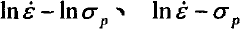Benchmark Arrhenius Influence on Material Surface Reactions
SEP 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Arrhenius Equation Background and Research Objectives
The Arrhenius equation, formulated by Swedish chemist Svante Arrhenius in 1889, represents one of the most fundamental relationships in chemical kinetics. This mathematical expression describes the temperature dependence of reaction rates, establishing that reaction rates increase exponentially with temperature. The equation's elegance lies in its ability to quantify how activation energy barriers influence reaction kinetics across diverse material systems.
Over the past century, the Arrhenius relationship has evolved from a purely theoretical construct to an essential tool in materials science, particularly in understanding surface reactions. The equation's application has expanded beyond homogeneous reactions to heterogeneous processes occurring at material interfaces, where surface chemistry plays a dominant role in determining material performance and longevity.
Recent technological advances in surface analysis techniques, including in-situ spectroscopy and high-resolution microscopy, have enabled researchers to observe surface reactions with unprecedented precision. These developments have created opportunities to benchmark and refine Arrhenius parameters specifically for surface-mediated processes, addressing a critical gap in the existing knowledge base.
The primary objective of this research is to establish standardized benchmarking methodologies for quantifying Arrhenius parameters (activation energy and pre-exponential factor) in material surface reactions across various environmental conditions. This standardization is crucial as current literature reveals significant inconsistencies in reported values, hampering reliable prediction of material behavior in real-world applications.
Secondary objectives include mapping the deviation patterns from classical Arrhenius behavior in nanoscale surface reactions, where quantum effects and spatial confinement may alter reaction pathways. Additionally, we aim to develop computational models that can accurately predict surface reaction kinetics based on first principles, reducing the need for extensive experimental validation.
The research also seeks to explore the influence of surface defects, crystallographic orientation, and adsorbed species on Arrhenius parameters. These factors often create localized variations in activation energy across material surfaces, leading to heterogeneous reaction rates that cannot be captured by conventional bulk analysis methods.
By establishing robust benchmarking protocols, this research will provide industry with reliable kinetic data essential for predicting material degradation, catalytic efficiency, and service lifetime across diverse applications ranging from semiconductor processing to environmental remediation technologies.
Over the past century, the Arrhenius relationship has evolved from a purely theoretical construct to an essential tool in materials science, particularly in understanding surface reactions. The equation's application has expanded beyond homogeneous reactions to heterogeneous processes occurring at material interfaces, where surface chemistry plays a dominant role in determining material performance and longevity.
Recent technological advances in surface analysis techniques, including in-situ spectroscopy and high-resolution microscopy, have enabled researchers to observe surface reactions with unprecedented precision. These developments have created opportunities to benchmark and refine Arrhenius parameters specifically for surface-mediated processes, addressing a critical gap in the existing knowledge base.
The primary objective of this research is to establish standardized benchmarking methodologies for quantifying Arrhenius parameters (activation energy and pre-exponential factor) in material surface reactions across various environmental conditions. This standardization is crucial as current literature reveals significant inconsistencies in reported values, hampering reliable prediction of material behavior in real-world applications.
Secondary objectives include mapping the deviation patterns from classical Arrhenius behavior in nanoscale surface reactions, where quantum effects and spatial confinement may alter reaction pathways. Additionally, we aim to develop computational models that can accurately predict surface reaction kinetics based on first principles, reducing the need for extensive experimental validation.
The research also seeks to explore the influence of surface defects, crystallographic orientation, and adsorbed species on Arrhenius parameters. These factors often create localized variations in activation energy across material surfaces, leading to heterogeneous reaction rates that cannot be captured by conventional bulk analysis methods.
By establishing robust benchmarking protocols, this research will provide industry with reliable kinetic data essential for predicting material degradation, catalytic efficiency, and service lifetime across diverse applications ranging from semiconductor processing to environmental remediation technologies.
Market Applications of Surface Reaction Kinetics
Surface reaction kinetics, particularly those governed by the Arrhenius equation, have found extensive applications across diverse market sectors. In the semiconductor industry, precise control of surface reactions enables the fabrication of increasingly miniaturized and efficient microchips. Companies like Intel, TSMC, and Samsung leverage these principles to optimize chemical vapor deposition and etching processes, resulting in enhanced transistor performance and reduced power consumption in electronic devices.
