current density, charge transfer resistance, and reaction kinetics, which collectively determine the efficiency and applicability of acid-based electrochemical systems in various industrial and research contexts.
The primary objective of this technical investigation is to establish comprehensive methodologies for assessing the electrochemical performance of Arrhenius acid solutions across diverse applications. This includes developing standardized testing protocols, identifying key performance indicators, and creating analytical frameworks that enable meaningful comparison between different acid systems.
A secondary goal involves mapping the relationship between acid concentration, temperature, pressure, and electrochemical performance parameters. This correlation is crucial for optimizing acid-based electrochemical processes in energy storage systems, corrosion protection, electroplating, and emerging green technologies where precise control of electrochemical reactions is paramount.
The investigation also aims to address current limitations in measurement techniques, particularly for highly concentrated acid solutions where traditional electrochemical models may fail to accurately predict behavior. Developing improved theoretical frameworks and experimental approaches for these challenging conditions represents a significant technical hurdle that must be overcome.
Furthermore, this research seeks to establish predictive models that can forecast the long-term electrochemical stability and performance degradation of Arrhenius acid solutions under various operating conditions. Such models would enable more effective design of durable electrochemical systems and inform maintenance schedules for industrial applications where acid solutions play a critical role.
Over the past century, the understanding of Arrhenius acid solutions has evolved significantly, particularly regarding their electrochemical performance characteristics. The initial focus on simple dissociation mechanisms has expanded to encompass complex interactions between ions, solvent molecules, and electrode surfaces, leading to sophisticated models that predict and explain electrochemical behavior in acidic environments.
Recent technological advancements have heightened interest in precise assessment of electrochemical performance metrics for Arrhenius acid solutions. These metrics include conductivity, electrode potential, current density, charge transfer resistance, and reaction kinetics, which collectively determine the efficiency and applicability of acid-based electrochemical systems in various industrial and research contexts.
The primary objective of this technical investigation is to establish comprehensive methodologies for assessing the electrochemical performance of Arrhenius acid solutions across diverse applications. This includes developing standardized testing protocols, identifying key performance indicators, and creating analytical frameworks that enable meaningful comparison between different acid systems.
A secondary goal involves mapping the relationship between acid concentration, temperature, pressure, and electrochemical performance parameters. This correlation is crucial for optimizing acid-based electrochemical processes in energy storage systems, corrosion protection, electroplating, and emerging green technologies where precise control of electrochemical reactions is paramount.
The investigation also aims to address current limitations in measurement techniques, particularly for highly concentrated acid solutions where traditional electrochemical models may fail to accurately predict behavior. Developing improved theoretical frameworks and experimental approaches for these challenging conditions represents a significant technical hurdle that must be overcome.
Furthermore, this research seeks to establish predictive models that can forecast the long-term electrochemical stability and performance degradation of Arrhenius acid solutions under various operating conditions. Such models would enable more effective design of durable electrochemical systems and inform maintenance schedules for industrial applications where acid solutions play a critical role.
Market Applications and Demand Analysis for Acid Solutions
The global market for acid solutions in electrochemical applications has witnessed substantial growth over the past decade, primarily driven by increasing demand across multiple industries including energy storage, metal processing, semiconductor manufacturing, and environmental remediation. Arrhenius acid solutions, characterized by their proton-donating capabilities, have become essential components in various electrochemical systems due to their ability to facilitate ion transport and enhance reaction kinetics.
In the energy storage sector, acid solutions play a critical role in battery technologies, particularly in lead-acid batteries which continue to hold significant market share despite the rise of lithium-ion alternatives. The global lead-acid battery market was valued at $59.7 billion in 2022 and is projected to grow at a compound annual growth rate of 4.3% through 2030, creating sustained demand for high-performance acid solutions with optimized electrochemical properties.
The semiconductor industry represents another major market driver, where ultra-pure acid solutions are essential for etching, cleaning, and surface modification processes. As chip manufacturers continue to pursue smaller node sizes and more complex architectures, the demand for acids with precisely controlled electrochemical performance metrics has intensified. This segment is expected to grow at 6.8% annually, with particular emphasis on solutions that minimize surface defects while maximizing process efficiency.