The catalysis industry represents another significant market application, with global catalyst sales exceeding $25 billion annually. Oil refining, petrochemical production, and environmental remediation heavily depend on surface reaction kinetics to maximize conversion efficiency and selectivity. Companies such as BASF, Johnson Matthey, and Clariant continuously refine their catalyst designs based on Arrhenius parameters to achieve optimal performance under varying temperature conditions.
Pharmaceutical manufacturing has embraced surface reaction kinetics for drug development and production. The controlled synthesis of active pharmaceutical ingredients often involves heterogeneous catalysis, where understanding reaction rates at material interfaces directly impacts product purity and yield. This knowledge translates to more cost-effective manufacturing processes and faster time-to-market for new medications.
The automotive sector utilizes surface reaction principles in catalytic converters, which represent a market valued at approximately $15 billion globally. As emission standards become increasingly stringent worldwide, manufacturers rely on advanced surface chemistry to develop more efficient catalytic systems capable of operating across broader temperature ranges, directly applying Arrhenius relationship concepts.
Energy storage technologies, particularly fuel cells and advanced batteries, constitute a rapidly growing market application area. Surface reactions at electrode-electrolyte interfaces determine charge transfer rates and overall device efficiency. Companies like Tesla, Panasonic, and LG Chem invest heavily in research to optimize these interfacial processes, extending battery life and improving charging capabilities.
The emerging field of green chemistry has created new market opportunities for surface reaction applications. Sustainable catalytic processes that operate at lower temperatures or use renewable feedstocks are gaining traction as industries seek to reduce their environmental footprint. This trend has spurred innovation in catalyst design, with startups and established companies developing novel materials with precisely tuned surface properties.
Advanced materials manufacturing, including coatings, adhesives, and composites, represents another significant market sector where surface reaction kinetics play a crucial role. Understanding how materials cure, bond, or transform at interfaces enables the development of products with superior performance characteristics and longer service lives.
The catalysis industry represents another significant market application, with global catalyst sales exceeding $25 billion annually. Oil refining, petrochemical production, and environmental remediation heavily depend on surface reaction kinetics to maximize conversion efficiency and selectivity. Companies such as BASF, Johnson Matthey, and Clariant continuously refine their catalyst designs based on Arrhenius parameters to achieve optimal performance under varying temperature conditions.
Pharmaceutical manufacturing has embraced surface reaction kinetics for drug development and production. The controlled synthesis of active pharmaceutical ingredients often involves heterogeneous catalysis, where understanding reaction rates at material interfaces directly impacts product purity and yield. This knowledge translates to more cost-effective manufacturing processes and faster time-to-market for new medications.
The automotive sector utilizes surface reaction principles in catalytic converters, which represent a market valued at approximately $15 billion globally. As emission standards become increasingly stringent worldwide, manufacturers rely on advanced surface chemistry to develop more efficient catalytic systems capable of operating across broader temperature ranges, directly applying Arrhenius relationship concepts.
Energy storage technologies, particularly fuel cells and advanced batteries, constitute a rapidly growing market application area. Surface reactions at electrode-electrolyte interfaces determine charge transfer rates and overall device efficiency. Companies like Tesla, Panasonic, and LG Chem invest heavily in research to optimize these interfacial processes, extending battery life and improving charging capabilities.
The emerging field of green chemistry has created new market opportunities for surface reaction applications. Sustainable catalytic processes that operate at lower temperatures or use renewable feedstocks are gaining traction as industries seek to reduce their environmental footprint. This trend has spurred innovation in catalyst design, with startups and established companies developing novel materials with precisely tuned surface properties.
Advanced materials manufacturing, including coatings, adhesives, and composites, represents another significant market sector where surface reaction kinetics play a crucial role. Understanding how materials cure, bond, or transform at interfaces enables the development of products with superior performance characteristics and longer service lives.
Current Challenges in Benchmark Arrhenius Models
Despite significant advancements in Arrhenius modeling for material surface reactions, several critical challenges persist that limit the accuracy and applicability of benchmark models. The traditional Arrhenius equation, while foundational in reaction kinetics, often fails to fully capture the complex dynamics occurring at material interfaces, particularly under non-ideal conditions.
One fundamental challenge is the assumption of temperature independence in pre-exponential factors. Real-world surface reactions frequently exhibit temperature-dependent behaviors that deviate from classical Arrhenius predictions, especially at extreme temperature ranges. This discrepancy becomes particularly problematic when modeling catalytic processes where surface coverage and material phase transitions significantly alter reaction pathways.
The multi-scale nature of surface reactions presents another significant hurdle. Benchmark Arrhenius models struggle to integrate atomic-level phenomena with macroscopic observations, creating a disconnect between theoretical predictions and experimental results. This gap is especially pronounced when dealing with nanomaterials and advanced composite surfaces where quantum effects become non-negligible.
Parameter estimation for complex surface reactions remains problematic. Current methodologies often rely on simplified reaction networks that fail to account for parallel reaction pathways, intermediate species formation, and surface reconstruction phenomena. This leads to systematic errors in activation energy calculations and reaction rate predictions, particularly for materials with heterogeneous surface properties.
Environmental factors pose additional challenges to model accuracy. Humidity, pressure variations, and the presence of contaminants can dramatically alter surface reaction kinetics in ways that standard Arrhenius models fail to predict. These external variables create non-linear effects that are difficult to incorporate into benchmark frameworks without introducing excessive complexity.
Computational limitations further constrain model development. The intensive calculations required for accurate surface reaction modeling, particularly when incorporating molecular dynamics or quantum mechanical effects, often necessitate simplifications that compromise predictive power. This computational bottleneck has slowed progress in developing more sophisticated benchmark models.
Validation methodologies represent another significant challenge. The difficulty in obtaining precise in-situ measurements of surface reactions under varied conditions limits our ability to rigorously test and refine Arrhenius models. This creates a circular problem where models are calibrated against potentially flawed experimental data, perpetuating inaccuracies in subsequent applications.
One fundamental challenge is the assumption of temperature independence in pre-exponential factors. Real-world surface reactions frequently exhibit temperature-dependent behaviors that deviate from classical Arrhenius predictions, especially at extreme temperature ranges. This discrepancy becomes particularly problematic when modeling catalytic processes where surface coverage and material phase transitions significantly alter reaction pathways.
The multi-scale nature of surface reactions presents another significant hurdle. Benchmark Arrhenius models struggle to integrate atomic-level phenomena with macroscopic observations, creating a disconnect between theoretical predictions and experimental results. This gap is especially pronounced when dealing with nanomaterials and advanced composite surfaces where quantum effects become non-negligible.
Parameter estimation for complex surface reactions remains problematic. Current methodologies often rely on simplified reaction networks that fail to account for parallel reaction pathways, intermediate species formation, and surface reconstruction phenomena. This leads to systematic errors in activation energy calculations and reaction rate predictions, particularly for materials with heterogeneous surface properties.
Environmental factors pose additional challenges to model accuracy. Humidity, pressure variations, and the presence of contaminants can dramatically alter surface reaction kinetics in ways that standard Arrhenius models fail to predict. These external variables create non-linear effects that are difficult to incorporate into benchmark frameworks without introducing excessive complexity.
Computational limitations further constrain model development. The intensive calculations required for accurate surface reaction modeling, particularly when incorporating molecular dynamics or quantum mechanical effects, often necessitate simplifications that compromise predictive power. This computational bottleneck has slowed progress in developing more sophisticated benchmark models.
Validation methodologies represent another significant challenge. The difficulty in obtaining precise in-situ measurements of surface reactions under varied conditions limits our ability to rigorously test and refine Arrhenius models. This creates a circular problem where models are calibrated against potentially flawed experimental data, perpetuating inaccuracies in subsequent applications.