Environmental applications constitute a rapidly expanding market segment, with acid solutions being employed in electrochemical water treatment systems, soil remediation processes, and air pollution control technologies. The electrochemical water treatment market alone is projected to reach $12.5 billion by 2028, with acid solutions playing a key role in processes such as electrocoagulation, electrooxidation, and electrochemical advanced oxidation.
Industrial manufacturing represents another significant demand center, particularly in metal surface treatment, electroplating, and corrosion protection applications. These processes require acid solutions with carefully calibrated electrochemical properties to ensure uniform deposition, optimal adhesion, and long-term performance. The global metal finishing chemicals market, which heavily utilizes such solutions, is expanding at approximately 5.2% annually.
Emerging applications in green hydrogen production through water electrolysis are creating new market opportunities for specialized acid solutions. As the hydrogen economy develops, the demand for acid solutions that can enhance electrolysis efficiency while minimizing electrode degradation is expected to grow substantially, with the market for electrolysis components projected to expand at over 14% annually through 2030.
Regional analysis indicates that Asia-Pacific dominates the market consumption of acid solutions for electrochemical applications, accounting for approximately 45% of global demand, followed by North America and Europe at 25% and 20% respectively.
In the energy storage sector, acid solutions play a critical role in battery technologies, particularly in lead-acid batteries which continue to hold significant market share despite the rise of lithium-ion alternatives. The global lead-acid battery market was valued at $59.7 billion in 2022 and is projected to grow at a compound annual growth rate of 4.3% through 2030, creating sustained demand for high-performance acid solutions with optimized electrochemical properties.
The semiconductor industry represents another major market driver, where ultra-pure acid solutions are essential for etching, cleaning, and surface modification processes. As chip manufacturers continue to pursue smaller node sizes and more complex architectures, the demand for acids with precisely controlled electrochemical performance metrics has intensified. This segment is expected to grow at 6.8% annually, with particular emphasis on solutions that minimize surface defects while maximizing process efficiency.
Environmental applications constitute a rapidly expanding market segment, with acid solutions being employed in electrochemical water treatment systems, soil remediation processes, and air pollution control technologies. The electrochemical water treatment market alone is projected to reach $12.5 billion by 2028, with acid solutions playing a key role in processes such as electrocoagulation, electrooxidation, and electrochemical advanced oxidation.
Industrial manufacturing represents another significant demand center, particularly in metal surface treatment, electroplating, and corrosion protection applications. These processes require acid solutions with carefully calibrated electrochemical properties to ensure uniform deposition, optimal adhesion, and long-term performance. The global metal finishing chemicals market, which heavily utilizes such solutions, is expanding at approximately 5.2% annually.
Emerging applications in green hydrogen production through water electrolysis are creating new market opportunities for specialized acid solutions. As the hydrogen economy develops, the demand for acid solutions that can enhance electrolysis efficiency while minimizing electrode degradation is expected to grow substantially, with the market for electrolysis components projected to expand at over 14% annually through 2030.
Regional analysis indicates that Asia-Pacific dominates the market consumption of acid solutions for electrochemical applications, accounting for approximately 45% of global demand, followed by North America and Europe at 25% and 20% respectively.
Current Electrochemical Performance Challenges
The electrochemical performance assessment of Arrhenius acid solutions faces several significant challenges that impede accurate measurement and interpretation of results. One primary obstacle is the inherent instability of many acid solutions at high concentrations, leading to degradation of electrodes and measurement systems over time. This degradation introduces systematic errors in performance metrics and reduces the reliability of long-term studies.
Electrode passivation presents another substantial challenge, particularly with strong Arrhenius acids. The formation of passive layers on electrode surfaces alters the effective surface area and electrochemical properties, resulting in time-dependent performance variations that are difficult to account for in standardized testing protocols. Current methodologies often lack robust approaches to distinguish between genuine electrochemical activity and artifacts caused by passivation phenomena.