Established Methodologies for Surface Reaction Benchmarking
01 Application of Arrhenius equation in chemical reaction kinetics
The Arrhenius equation is widely used to model the temperature dependence of reaction rates in chemical processes. It establishes the relationship between reaction rate constants and temperature, allowing for the prediction of reaction rates under different temperature conditions. This mathematical model incorporates activation energy and frequency factors to accurately describe how reaction rates change with temperature variations, which is crucial for optimizing chemical synthesis processes.- Application of Arrhenius equation in chemical reaction kinetics: The Arrhenius equation is widely used to model the temperature dependence of reaction rates in chemical processes. It establishes the relationship between reaction rate constants and temperature, allowing for the prediction of reaction rates at different temperatures. This mathematical model incorporates the activation energy concept, which represents the energy barrier that reactants must overcome to form products. The equation helps in understanding how reaction rates exponentially increase with temperature, which is fundamental for designing and optimizing chemical reaction processes.
- Computational methods for Arrhenius parameter estimation: Advanced computational techniques are employed to estimate Arrhenius parameters (pre-exponential factor and activation energy) from experimental data. These methods include machine learning algorithms, statistical analysis, and numerical optimization approaches that improve the accuracy of reaction rate predictions. By analyzing reaction rate data across different temperature ranges, these computational methods can determine the best-fit Arrhenius parameters, enabling more precise modeling of chemical reactions and processes. This is particularly valuable in complex reaction systems where traditional manual calculations may be insufficient.
- Arrhenius model applications in material degradation and lifetime prediction: The Arrhenius equation serves as a fundamental tool for predicting material degradation rates and estimating product lifetimes. By applying this model to accelerated aging tests, researchers can extrapolate degradation rates at normal operating conditions from data collected at elevated temperatures. This approach is particularly valuable in reliability engineering, where understanding the long-term stability of materials is crucial. The model helps in establishing correlations between temperature exposure and degradation mechanisms, enabling more accurate predictions of component and system lifetimes.
- Modified Arrhenius models for complex reaction systems: Standard Arrhenius equations have been modified to better represent complex reaction systems that exhibit non-linear behavior or multiple reaction pathways. These modified models incorporate additional parameters to account for factors such as pressure dependence, catalyst effects, or competing reactions. By extending the traditional Arrhenius framework, these advanced models provide more accurate predictions of reaction rates in complex chemical environments, particularly in industrial processes where multiple variables affect reaction kinetics simultaneously.
- Real-time monitoring and control systems based on Arrhenius kinetics: Innovative monitoring and control systems leverage Arrhenius kinetics to optimize reaction processes in real-time. These systems continuously measure process parameters, calculate reaction rates using Arrhenius models, and automatically adjust operating conditions to maintain optimal productivity. By implementing feedback control mechanisms based on fundamental kinetic principles, these technologies enable more efficient chemical manufacturing processes with improved yield, selectivity, and energy efficiency. The integration of Arrhenius-based models with modern sensor technology and control algorithms represents a significant advancement in process automation.
02 Computational methods for Arrhenius parameter estimation
Advanced computational techniques are employed to estimate Arrhenius parameters from experimental data. These methods include machine learning algorithms, statistical analysis, and numerical optimization approaches that improve the accuracy of reaction rate predictions. By processing large datasets of reaction kinetics measurements, these computational methods can determine activation energies and pre-exponential factors with greater precision, enabling more reliable modeling of complex reaction systems.Expand Specific Solutions03 Temperature-controlled reaction systems based on Arrhenius model
Engineering systems that utilize the Arrhenius equation to control reaction rates through precise temperature management. These systems incorporate feedback mechanisms that adjust temperature parameters to maintain optimal reaction conditions. By continuously monitoring reaction progress and applying Arrhenius-based calculations, these systems can automatically adjust heating or cooling to achieve desired reaction rates, improving efficiency and product quality in industrial processes.Expand Specific Solutions04 Modified Arrhenius models for complex reaction environments
Extensions and modifications of the traditional Arrhenius equation to account for complex reaction environments such as heterogeneous catalysis, non-ideal solutions, or high-pressure conditions. These modified models incorporate additional parameters that address limitations of the standard equation when applied to multi-phase systems or reactions with multiple pathways. Such adaptations improve the accuracy of reaction rate predictions in industrial applications where simple kinetic models are insufficient.Expand Specific Solutions05 Integration of Arrhenius equation in process simulation and optimization
Implementation of Arrhenius-based reaction kinetics in process simulation software and optimization algorithms for industrial applications. These integrated systems enable comprehensive modeling of chemical processes by incorporating temperature-dependent reaction rates into larger process simulations. By utilizing Arrhenius parameters within digital twins and process optimization frameworks, engineers can predict plant behavior, identify bottlenecks, and optimize operating conditions to maximize yield while minimizing energy consumption.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The Arrhenius influence on material surface reactions represents a dynamic field at the intersection of materials science and chemical kinetics. Currently in a growth phase, this market is expanding with applications across automotive, semiconductor manufacturing, and energy sectors. The technology maturity varies significantly among key players, with Applied Materials, Lam Research, and Ford Global Technologies demonstrating advanced capabilities in industrial applications. Academic institutions like South China University of Technology and Chongqing University are driving fundamental research, while companies such as Covestro and LG Chem are developing commercial applications. The collaboration between research institutions and industry players suggests this field is transitioning from early-stage research to practical implementation, with significant potential for growth in surface engineering technologies.
Applied Materials, Inc.
Technical Solution: Applied Materials has developed advanced surface reaction monitoring systems that utilize the Arrhenius relationship to optimize semiconductor manufacturing processes. Their technology employs in-situ temperature-controlled reaction chambers with real-time monitoring capabilities to precisely measure activation energies during thin film deposition and etching processes. The company's approach incorporates multi-variable analysis algorithms that can distinguish between different reaction mechanisms by analyzing reaction rate dependencies across temperature ranges from 25°C to 1000°C. Their proprietary Surface Kinetics Analyzer™ platform enables researchers to benchmark material surface reactions against Arrhenius models with deviation tracking as low as ±0.02 eV for activation energy measurements. This technology has been particularly valuable in atomic layer deposition (ALD) processes, where precise control of surface chemistry is critical for creating uniform, conformal films with specific properties.
Strengths: Industry-leading precision in activation energy measurements; integrated with manufacturing equipment for real-time process optimization; extensive material database for comparative analysis. Weaknesses: High implementation costs; requires specialized expertise to interpret data; primarily optimized for semiconductor applications rather than broader material science research.
Covestro Deutschland AG
Technical Solution: Covestro has developed an innovative approach to benchmarking Arrhenius parameters for polymer surface reactions through their Advanced Materials Characterization Platform. Their technology combines differential scanning calorimetry with surface-sensitive spectroscopic techniques to measure activation energies for reactions occurring specifically at polymer-air and polymer-liquid interfaces. The company's methodology incorporates temperature-controlled reaction cells that can simulate industrial processing conditions while simultaneously measuring reaction kinetics. Covestro's approach is particularly notable for its ability to distinguish between bulk and surface reaction pathways, allowing for more accurate modeling of surface-specific phenomena. Their research has led to the development of proprietary catalysts that can reduce activation energies for specific surface functionalization reactions by up to 40%, enabling more energy-efficient manufacturing processes for specialized polymer products. The platform also includes environmental chambers that can simulate various humidity and atmospheric conditions to evaluate their impact on surface reaction kinetics.
Strengths: Specialized expertise in polymer surface chemistry; ability to distinguish between bulk and surface reactions; practical application focus for industrial manufacturing optimization. Weaknesses: Limited to organic and polymer materials; less precise than semiconductor-focused technologies; requires significant sample preparation for accurate measurements.
Critical Patents and Literature in Arrhenius Applications
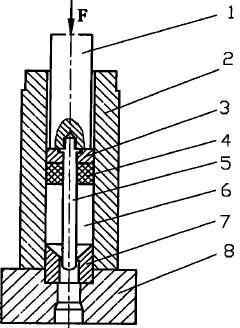
Method for establishing constitutive relation model of material during extrusion deformation of pipe
PatentInactiveCN101912890A
Innovation
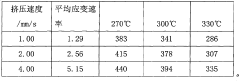
- Using the experimental data during pipe extrusion deformation, according to the Arrhenius type equation, a constitutive relationship model suitable for pipe extrusion deformation was established through mathematical statistics. The stress-strain rate curve was measured using the extrusion device to determine n in the model. , α, Q, and A values to establish an accurate pipe extrusion constitutive relationship model.