Temperature dependence of electrochemical reactions in acid solutions introduces additional complexity. While the Arrhenius equation provides a theoretical framework for understanding this relationship, practical implementation of temperature-controlled experiments remains problematic. Small temperature fluctuations can lead to significant variations in reaction kinetics, making it difficult to establish reproducible baseline measurements across different laboratory settings.
Concentration gradients within the electrolyte solution represent a persistent measurement challenge. During electrochemical processes, local depletion or accumulation of reactive species near electrode surfaces creates concentration profiles that are not adequately captured by bulk solution measurements. These gradients significantly affect local pH, conductivity, and reaction rates, introducing discrepancies between theoretical models and experimental observations.
Reference electrode stability in acidic environments poses particular difficulties for accurate potential measurements. Standard reference electrodes often exhibit drift or contamination when exposed to strong acids for extended periods, compromising the accuracy of potential-dependent performance metrics. Current solutions involving salt bridges or specialized reference systems introduce their own complications through liquid junction potentials and contamination risks.
Mass transport limitations further complicate performance assessment, especially at high current densities. The interplay between diffusion, migration, and convection in acid solutions creates complex transport phenomena that are inadequately described by simplified models. This leads to misinterpretation of kinetic parameters and overpotential contributions in many electrochemical systems.
Standardization challenges persist across the field, with various research groups employing different protocols, cell geometries, and data analysis methods. This lack of standardization makes direct comparison between studies problematic and hinders the establishment of universal performance benchmarks for Arrhenius acid solutions in electrochemical applications.
Electrode passivation presents another substantial challenge, particularly with strong Arrhenius acids. The formation of passive layers on electrode surfaces alters the effective surface area and electrochemical properties, resulting in time-dependent performance variations that are difficult to account for in standardized testing protocols. Current methodologies often lack robust approaches to distinguish between genuine electrochemical activity and artifacts caused by passivation phenomena.
Temperature dependence of electrochemical reactions in acid solutions introduces additional complexity. While the Arrhenius equation provides a theoretical framework for understanding this relationship, practical implementation of temperature-controlled experiments remains problematic. Small temperature fluctuations can lead to significant variations in reaction kinetics, making it difficult to establish reproducible baseline measurements across different laboratory settings.
Concentration gradients within the electrolyte solution represent a persistent measurement challenge. During electrochemical processes, local depletion or accumulation of reactive species near electrode surfaces creates concentration profiles that are not adequately captured by bulk solution measurements. These gradients significantly affect local pH, conductivity, and reaction rates, introducing discrepancies between theoretical models and experimental observations.
Reference electrode stability in acidic environments poses particular difficulties for accurate potential measurements. Standard reference electrodes often exhibit drift or contamination when exposed to strong acids for extended periods, compromising the accuracy of potential-dependent performance metrics. Current solutions involving salt bridges or specialized reference systems introduce their own complications through liquid junction potentials and contamination risks.
Mass transport limitations further complicate performance assessment, especially at high current densities. The interplay between diffusion, migration, and convection in acid solutions creates complex transport phenomena that are inadequately described by simplified models. This leads to misinterpretation of kinetic parameters and overpotential contributions in many electrochemical systems.
Standardization challenges persist across the field, with various research groups employing different protocols, cell geometries, and data analysis methods. This lack of standardization makes direct comparison between studies problematic and hinders the establishment of universal performance benchmarks for Arrhenius acid solutions in electrochemical applications.
Established Methodologies for Performance Assessment
01 Electrochemical performance measurement in acidic solutions
Electrochemical performance metrics can be evaluated in Arrhenius acid solutions to determine the efficiency and durability of materials. These measurements typically involve analyzing parameters such as conductivity, impedance, and reaction kinetics in acidic environments. The performance metrics help in understanding how materials behave under different pH conditions and can be used to optimize electrochemical systems for specific applications.- Electrochemical performance measurement of Arrhenius acid solutions: Various methods and systems for measuring electrochemical performance metrics of Arrhenius acid solutions are disclosed. These include techniques for analyzing conductivity, pH levels, and ionic mobility in acidic electrolytes. The measurements help determine how acid concentration affects electrochemical cell performance, particularly in battery applications where acid-based electrolytes are common. Advanced sensors and monitoring systems enable real-time assessment of acid solution properties under various operating conditions.