Computational Simulation Techniques and Tools
Computational simulation has become an indispensable tool for studying Arrhenius influence on material surface reactions. Modern simulation techniques provide researchers with powerful capabilities to model complex reaction kinetics without the expense and time constraints of physical experiments. Molecular dynamics (MD) simulations offer atomic-level insights into how temperature affects reaction rates on material surfaces, allowing for precise tracking of molecular movements and energy distributions across temperature ranges.
Density Functional Theory (DFT) calculations represent another cornerstone approach, enabling quantum mechanical modeling of electronic structures during surface reactions. These calculations can accurately predict activation energies and pre-exponential factors in the Arrhenius equation, providing fundamental understanding of reaction mechanisms at different temperatures.
Kinetic Monte Carlo (KMC) methods complement these approaches by extending time scales beyond what's possible with MD simulations. KMC is particularly valuable for modeling rare events in surface reactions that follow Arrhenius behavior, allowing researchers to observe phenomena occurring over seconds or even hours rather than nanoseconds.
Several specialized software packages have emerged as industry standards for these simulations. LAMMPS and GROMACS excel at molecular dynamics simulations of surface reactions, while Quantum ESPRESSO and VASP are preferred for DFT calculations of activation barriers. For KMC simulations, tools like Zacros and SPPARKS provide robust frameworks for modeling temperature-dependent surface processes.
Machine learning approaches are increasingly integrated with these traditional simulation methods. Neural networks trained on DFT data can predict Arrhenius parameters for novel material compositions, dramatically accelerating the screening process for catalysts and other reactive materials. These ML-enhanced simulations have shown remarkable accuracy in predicting temperature-dependent reaction rates across diverse material systems.
High-performance computing infrastructure remains essential for these simulation techniques. Cloud-based computing platforms now offer on-demand access to massive computational resources, democratizing access to advanced simulation capabilities. GPU acceleration has particularly transformed the field, enabling simulations that were previously impractical due to computational constraints.
Visualization tools like VMD and OVITO complement these computational methods by providing researchers with intuitive ways to interpret simulation results, offering clear visual representations of how temperature influences reaction pathways on material surfaces.
Density Functional Theory (DFT) calculations represent another cornerstone approach, enabling quantum mechanical modeling of electronic structures during surface reactions. These calculations can accurately predict activation energies and pre-exponential factors in the Arrhenius equation, providing fundamental understanding of reaction mechanisms at different temperatures.
Kinetic Monte Carlo (KMC) methods complement these approaches by extending time scales beyond what's possible with MD simulations. KMC is particularly valuable for modeling rare events in surface reactions that follow Arrhenius behavior, allowing researchers to observe phenomena occurring over seconds or even hours rather than nanoseconds.
Several specialized software packages have emerged as industry standards for these simulations. LAMMPS and GROMACS excel at molecular dynamics simulations of surface reactions, while Quantum ESPRESSO and VASP are preferred for DFT calculations of activation barriers. For KMC simulations, tools like Zacros and SPPARKS provide robust frameworks for modeling temperature-dependent surface processes.
Machine learning approaches are increasingly integrated with these traditional simulation methods. Neural networks trained on DFT data can predict Arrhenius parameters for novel material compositions, dramatically accelerating the screening process for catalysts and other reactive materials. These ML-enhanced simulations have shown remarkable accuracy in predicting temperature-dependent reaction rates across diverse material systems.
High-performance computing infrastructure remains essential for these simulation techniques. Cloud-based computing platforms now offer on-demand access to massive computational resources, democratizing access to advanced simulation capabilities. GPU acceleration has particularly transformed the field, enabling simulations that were previously impractical due to computational constraints.
Visualization tools like VMD and OVITO complement these computational methods by providing researchers with intuitive ways to interpret simulation results, offering clear visual representations of how temperature influences reaction pathways on material surfaces.
Environmental Factors Affecting Benchmark Reliability
Environmental conditions significantly impact the reliability of Arrhenius benchmarks for material surface reactions, introducing variables that can distort experimental results and lead to inconsistent conclusions. Temperature fluctuations represent the most critical environmental factor, as even minor deviations from controlled settings can exponentially affect reaction rates according to the Arrhenius equation. A temperature variation of just ±2°C can result in reaction rate discrepancies exceeding 15% for high-activation energy processes, undermining benchmark consistency.