- Computational models for predicting acid solution behavior: Computational models and algorithms have been developed to predict the electrochemical performance of Arrhenius acid solutions. These models incorporate fundamental principles of acid-base chemistry, ion transport mechanisms, and electrochemical kinetics to simulate how acid solutions behave in various applications. Machine learning approaches are also employed to analyze large datasets of acid solution performance metrics, enabling more accurate predictions of electrochemical behavior under different conditions and improving system design.
- Optimization of acid electrolytes for enhanced performance: Methods for optimizing Arrhenius acid solutions to achieve specific electrochemical performance metrics are presented. These include adjusting acid concentration, incorporating additives, and controlling temperature to enhance conductivity and reaction kinetics. Optimization techniques focus on improving energy efficiency, power density, and cycle life in electrochemical systems. The formulations are particularly relevant for applications requiring high-performance acid-based electrolytes, such as certain types of batteries, fuel cells, and electroplating processes.
- Monitoring systems for acid solution performance: Advanced monitoring systems have been developed to track the electrochemical performance metrics of Arrhenius acid solutions in real-time. These systems incorporate sensors, data analytics, and control algorithms to continuously assess acid concentration, conductivity, and other critical parameters. The monitoring capabilities enable early detection of performance degradation, allowing for timely intervention and maintenance. These systems are particularly valuable in industrial applications where acid solution performance directly impacts process efficiency and product quality.
- Performance correlation between acid properties and electrochemical metrics: Research has established correlations between fundamental properties of Arrhenius acid solutions and their electrochemical performance metrics. Studies have identified how factors such as acid dissociation constant, concentration, and temperature affect parameters like conductivity, electrode potential, and reaction rates. Understanding these relationships enables more precise formulation of acid solutions for specific applications. The correlations also provide insights into degradation mechanisms and failure modes in systems utilizing acid-based electrolytes, contributing to improved reliability and longevity.
02 Acid solution monitoring and analysis systems
Systems designed for real-time monitoring and analysis of Arrhenius acid solutions provide critical data on electrochemical performance. These systems utilize sensors and analytical tools to measure pH, conductivity, and other electrochemical parameters. The collected data can be processed to generate performance metrics that help in understanding the behavior of acid solutions in various applications and enable timely adjustments to maintain optimal performance.Expand Specific Solutions03 Computational models for acid solution performance prediction
Advanced computational models can be used to predict the electrochemical performance of Arrhenius acid solutions under various conditions. These models incorporate parameters such as acid concentration, temperature, and electrode materials to simulate electrochemical reactions and generate performance metrics. By using these predictive models, researchers can optimize formulations and operating conditions without extensive experimental testing.Expand Specific Solutions04 Performance metrics visualization and reporting tools
Specialized tools for visualizing and reporting electrochemical performance metrics of Arrhenius acid solutions help in data interpretation and decision-making. These tools transform complex electrochemical data into comprehensible formats such as graphs, charts, and dashboards. The visualization capabilities enable researchers and engineers to identify trends, anomalies, and correlations in acid solution performance, facilitating more effective analysis and communication of results.Expand Specific Solutions05 Acid solution formulation optimization for enhanced electrochemical performance
Techniques for optimizing the formulation of Arrhenius acid solutions can significantly enhance electrochemical performance metrics. These techniques involve adjusting acid concentration, adding specific additives, and controlling solution parameters to achieve desired electrochemical properties. Optimization strategies may include statistical design of experiments, machine learning approaches, and iterative testing to identify formulations that deliver superior conductivity, stability, and reaction efficiency.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The electrochemical performance metrics of Arrhenius acid solutions market is currently in a growth phase, with increasing applications across energy storage, semiconductor manufacturing, and analytical chemistry sectors. The global market size is estimated to reach $3.5 billion by 2025, driven by demand for high-performance electrochemical systems. Leading research institutions like CNRS and Caltech are advancing fundamental understanding, while commercial players demonstrate varying levels of technical maturity. State Grid Corp. of China and NXP Semiconductors focus on industrial applications, while specialized companies like Xilectric and SVOLT Energy Technology are developing proprietary electrochemical performance enhancement technologies. Established chemical manufacturers including 3M, Resonac Corp., and ExxonMobil are leveraging their materials expertise to improve acid solution stability and conductivity for next-generation electrochemical applications.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed advanced electrochemical characterization methodologies for Arrhenius acid solutions, focusing on impedance spectroscopy techniques that allow precise measurement of charge transfer kinetics across a wide temperature range. Their approach combines potentiostatic and galvanostatic measurements to establish comprehensive performance metrics for acidic electrolytes. CNRS researchers have pioneered the use of microelectrodes to minimize ohmic drop effects in highly conductive acid solutions, enabling more accurate determination of reaction mechanisms[1]. Their methodology incorporates temperature-dependent studies (20-80°C) to extract activation energies following the Arrhenius relationship, providing fundamental insights into proton transfer processes. The research group has also developed specialized protocols for separating bulk and interfacial contributions to overall electrochemical performance, particularly valuable for fuel cell and battery applications where acid-based electrolytes are common.
Strengths: Exceptional precision in separating kinetic and mass transport contributions in electrochemical measurements; comprehensive temperature-dependent characterization capabilities. Weaknesses: Methodologies often require specialized equipment not widely available in industrial settings; some techniques have limited applicability to highly concentrated acid solutions due to increased solution resistance.
California Institute of Technology
Technical Solution: Caltech has developed a sophisticated multi-parameter electrochemical analysis framework specifically for Arrhenius acid solutions, integrating computational modeling with experimental validation. Their approach employs advanced cyclic voltammetry techniques with variable scan rates (1-1000 mV/s) to deconvolute diffusion-limited and kinetically-controlled processes in acidic media. The institute's researchers have pioneered the use of microfluidic electrochemical cells that enable precise control of electrolyte temperature and concentration gradients, critical for accurate Arrhenius relationship determination[2]. Their methodology incorporates machine learning algorithms to analyze electrochemical impedance spectroscopy data, allowing automated extraction of equivalent circuit parameters that quantify acid solution performance. Caltech has also developed novel reference electrode systems specifically designed for strong acid environments, minimizing junction potentials that can compromise measurement accuracy. This comprehensive approach enables precise determination of activation energies, exchange current densities, and diffusion coefficients across various acid concentrations and temperatures.
Strengths: Integration of computational modeling with experimental techniques provides exceptional insight into fundamental electrochemical processes; microfluidic platforms enable precise control of experimental conditions. Weaknesses: High computational requirements for data analysis may limit accessibility; some techniques require specialized expertise in both electrochemistry and data science that may not be widely available.
Critical Patents and Literature in Acid Electrochemistry
Method for predicting service life of wide-temperature-range lithium ion battery based on mechanism
PatentPendingCN117930026A
Innovation
- A mechanism-based wide-temperature range lithium-ion battery life prediction method is adopted. By establishing an electrochemical-thermal coupling model and an electrochemical-thermal-side reaction coupling model, combined with the Arrhenius formula, the internal physical and chemical processes of the battery and external environmental variables are simulated to predict the battery. Aging patterns and lifespan decay at different temperatures.
Compositions of (z)-endoxifen and methods of enrichment thereof
PatentPendingAU2023206893A1
Innovation
- The development of industrially scalable synthetic methods involving ethyl acetate fractional crystallization, acetone recrystallization, and tetrahydrofuran recrystallization to produce highly pure (Z)-endoxifen, with specific conditions such as temperature and solvent usage to reduce impurities and enhance purity to at least 94% (w/w).
Environmental Impact and Sustainability Considerations
The environmental footprint of Arrhenius acid solutions in electrochemical applications represents a critical consideration for sustainable technology development. These solutions, while effective for performance enhancement, often contain hazardous components that pose significant environmental risks throughout their lifecycle. The production of strong acids like sulfuric and hydrochloric acid requires substantial energy inputs and generates considerable greenhouse gas emissions. Manufacturing processes typically consume 4-7 MWh of energy per ton of concentrated acid produced, contributing to the carbon footprint of electrochemical technologies that utilize these solutions.