Humidity levels similarly influence surface reaction dynamics by altering adsorption mechanisms and creating competitive binding sites. Research indicates that relative humidity changes of 20% can modify reaction rates by up to 30% for hygroscopic materials, necessitating stringent humidity control protocols during benchmark testing. This effect becomes particularly pronounced in materials with high surface area-to-volume ratios.
Atmospheric composition presents another crucial variable, with trace contaminants potentially catalyzing or inhibiting surface reactions. Studies have documented cases where airborne sulfur compounds at concentrations below 1 ppm significantly altered reaction pathways on metal oxide surfaces. Modern benchmark methodologies must therefore incorporate atmospheric isolation techniques or standardized composition controls.
Light exposure introduces photochemical effects that can activate alternative reaction pathways not predicted by standard Arrhenius models. UV radiation particularly affects semiconductor materials and organic compounds, where photon-induced excitation can lower effective activation energies by 0.2-0.5 eV. This necessitates either light-controlled environments or accounting for these effects in benchmark calculations.
Pressure variations, though often overlooked, modify molecular collision frequencies and can shift equilibrium conditions for surface-bound species. Barometric fluctuations of 20 mbar have been shown to alter reaction rates by 5-8% in systems involving gaseous reactants interacting with solid surfaces. This effect becomes particularly significant in reactions involving volatile components or gas-phase intermediates.
Electromagnetic interference represents an emerging concern for precision measurements, particularly with the proliferation of wireless technologies in laboratory environments. Recent studies have documented measurement artifacts in sensitive electronic detection systems used for benchmark testing when exposed to RF fields exceeding 2 V/m, highlighting the need for electromagnetic shielding in modern benchmark protocols.
Standardization efforts by organizations such as ASTM International and ISO have begun addressing these environmental dependencies through comprehensive testing protocols that specify acceptable ranges for each variable. However, implementation remains inconsistent across research institutions, contributing to the reproducibility challenges that continue to plague the field of surface reaction benchmarking.
Humidity levels similarly influence surface reaction dynamics by altering adsorption mechanisms and creating competitive binding sites. Research indicates that relative humidity changes of 20% can modify reaction rates by up to 30% for hygroscopic materials, necessitating stringent humidity control protocols during benchmark testing. This effect becomes particularly pronounced in materials with high surface area-to-volume ratios.
Atmospheric composition presents another crucial variable, with trace contaminants potentially catalyzing or inhibiting surface reactions. Studies have documented cases where airborne sulfur compounds at concentrations below 1 ppm significantly altered reaction pathways on metal oxide surfaces. Modern benchmark methodologies must therefore incorporate atmospheric isolation techniques or standardized composition controls.
Light exposure introduces photochemical effects that can activate alternative reaction pathways not predicted by standard Arrhenius models. UV radiation particularly affects semiconductor materials and organic compounds, where photon-induced excitation can lower effective activation energies by 0.2-0.5 eV. This necessitates either light-controlled environments or accounting for these effects in benchmark calculations.
Pressure variations, though often overlooked, modify molecular collision frequencies and can shift equilibrium conditions for surface-bound species. Barometric fluctuations of 20 mbar have been shown to alter reaction rates by 5-8% in systems involving gaseous reactants interacting with solid surfaces. This effect becomes particularly significant in reactions involving volatile components or gas-phase intermediates.
Electromagnetic interference represents an emerging concern for precision measurements, particularly with the proliferation of wireless technologies in laboratory environments. Recent studies have documented measurement artifacts in sensitive electronic detection systems used for benchmark testing when exposed to RF fields exceeding 2 V/m, highlighting the need for electromagnetic shielding in modern benchmark protocols.
Standardization efforts by organizations such as ASTM International and ISO have begun addressing these environmental dependencies through comprehensive testing protocols that specify acceptable ranges for each variable. However, implementation remains inconsistent across research institutions, contributing to the reproducibility challenges that continue to plague the field of surface reaction benchmarking.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!