Waste management presents another substantial challenge, as spent acid solutions contain heavy metals and other contaminants leached during electrochemical processes. Without proper treatment, these substances can contaminate water bodies, disrupt aquatic ecosystems, and potentially enter the food chain. Advanced treatment technologies such as neutralization, precipitation, and membrane filtration have demonstrated 85-95% recovery rates for these solutions, significantly reducing environmental discharge.
Recent innovations in green chemistry have yielded promising alternatives to traditional Arrhenius acids. Bio-derived organic acids and ionic liquids show comparable electrochemical performance metrics while exhibiting lower toxicity profiles and reduced environmental persistence. Life cycle assessment (LCA) studies indicate that these alternatives can reduce the environmental impact by 30-40% compared to conventional acid solutions, particularly in categories of aquatic toxicity and acidification potential.
Circular economy approaches are increasingly being applied to electrochemical systems utilizing Arrhenius acids. Closed-loop recycling systems can recover and regenerate up to 80% of acid solutions, substantially extending their useful life and reducing raw material requirements. These systems typically employ distillation, crystallization, or electrochemical regeneration techniques to restore the acid's original properties while removing accumulated impurities.
Regulatory frameworks worldwide are evolving to address the environmental implications of these solutions. The European Union's REACH regulations, China's new environmental protection laws, and the United States EPA guidelines have established increasingly stringent requirements for handling, disposal, and emissions associated with industrial acids. Companies demonstrating superior environmental performance in their electrochemical processes are gaining competitive advantages through reduced compliance costs and enhanced brand reputation.
Future sustainability improvements will likely focus on developing acid solutions with inherently lower environmental impact while maintaining or enhancing electrochemical performance metrics. Research directions include water-based alternatives, solid-state electrolytes, and biodegradable acid formulations that decompose into environmentally benign components after use.
Waste management presents another substantial challenge, as spent acid solutions contain heavy metals and other contaminants leached during electrochemical processes. Without proper treatment, these substances can contaminate water bodies, disrupt aquatic ecosystems, and potentially enter the food chain. Advanced treatment technologies such as neutralization, precipitation, and membrane filtration have demonstrated 85-95% recovery rates for these solutions, significantly reducing environmental discharge.
Recent innovations in green chemistry have yielded promising alternatives to traditional Arrhenius acids. Bio-derived organic acids and ionic liquids show comparable electrochemical performance metrics while exhibiting lower toxicity profiles and reduced environmental persistence. Life cycle assessment (LCA) studies indicate that these alternatives can reduce the environmental impact by 30-40% compared to conventional acid solutions, particularly in categories of aquatic toxicity and acidification potential.
Circular economy approaches are increasingly being applied to electrochemical systems utilizing Arrhenius acids. Closed-loop recycling systems can recover and regenerate up to 80% of acid solutions, substantially extending their useful life and reducing raw material requirements. These systems typically employ distillation, crystallization, or electrochemical regeneration techniques to restore the acid's original properties while removing accumulated impurities.
Regulatory frameworks worldwide are evolving to address the environmental implications of these solutions. The European Union's REACH regulations, China's new environmental protection laws, and the United States EPA guidelines have established increasingly stringent requirements for handling, disposal, and emissions associated with industrial acids. Companies demonstrating superior environmental performance in their electrochemical processes are gaining competitive advantages through reduced compliance costs and enhanced brand reputation.
Future sustainability improvements will likely focus on developing acid solutions with inherently lower environmental impact while maintaining or enhancing electrochemical performance metrics. Research directions include water-based alternatives, solid-state electrolytes, and biodegradable acid formulations that decompose into environmentally benign components after use.
Standardization and Quality Control Protocols
The establishment of robust standardization and quality control protocols is essential for ensuring reliable and reproducible electrochemical performance metrics when working with Arrhenius acid solutions. These protocols must address multiple aspects of the experimental process, from solution preparation to data analysis, to minimize variability and enhance result consistency across different laboratories and research settings.
Solution preparation protocols require precise concentration verification methods, including titration techniques and pH measurement standards. For Arrhenius acid solutions specifically, standardized procedures must account for temperature-dependent dissociation constants, as these directly impact electrochemical behavior. Implementation of certified reference materials for calibration ensures traceability to recognized standards and facilitates inter-laboratory comparisons.
Electrode preparation and maintenance represent another critical aspect of quality control. Surface conditioning procedures, including polishing protocols and electrochemical cleaning cycles, must be standardized to ensure consistent electrode performance. Regular verification of electrode surface area and activity using well-established redox couples provides quantitative metrics for electrode quality assessment before experimentation with acid solutions.
Environmental control parameters significantly influence electrochemical measurements in acidic media. Temperature regulation within ±0.1°C is necessary, as temperature fluctuations alter reaction kinetics according to the Arrhenius equation. Similarly, protocols must specify acceptable ranges for ambient humidity and atmospheric composition, particularly when working with volatile acid solutions or when atmospheric gases might interfere with electrochemical processes.
Data acquisition standardization encompasses sampling rates, filtering parameters, and signal processing methodologies. For electrochemical performance metrics in Arrhenius acid solutions, protocols should define minimum requirements for potentiostat specifications, including input impedance, bandwidth, and current resolution. Statistical analysis guidelines must establish confidence intervals, outlier identification criteria, and minimum replication requirements to ensure robust conclusions.
Documentation requirements constitute the foundation of quality assurance. Comprehensive records must include detailed experimental conditions, equipment specifications, calibration histories, and raw data preservation formats. Electronic laboratory notebooks with appropriate metadata tagging facilitate data sharing and experiment reproducibility, while version control systems ensure traceability of protocol modifications over time.
Regular proficiency testing through round-robin experiments among participating laboratories helps identify systematic errors and refine protocols. These collaborative exercises, particularly important for complex electrochemical systems involving Arrhenius acids, provide valuable feedback on protocol robustness and highlight areas requiring further standardization efforts.
Solution preparation protocols require precise concentration verification methods, including titration techniques and pH measurement standards. For Arrhenius acid solutions specifically, standardized procedures must account for temperature-dependent dissociation constants, as these directly impact electrochemical behavior. Implementation of certified reference materials for calibration ensures traceability to recognized standards and facilitates inter-laboratory comparisons.
Electrode preparation and maintenance represent another critical aspect of quality control. Surface conditioning procedures, including polishing protocols and electrochemical cleaning cycles, must be standardized to ensure consistent electrode performance. Regular verification of electrode surface area and activity using well-established redox couples provides quantitative metrics for electrode quality assessment before experimentation with acid solutions.
Environmental control parameters significantly influence electrochemical measurements in acidic media. Temperature regulation within ±0.1°C is necessary, as temperature fluctuations alter reaction kinetics according to the Arrhenius equation. Similarly, protocols must specify acceptable ranges for ambient humidity and atmospheric composition, particularly when working with volatile acid solutions or when atmospheric gases might interfere with electrochemical processes.
Data acquisition standardization encompasses sampling rates, filtering parameters, and signal processing methodologies. For electrochemical performance metrics in Arrhenius acid solutions, protocols should define minimum requirements for potentiostat specifications, including input impedance, bandwidth, and current resolution. Statistical analysis guidelines must establish confidence intervals, outlier identification criteria, and minimum replication requirements to ensure robust conclusions.
Documentation requirements constitute the foundation of quality assurance. Comprehensive records must include detailed experimental conditions, equipment specifications, calibration histories, and raw data preservation formats. Electronic laboratory notebooks with appropriate metadata tagging facilitate data sharing and experiment reproducibility, while version control systems ensure traceability of protocol modifications over time.
Regular proficiency testing through round-robin experiments among participating laboratories helps identify systematic errors and refine protocols. These collaborative exercises, particularly important for complex electrochemical systems involving Arrhenius acids, provide valuable feedback on protocol robustness and highlight areas requiring further standardization efforts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!